Published online Oct 7, 2013. doi: 10.3748/wjg.v19.i37.6144
Revised: July 18, 2013
Accepted: August 4, 2013
Published online: October 7, 2013
Processing time: 199 Days and 1 Hours
Hepatocellular carcinoma (HCC) is one of the most frequent tumors worldwide. The majority of HCC cases occur in patients with chronic liver disease. Despite regular surveillance to detect small HCC in these patients, HCC is often diagnosed at an advanced stage. Because HCC is highly resistant to conventional systemic therapies, the prognosis for advanced HCC patients remains poor. The introduction of sorafenib as the standard systemic therapy has unveiled a new direction for future research regarding HCC treatment. However, given the limited efficacy of the drug, a need exists to look beyond sorafenib. Many molecular targeted agents that inhibit different pathways involved in hepatocarcinogenesis are under various phases of clinical development, and novel targets are being assessed in HCC. This review aims to summarize the efforts to target molecular components of the signaling pathways that are responsible for the development and progression of HCC and to discuss perspectives on the future direction of research.
Core tip: Sorafenib is the first drug to prolong survival of patients with advanced hepatocellular carcinoma (HCC). This advance has shifted the paradigm of systemic treatment for HCC toward molecular targeted therapy. This review aims to summarize the efforts to target molecular components of the signaling pathways that are responsible for the development and progression of HCC and to discuss perspectives on the future direction of research.
- Citation: Shin JW, Chung YH. Molecular targeted therapy for hepatocellular carcinoma: Current and future. World J Gastroenterol 2013; 19(37): 6144-6155
- URL: https://www.wjgnet.com/1007-9327/full/v19/i37/6144.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i37.6144
Hepatocellular carcinoma (HCC) is a common solid cancer and the third most frequent cause of cancer-related mortality worldwide. The 5-year relative survival rate for patients with HCC is only 7%, and very few patients with symptomatic disease survive for > 1 year[1]. One of the primary reasons for the poor prognosis of patients with HCC is the lack of effective treatment options, especially for those with advanced disease. Although surgery and percutaneous ablation can achieve long-term control in some patients with early HCC, fewer than 40% of patients are diagnosed at early stages; hence, only a minority of HCC patients are eligible for potentially curative therapies, such as resection, transplantation, or percutaneous ablation[2]. Furthermore, systemic therapies (such as standard chemotherapeutic agents) do not provide significant efficacy in HCC based on randomized trials[3].
In recent years, improved knowledge of the oncogenic processes and signaling pathways that regulate tumor cell proliferation, differentiation, angiogenesis, invasion and metastasis has led to the identification of several potential therapeutic targets, which has driven the development of molecularly targeted therapies. An ideal cancer target meets the following criteria: (1) the target is relatively specific for cancer cells (not expressed or expressed at very low levels in normal cells but overexpressed in cancer cells). Meanwhile, overexpression of the target is associated with malignant biological phenotypes and/or poor prognosis; (2) inhibition of the target is efficacious (the target plays an essential role in cancer initiation and progression, and inhibition of expression or activity of the target induces growth suppression and/or apoptosis in cancer cells); and (3) the target is “drugable” as an enzyme (e.g., a kinase) or a cell surface molecule (e.g., a membrane-bound receptor) that can be easily screened for small-molecule inhibitors or targeted by a specific antibody[4].
The aim of this article is to review the efforts to target molecular components of the signaling pathways that are responsible for the development and progression of HCC and to summarize the evidence for the clinical activity of these agents in patients with HCC.
Hepatocarcinogenesis is a multistep process initiated by external stimuli that lead to genetic changes in hepatocytes or stem cells, resulting in proliferation, apoptosis, dysplasia and neoplasia. The majority of HCC cases are related to chronic viral infections. However, the mechanisms by which hepatitis B virus (HBV) or hepatitis C virus (HCV) induce malignant transformation seem to differ. HBV DNA integrates into the host genome, inducing chromosome instability and insertional mutations that may activate various oncogenes, such as cyclin A[5-7]. Viral proteins, in particular X protein (HBx), act as transactivators to upregulate several oncogenes (such as c-myc and c-jun) and transcriptional factors [(such as nuclear factor-κB][8-10]. Additionally, HBx activates promoters of genes encoding IL-8, tumor necrosis factor (TNF), transforming growth factor (TGF)-β and epidermal growth factor receptor (EGFR)[11]. HBx can also stimulate several signal transduction pathways, including the JAK/STAT, RAS/RAF/MAPK, and Wnt/β-catenin pathways[12-14].
The contributions of HCV to hepatocarcinogenesis are mediated by viral proteins, including core, NS3 and NS5A proteins. HCV core protein can promote apoptosis or cell proliferation through interaction with p53 or upregulation of Wnt-1 at the transcriptional level[15,16]. NS4A and NS4B proteins mediate translational inhibition and degradation of various cellular proteins[17]. Cirrhosis is present in approximately 80%-90% of HCC patients and constitutes the largest single risk factor. In cirrhotic liver, changes in fat metabolism associated with the activation of adipocyte-like pathways are thought to be involved in neoplastic transformation[18].
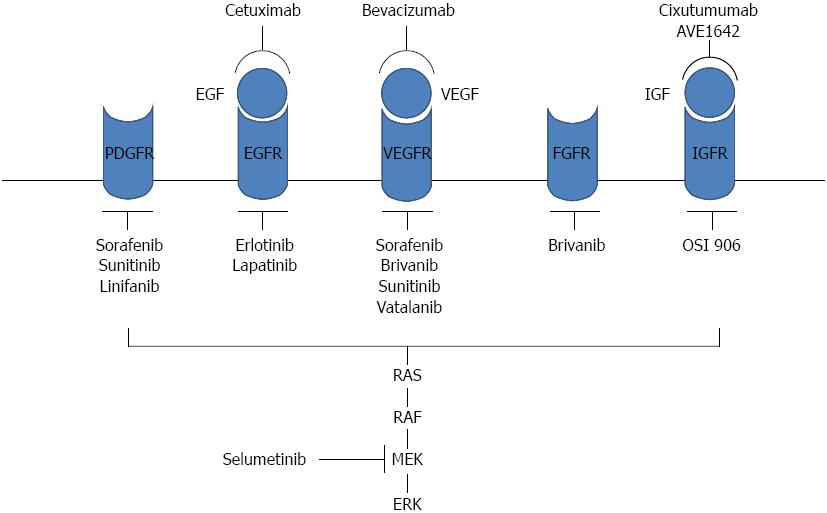
The Raf/MAPK/extracellular-signal-regulated kinase (ERK) pathway is an important pro-survival signaling pathway that is primarily involved in cell growth and survival and regulates cell differentiation. This pathway transduces extracellular signals form membrane-bound tyrosine kinase receptors, such as EGFR, insulin-like growth factor receptor (IGFR), vascular endothelial growth factor receptor (VEGFR), c-Met and platelet-derived growth factor receptor (PDGFR), to the nucleus. Growth factor binding results in receptor phosphorylation, which activates an adapter molecule complex known as GRB2/SHC/SOS. This sequence in turn activates the RAF/mitogen/extracellular protein kinase (MEK)/ERK pathway, which triggers a cascade of specific phosphorylation events[19]. Within this pathway, the small GTPase RAS and the serine/threonine kinase Raf are the key signal regulators[20]. Intermediate signaling is regulated by MEK1 and MEK2, which are responsible for phosphorylating and activating the final downstream signaling molecules extracellular-regulated protein kinases (ERK)1 and ERK2[21]. ERK1/2 regulates cellular activity by acting on more than 100 substrates in the cytoplasm and nucleus. RAS also regulates the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway, the phospholipase C/protein kinase C pathway and the ral guanine nucleotide dissociation stimulator pathway[22,23].
Up-regulated activation of the Raf/MAPK/ERK signaling pathway has been well documented in HCC and correlates with advanced stage[24,25]. Mechanisms for the increased activity of the Raf/MAPK/ERK signaling pathway in HCC include down-regulation of Raf kinase inhibitor protein (a suppressor of the Raf/MAPK/ERK pathway) and induction by hepatitis viral proteins (such as the hepatitis B X protein and the hepatitis C core protein)[26-28].
Targeting Raf kinase is one of the most promising targeted approaches for the treatment of HCC. Sorafenib has strong inhibitory activity against Raf-1 (C-Raf) kinase and B-Raf (wild-type B-Raf and mutant V600E B-Raf) and has been shown to inhibit other serine/threonine kinases, the pro-angiogenic receptor tyrosine kinases VEGFR, PDGFR and FGFR1, and tyrosine kinases such as c-kit, Flt-3 and RET, which are involved in tumor progression and overall prognosis (Figure 1)[29].
Selumetinib (AZD6244) is an oral non-ATP-competitive small-molecule inhibitor of the mitogen-activated protein kinase MEK1/2. A recent study has shown that selumetinib plus rapamycin exerts antitumor and antiangiogenic effects in preclinical models of human HCC[30]. In a phase I/II study of selumetinib in combination with sorafenib in advanced HCC, the objective responses were 3 partial response (PR), 6 stable disease (SD) and 2 progressive disease (PD) among 11 patients, and the common toxicities were diarrhea, rash, fatigue, and hypertension[31].
Another phase I/II study has evaluated the combination of the MEK inhibitor RDEA119 and sorafenib in patients with advanced cancer (NCT00785226).
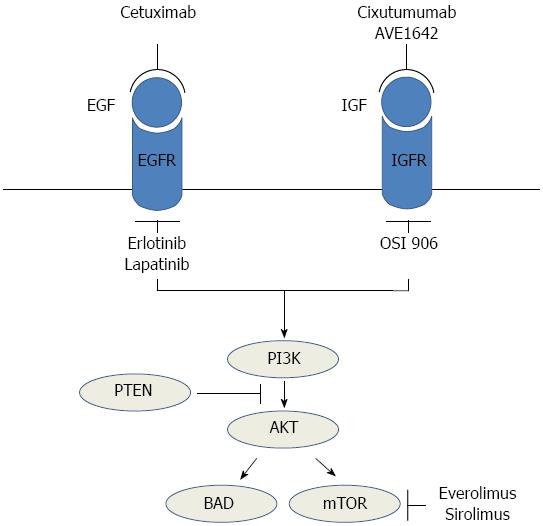
The PI3K/Akt/mTOR pathway also plays an important role in cell growth, survival regulation, metabolism and anti-apoptosis[32]. The binding of growth factors (such as IGF and EGF) to their receptors activates PI3K[19]. PI3K subsequently produces the lipid second messenger PIP3b (phospho-inositol triphosphate), which in turn activates the serine/threonine kinase AKT. Activated AKT also phosphorylates several cytoplasmic proteins, most notably mTOR and BCL-2-associated death promoter[19]. The activation of mTOR increases cellular proliferation, and inactivation of BAD decreases apoptosis and increases cell survival[21]. In normal tissue, this pathway is negatively regulated by the tumor suppressor phosphatase on chromosome 10 [phosphatase and tensin homolog (PTEN)], which targets the lipid products of PI3K for dephosphorylation[21].
Expression of both IGF and the IGF receptor is up-regulated in HCC and cirrhotic liver, resulting in stimulation of the PI3K/AKT/mTOR signaling pathway in addition to activation of the RAF/MEK/ERK and WNT/β-catenin pathways[33,34]. Anomalies in PTEN function may lead to overactivation of the PI3K/AKT/mTOR pathway in HCC. PTEN expression is reduced in nearly half of all HCC tumors, resulting in constitutive activation of the PI3K/AKT/mTOR pathway[35]. Decreased PTEN expression has been shown to correlate with increased tumor grade, advanced disease stage and reduced overall survival (OS) in patients with HCC[35]. In a mutation analysis of HCC samples, activation of the IGF pathway, upregulation of EGF, dysregulation of PTEN, and aberrant mTOR signaling were present in half of the samples; inhibition of mTOR activity with a rapamycin analog (everolimus) was effective in improving survival and suppressing recurrence[36]. These results suggest that mTOR pathway activation plays a crucial role in the pathogenesis of HCC (Figure 2).
The PI3K inhibitor RG7321 and the Akt inhibitor perifosine target the PI3K/Akt/mTOR pathway and are in early stages of clinical development. The mTOR inhibitors everolimus (RAD001), sirolimus (Rapamune) and temsirolimus (CCI-779) have been studied for efficacy and safety in patients with advanced HCC. Everolimus has produced a median progression-free survival (PFS) of 3.8 mo and OS of 8.4 mo in phase I/II testing in patients with advanced HCC[37]. A phase III study to compare everolimus and placebo and a phase I/randomized phase II study (sorafenib + everolimus vs sorafenib alone) to test the efficacy and tolerance of sorafenib in combination with everolimus are underway (NCT01035229). In a phase II study of sirolimus in patients with advanced HCC, sirolimus exhibited some antitumor activity in patients with advanced HCC[38]. However, larger studies are required to determine the value of this agent.
Temsirolimus, an mTOR inhibitor, has been approved for the treatment of advanced renal cell carcinoma. The efficacy and potential utility of this agent in HCC is currently under investigation (NCT01079767).
Normal angiogenesis is maintained by a balance between proangiogenic and anti-angiogenic factors[39]. The angiogenic balance is disturbed in HCC. Angiogenesis is important for HCC growth and metastasis and occurs as a result of complex alterations that involve promoting factors [such as VEGF, angiopoietin and fibroblast growth factor (FGF), inhibitory factors, including thrombospondin (TSP) and angiostatin], and the surrounding tissue. A number of angiogenic growth factors, including VEGF-A, angiopoietin-2 and PDGF, have been shown to be upregulated in HCC tumors at the gene expression level and plasma protein level in patients with HCC compared with cirrhotic patients[40]. The principal angiogenic factors involved are VEGFs, PDGFs, TGF-alpha and -beta, basic FGF, EGF, hepatocyte growth factor (HGF), angiopoietins and interleukin-4 and -8[39,41]. These growth factors and cytokines induce angiogenic signaling through a variety of mechanisms, including activation of the RAF/MEK/ERK, PI3K/AKT/mTOR and JAK/signal transducer and activator pathways.
Increased VEGF expression has been reported in cirrhotic and dysplastic liver tissue, suggesting a possible role for VEGF-mediated angiogenesis in hepatocarcinogenesis[42]. VEGF clearly plays an important regulatory role in HCC; high levels of VEGF expression have been linked with HCC tumor grade, poor outcome after resection, disease recurrence, poor disease-free survival (DFS) and OS, vascular invasion and portal vein emboli[43-46]. Expression of FGF-2 is also elevated in patients with HCC, and FGF-2 expression in HCC correlates with tumor microvessel density and postoperative recurrence rate[47-49]. Tumor angiogenin expression correlates with microvascular density in patients with HCC, and high serum angiogenin levels are associated with decreased survival at 5 years[50].
The VEGF pathway can be targeted through two approaches: anti-VEGF monoclonal antibodies or inhibitors of the receptor tyrosine kinase associated with VEGFR. The anti-VEGF monoclonal antibody bevacizumab was the first angiogenesis inhibitor to be approved as an antineoplastic agent. Bevacizumab has shown encouraging early evidence of efficacy in patients with advanced HCC[51,52]. The combination of bevacizumab with either cytotoxic agents (gemcitabine, oxaliplatin, and capecitabine) or erlotinib has also shown encouraging results in four phase II trials in patients with advanced HCC[53-56].
Sorafenib is an orally available multikinase inhibitor that was originally designed to target VEGFR-1, -2, -3, PDGFR and c-kit. In a phase II study of patients with advanced inoperable HCC, sorafenib induced a PR in 2% of the patients. The median time to progression (TTP) was 4.2 mo and median OS was 9.2 mo[57]. In the phase III SHARP (Sorafenib HCC Assessment Randomized Protocol) trial, sorafenib (400 mg twice daily) significantly prolonged OS compared with placebo in patients with advanced HCC (10.7 mo in the sorafenib group vs 7.9 mo in the placebo group)[58]. The median time to radiological progression was significantly longer in the sorafenib group (5.5 mo vs 2.8 mo). In another randomized phase III study performed in the Asia-Pacific region, the OS was 6.5 mo in the sorafenib group compared with 4.2 mo in the placebo group (the hazard ratio in the sorafenib group was 0.68, P = 0.014)[59]. Sorafenib is the only targeted therapy to have been approved for clinical use in several countries, including the United States and in Europe. Although sorafenib improved OS in patients with HCC, the associated toxicities may significantly affect patients’ quality of life. High rates of dermatologic side effects have commonly been reported with sorafenib, the most clinically significant of which is hand-foot skin reaction[60]. Despite initial responses to sorafenib, most HCC patients experience a loss of efficacy. No effective second-line treatment options currently exist for patients who are resistant or refractory to and/or intolerant of sorafenib.
Beyond sorafenib, sunitinib is the most studied multikinase inhibitor targeting VEGFR-1 and VEGFR-2. Sunitinib also displays inhibitory activities against other receptor tyrosine kinases, including PDGFR-a/b, c-KIT, FLT3, and RET kinases. Sunitinib is currently indicated for the treatment of renal cell carcinoma and gastrointestinal stromal tumors[61-63]. Two phase II studies of sunitinib in patients with advanced HCC have been performed. In the first study, the PR rate was 2.9%, and 50% of the patients achieved stable disease[64]. In a second phase II study, one (2.7%) patient experienced a confirmed PR and 13 (35%) of 37 patients achieved stable disease[65]. A phase III study comparing sunitinib with sorafenib (NCT00699374) was discontinued due to a greater incidence of adverse events in the sunitinib group and because sunitinib failed to demonstrate superiority or non-inferiority to sorafenib in extending the survival of patients with advanced HCC.
Brivanib is a dual inhibitor of VEGFR and fibroblast growth factor receptor signaling pathways. Because FGF signaling may contribute to acquired “resistance” or compensatory signaling during anti-VEGFR therapy, the simultaneous inhibition of these 2 pathways by brivanib may both delay initial progression in response to antiangiogenic therapy (as first-line treatment) and successfully treat tumors that have already progressed during the course of anti-VEGFR therapy (as second-line treatment)[66,67]. Brivanib has demonstrated a disease control rate of 51% and an OS of 10 mo as first-line monotherapy in a phase II trial of predominantly Asian patients with HCC[68]. In another phase II trial of brivanib in patients with HCC who had been treated with sorafenib, brivanib caused a tumor response rate of 4.3% and disease control rate of 45.7%[69].
Large randomized phase III Brivanib Study in Patients at Risk (BRISK) HCC program trials have been conducted to evaluate the role of brivanib in advanced HCC (BRISK-FL, BRISK-PS and BRISK-APS). The BRISK-PS trial evaluated brivanib vs placebo in patients who had failed or were intolerant to sorafenib therapy (NCT00825955). This study did not meet its primary end point of improving OS, but treatment with brivanib showed improvements in the response rate[70]. The BRISK-FL trial (NCT00858871) directly compared the clinical outcomes of brivanib vs sorafenib in patients with advanced HCC who received no prior systemic therapy. The median OS was 9.5 mo in the brivanib arm compared with 9.9 mo in the sorafenib arm, which was not a statistically significant difference. No significant survival differences were observed between subgroups based on geographic regions, cause of HCC or disease severity. The study did not meet its primary OS objective based upon a non-inferiority statistical design[71].
Vatalanib (PTK787) is a potent tyrosine kinase inhibitor that binds directly to the ATP-binding sites of VEGF receptors. Vatalanib inhibits both Flt-1 and Flk-1/KDR and other class III receptor tyrosine kinases, such as PDGFR-β, Flt-4, c-kit, and c-fms[72]. In a phase I/II study of vatalanib combined with doxorubicin in patients with advanced HCC, the overall response rate was 26.0%, with all of the responding patients achieving PR. Another 20% of the patients achieved SD for at least 12 wk[73].
Linifanib (ABT-869) is a novel receptor tyrosine kinase inhibitor with potent activity against members of the VEGFR and PDGFR families[74]. In a phase II study of linifanib in advanced HCC, the estimated objective response rate was 9.1%, the median time to disease progression was 3.7 mo, and the median OS was 9.7 mo[75]. An open-label, randomized phase III study of the efficacy and tolerability of linifanib vs sorafenib in advanced HCC (NCT01009593) was conducted. The OS of linifanib given as monotherapy once daily was similar to sorafenib given twice daily per standard of care[76].
TSU-68 is an oral tyrosine kinase inhibitor of FGFRs, VEGFRs and PDGFR and has demonstrated some clinical efficacy in a phase I/II trial of heavily pretreated patients with advanced HCC. Treatment of patients with unresectable or metastatic HCC with TSU-68 was associated with disease stabilization or improvement in 51% of the patients[77]. A randomized placebo-controlled phase III trial in Japan, South Korea and Taiwan is currently recruiting patients with unresectable HCC and will evaluate transcatheter arterial chemoembolization (TACE) in combination with either TSU-68 or placebo.
Cediranib (AZD2171) is another selective inhibitor of VEGFR-1, -2 and -3. Cediranib also exhibits activity against c-kit, PDGFR-β, and FLT4 at nanomolar concentrations. In a phase II clinical study of advanced HCC, the median OS was 5.8 mo. No patients experienced confirmed response. The median time to progression was 2.8 mo[78].
EGFR, a member of the human epidermal growth factor receptor (HER) family, contains an intracellular tyrosine kinase domain which can trigger signal transduction through the MAPK and PI3K/Akt/mTOR pathways. Thus, these receptors contribute to cell growth, differentiation, survival and adhesion[79]. EGFR overexpression has been reported in HCC. Immunohistochemical analysis by Buckley et al[80] revealed that EGFR was overexpressed in 50 (66%) of 76 HCCs, and fluorescence in situ hybridization (FISH) showed additional EGFR gene copies in 17 (45%) of 38 HCCs. EGFR-targeting drugs include anti-EGFR antibodies (such as cetuximab and panitumumab) and inhibitors of EGFR tyrosine kinases (such as erlotinib, lapatinib and gefitinib); these drugs have been used widely for the treatment of HCC.
Cetuximab is a recombinant chimeric monoclonal antibody that targets the extracellular domain of EGFR. In a phase II clinical trial of cetuximab in patients with advanced HCC, the median OS was 9.6 mo and the median PFS was 1.4 mo. The treatment was generally well tolerated. No treatment-related grade 4-5 toxicities occurred. Grade 3 aspartate aminotransferase, hypomagnesemia, and fever without neutropenia were each noted in 1 patient[81]. A randomized trial comparing gemcitabine-oxaliplatin (GEMOX) alone with a GEMOX-cetuximab combination is ongoing to define the real contribution of anti-EGFR therapy.
Erlotinib is a potent and reversible inhibitor of EGFR tyrosine kinase. In an in vitro study, erlotinib potently suppressed the growth of human EGFR-expressing HCC cell lines. Erlotinib has been shown to inhibit the RAF/MEK/ERK signaling pathway and block signal transducer and activator of transcription-mediated signaling[82]. A phase III placebo-controlled, double-blind SEARCH (Sorafenib and Erlotinib, a Randomized Trial Protocol for the Treatment of Patients with HCC) trial has been conducted in patients with advanced HCC. Three hundred sixty-two patients received sorafenib plus erlotinib and 358 received sorafenib plus placebo. No significant differences were observed in OS or TTP between the arms. Erlotinib, when added to sorafenib as the standard of care in advanced HCC, did not prolong overall survival[83].
Lapatinib is a dual inhibitor of EGFR and HER-2/NEU that acts by docking into the ATP binding site of the two receptors[84]. Phase II results have indicated that lapatinib is well tolerated and have shown preliminary evidence of antitumor activity in HCC[85]. Among 40 patients with advanced HCC, the response rate was 5%, median PFS was 2.3 mo and median OS was 6.2 mo.
The IGF/IGFR signaling pathway regulates several cellular processes, including proliferation, motility and inhibition of apoptosis[86]. Ligand binding to IGF-1R triggers rapid receptor autophosphorylation, which in turn initiates downstream cellular effectors, ultimately leading to activation of PI3K, protein kinase B and the RAF/MEK/ERK pathway[87]. In HCC, dysregulation of IGF signaling occurs predominantly at the level of IGF-2. IGF-2 is overexpressed in 16%-40% of human HCCs, and IGF-2R (an alternative receptor for IGF-2) is underexpressed in approximately 80% of HCCs[88,89]. Associations have been reported between disease stage, metastasis and survival and the functions of IGF and IGFR in HCC[90,91]. Several strategies to target this system, including monoclonal antibodies against the IGF-1 receptor (IGF-1R) and small molecule inhibitors of the tyrosine kinase function of IGF-1R, are under active investigation.
Pre-clinical evidence obtained from HCC cells has shown that IMC-A12 (cituxumumab), a human monoclonal antibody that blocks IGF-1R. A phase I study of IMC-A12 yielded a partial response in HCC[92]. However, a subsequent phase II study in patients with advanced HCC showed that IMC-A12 is inactive as a monotherapy[93]. Up to 46% of the patients developed grade 3-4 hyperglycemia in this study. Hyperglycemia may be the dose limiting toxicity of IGF-1R monoclonal antibodies.
BIIB022 is an anti-IGF-1R monoclonal antibody that blocks binding of both IGF-1 and IGF-2 to IGF-1R. This agent does not appear to cause hyperglycemia, which is a common side effect of receptor-specific antibodies. A planned phase I/II study comparing sorafenib with or without BIIB022 in patients with advanced HCC was terminated due to a business decision by the sponsor company.
AVE1642 is another monoclonal antibody that specifically blocks IGF-1R signaling. This agent has been evaluated in combination with sorafenib in a phase I study in advanced HCC patients[94]. Long-lasting disease stabilization was observed in most patients with PD.
OSI-906 is a novel potent dual tyrosine kinase inhibitor of both IGF-1R and insulin receptor. The unique advantage of OSI-906 over the previous class of anti-IGF drugs is its ability to minimize IGF-2 activity in situations in which IGF-1R inhibition alone is not sufficient. The phase II study of second-line treatment for advanced HCC patients who failed first-line treatment with sorafenib (NCT01101906) was terminated because the sponsor decided not to pursue the development of this drug.
The HGF/Met pathway is involved in tumor growth, invasion and angiogenesis in various types of cancer[95]. c-Met is a tyrosine kinase receptor for the HGF ligand. HGF-induced activation of c-MET ultimately leads to the activation of downstream effecter molecules, including phospholipase C, PI3K and ERK[96]. c-MET overexpression has been observed in 20%-48% of HCC, and overexpression has been linked with decreased 5-year survival in patients with HCC (FIgure 3)[97-99].
Tivantinib (ARQ 197) is a selective, oral MET receptor tyrosine kinase inhibitor with broad-spectrum antitumor activity as single agent. MET overexpression has been shown to be a negative prognostic factor in HCC after sorafenib failure. Tivantinib demonstrated a nearly doubling of PFS and OS in the MET high group compared to placebo in a phase II study as second-line treatment in patients with advanced HCC[100]. The activity of tivantinib in combination with sorafenib is also promising. Adverse events include hematological toxicity, asthenia and loss of appetite. The initially high incidence of neutropenia in patients with HCC led to dose reduction from 360 mg bid to 240 mg bid. Currently, a pivotal phase III study in advanced, MET-high HCC after sorafenib failure is planned.
A major and early carcinogenic event in the development of HCC seems to be the abnormal regulation of the transcription factor β-catenin, a key component of the WNT signaling pathway.
During normal cell homeostasis, Wnt proteins are absent. Initiation of Wnt signaling leads to a series of events that cause loss of β-catenin phosphorylation, which prevents its degradation. β-catenin then accumulates in the cytoplasm and translocates into the nucleus. Hepatocytes with nuclear translocation of β-catenin display abnormal cellular proliferation and express membrane proteins involved in HCC, metastatic behavior, and cancer stem cells[101]. A high incidence of β-catenin mutations (nearly 40%) has been observed in HCC cases that occur in patients with HCV. HCC cases that occur in HBV patients display β-catenin activation that is induced in a mutation-dependent manner by the expression of HBx protein[102,103]. Agents targeting Wnt-β-catenin are under development. Preliminary studies targeting the Wnt-β-catenin pathway have demonstrated a potential space for new novel therapies to treat HCC.
The Jak/Stat pathway is activated by more than 40 cytokines and growth factors and is involved in multiple cell functions, including differentiation, proliferation, and apoptosis[104]. In this pathway, cytokines induce phosphorylation of the Janus tyrosine kinases (Jak1, 2 and 3 and Tyk2), which is followed by activation of Stat1-6[105]. The phosphorylation of Jak1, Jak2, and Tyk2 tyrosine kinases is not detected in normal livers but increases significantly between surrounding non-neoplastic liver and HCCs[106]. Activation of Stat1, Stat3, and Stat5 has been shown to be significantly higher in tumors than in the respective surrounding livers; pStat3 is higher in HCC with poor prognosis than in HCC with better prognosis[106]. The levels of Jak/Stat targets, including Bcl-xl, Mcl-1, cyclin D1, and c-Myc, are markedly elevated in the majority of HCCs. A phase I study of the JAK2 inhibitor AZD1480 in advanced solid malignancy (including HCC) is planned (NCT01219543).
Molecular targeted agents that have been introduced into clinical use in recent years have been approved for the treatment of a specific cancer and then frequently used to treat various other types of cancer (Table 1). Genetic alterations clearly play a major role in hepatocarcinogenesis, and abnormalities in several critical molecular signaling pathways have been identified as contributing to tumor development and progression[107,108].
| Molecular targets | Therapeutic agents |
| VEGF/VEGFR | Sorafenib |
| Bevacizumab | |
| Vatalanib (PTK787) | |
| Cediranib (AZD2171) | |
| Brivanib | |
| Sunitinib | |
| Linifanib (ABT869) | |
| EGF/EGFR | Cetuximab |
| Erlotinib | |
| Lapatinib | |
| IGF/IGFR | OSI-906 |
| IMC-A12 | |
| AVE1642 | |
| BIIB022 | |
| Ras/Raf/MEK/ERK | Sorafenib |
| Selumetinib (AZD6244) | |
| PI3K/Akt/mTOR | AZD8055 |
| Everolimus | |
| Sirolimus | |
| Temsirolimus | |
| Wnt-β-catenin | PFK118-310 |
| PFK115-584 | |
| CGP049090 | |
| MET | Tivanitib |
Currently, sorafenib is the only effective systemic treatment option for advanced HCC. While the drug is effective for patients with advanced HCC, sorafenib prolongs life expectancy for only approximately three mo. To move beyond sorafenib monotherapy, a potential role for this agent in the adjuvant setting following surgical resection, radiofrequency ablation, or TACE or in combination with other targeted agents or chemotherapy is under investigation.
Several new promising multi-targeted molecules have been developed and are currently under investigation for the treatment of HCC (Table 2). Unfortunately, HCCs are refractory to many targeted therapies. Therefore, resistance to treatment remains the major challenge for targeted therapy. Many resistance mechanisms have been identified, including epigenetic changes, alternative splicing, target inactivation, upregulation of alternative pathways (by cellular adaptation to the pathway being targeted), and a range of mutations. A combination of different agents or a single ‘‘unspecific’’ inhibitor of several pathways may offer advantages to overcome resistance. Combinations of targeted agents with chemotherapy regimens also remain to be further explored. Molecular targeted therapy blocking angiogenesis has demonstrated somewhat promising results, but the efficacy of these agents is limited by survival pathways induced by hypoxia. Thus, the inhibition of hypoxia-induced survival signals might be required for targeted agents to block angiogenesis as an adjuvant therapy following TACE. Additionally, exploring potential markers that can help in identifying the patients who are most likely to respond (or to at least identify those who will not respond) to treatment is critical. Future development of genomic analysis of HCC will aid in the identification of specific biomarkers for patient selection for either single agent or combination molecular targeted therapies.
| Molecular targets/agents | Phase | Efficacy | Ref. |
| VEGF/VEGFR | |||
| Sorafenib | Phase III SHARP | Median OS: 10.7 mo vs 7.9 mo | [58] |
| Sorafenib vs placebo | |||
| Phase III (Asian) | Median OS:6.5 mo vs 4.2 mo | [59] | |
| Sunitinib | Phase II | Median PFS: 3.9 mo | [65] |
| Median OS: 9.8 mo | |||
| Phase III | Median OS: 7.9 mo vs 10.2 mo | ||
| Sunitinib vs sorafenib | |||
| Brivanib | Phase II, first-line | Median PFS: 2.8 mo | [68] |
| Median OS: 10 mo | |||
| Phase II, second-line | Median PFS: 2.7 mo | [69] | |
| Median OS: 9.8 mo | |||
| Phase III (BRISK-PS) | Median OS: 9.4 mo vs 8.3 mo | [70] | |
| Brivanib vs placebo | TTP: 4.2 mo vs 2.7 mo | ||
| RR: 12% vs 2% | |||
| Phase III (BRISK-FL) | Median OS: 9.5 mo vs 9.9 mo | [71] | |
| Brivanib vs placebo | TTP: 4.2 mo vs 4.1 mo | ||
| RR: 12% vs 8% | |||
| Vatalanib (PTK787) | Phase I/II, combined with doxorubicin | OS: 7.3 mo | [73] |
| PFS: 5. 4 mo | |||
| Inifanib (ABT-869) | Phase II | TTP: 3.7 mo | [75] |
| Median OS: 9.7 mo | |||
| Cediranib (AZD2171) | Phase II | Median OS: 5.8 mo | [78] |
| TTP: 2.8 mo | |||
| EGF/EGFR | |||
| Cetuximab | Phase II | Median OS: 9.6 mo | [81] |
| Median PFS: 1.4 mo | |||
| Erlotinib | Phase III (SEARCH) | Median OS: 9.5 mo vs 8.5 mo | [83] |
| Sorafenib/erlotinib vs orafenib/placebo | TTP: 3.2 mo vs 4.0 mo | ||
| Lapatinib | Phase II | Median PFS: 2.3 mo | [85] |
| Median OS: 6.2 mo | |||
| Phase III | Median OS: 9.1 mo vs 9.8 mo | ||
| Lipatinib vs sorafenib | |||
| IGF/IGFR | |||
| Cituxumumab (IMC-A12) | Phase II | Median OS: 8 mo | [93] |
| Ras/Raf/MEK/ERK | |||
| Selumetinib (AZD6244) | Phase I/II | 11 patients enrolled | [31] |
| PR in 3, SD in 6, PD in 2 patients | |||
| PI3K/Akt/mTOR | |||
| Everolimus | Phase I/II | Median PFS: 3.8 mo | [37] |
| Median OS: 8.4 mo | |||
| Sirolimus | Phase II | Median PFS: 15.3 wk | [38] |
| Median OS: 26.4 wk | |||
| MET | |||
| Tivantinib | Randomized Phase II | [100] | |
| Tivantinib vs placebo | |||
| ITT population | Median TTP: 6.9 wk vs 6.0 wk | ||
| Median OS: 6.6 mo vs 6.2 wk | |||
| c-Met high | Median TTP: 11.7 wk vs 6.1 wk | ||
| Median OS: 7.2 mo vs 3.8 wk |
P- Reviewer Sangro B S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1816] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2270] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 4. | Sarker D, Molife R, Evans TR, Hardie M, Marriott C, Butzberger-Zimmerli P, Morrison R, Fox JA, Heise C, Louie S. A phase I pharmacokinetic and pharmacodynamic study of TKI258, an oral, multitargeted receptor tyrosine kinase inhibitor in patients with advanced solid tumors. Clin Cancer Res. 2008;14:2075-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Brechot C, Pourcel C, Louise A, Rain B, Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980;286:533-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 422] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Minami M, Daimon Y, Mori K, Takashima H, Nakajima T, Itoh Y, Okanoue T. Hepatitis B virus-related insertional mutagenesis in chronic hepatitis B patients as an early drastic genetic change leading to hepatocarcinogenesis. Oncogene. 2005;24:4340-4348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Wang J, Chenivesse X, Henglein B, Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990;343:555-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 489] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Balsano C, Avantaggiati ML, Natoli G, De Marzio E, Will H, Perricaudet M, Levrero M. Full-length and truncated versions of the hepatitis B virus (HBV) X protein (pX) transactivate the cmyc protooncogene at the transcriptional level. Biochem Biophys Res Commun. 1991;176:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Twu JS, Lai MY, Chen DS, Robinson WS. Activation of protooncogene c-jun by the X protein of hepatitis B virus. Virology. 1993;192:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Chirillo P, Falco M, Puri PL, Artini M, Balsano C, Levrero M, Natoli G. Hepatitis B virus pX activates NF-kappa B-dependent transcription through a Raf-independent pathway. J Virol. 1996;70:641-646. [PubMed] |
| 11. | Andrisani OM, Barnabas S. The transcriptional function of the hepatitis B virus X protein and its role in hepatocarcinogenesis (Review). Int J Oncol. 1999;15:373-379. [PubMed] |
| 12. | Lee YH, Yun Y. HBx protein of hepatitis B virus activates Jak1-STAT signaling. J Biol Chem. 1998;273:25510-25515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Benn J, Schneider RJ. Hepatitis B virus HBx protein activates Ras-GTP complex formation and establishes a Ras, Raf, MAP kinase signaling cascade. Proc Natl Acad Sci USA. 1994;91:10350-10354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 333] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Cha MY, Kim CM, Park YM, Ryu WS. Hepatitis B virus X protein is essential for the activation of Wnt/beta-catenin signaling in hepatoma cells. Hepatology. 2004;39:1683-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Yamanaka T, Kodama T, Doi T. Subcellular localization of HCV core protein regulates its ability for p53 activation and p21 suppression. Biochem Biophys Res Commun. 2002;294:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Fukutomi T, Zhou Y, Kawai S, Eguchi H, Wands JR, Li J. Hepatitis C virus core protein stimulates hepatocyte growth: correlation with upregulation of wnt-1 expression. Hepatology. 2005;41:1096-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Florese RH, Nagano-Fujii M, Iwanaga Y, Hidajat R, Hotta H. Inhibition of protein synthesis by the nonstructural proteins NS4A and NS4B of hepatitis C virus. Virus Res. 2002;90:119-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Terasaki S, Kaneko S, Kobayashi K, Nonomura A, Nakanuma Y. Histological features predicting malignant transformation of nonmalignant hepatocellular nodules: a prospective study. Gastroenterology. 1998;115:1216-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene. 2006;25:3866-3884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351 Pt 2:289-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 21. | Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin Liver Dis. 2005;25:212-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Harden TK, Sondek J. Regulation of phospholipase C isozymes by ras superfamily GTPases. Annu Rev Pharmacol Toxicol. 2006;46:355-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | To MD, Perez-Losada J, Mao JH, Balmain A. Crosstalk between Pten and Ras signaling pathways in tumor development. Cell Cycle. 2005;4:1185-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Huynh H, Nguyen TT, Chow KH, Tan PH, Soo KC, Tran E. Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol. 2003;3:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, Ueki T, Hirano T, Yamamoto H, Fujimoto J. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 291] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Chung TW, Lee YC, Kim CH. Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: involvement of invasive potential. FASEB J. 2004;18:1123-1125. [PubMed] |
| 27. | Erhardt A, Hassan M, Heintges T, Häussinger D. Hepatitis C virus core protein induces cell proliferation and activates ERK, JNK, and p38 MAP kinases together with the MAP kinase phosphatase MKP-1 in a HepG2 Tet-Off cell line. Virology. 2002;292:272-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Lee HC, Tian B, Sedivy JM, Wands JR, Kim M. Loss of Raf kinase inhibitor protein promotes cell proliferation and migration of human hepatoma cells. Gastroenterology. 2006;131:1208-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129-3140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1149] [Cited by in RCA: 1109] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 30. | Huynh H. AZD6244 (ARRY-142886) enhances the antitumor activity of rapamycin in mouse models of human hepatocellular carcinoma. Cancer. 2010;116:1315-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Choo S, Ng Q, Chen W, Tham C, Yong W, Wang L, Koh T, Goh B, Thng C, Huynh H. A phase I/II study of AZD6244 in combination with sorafenib in advanced hepatocellular carcinoma. J Clin Oncol. 2012;30:A4100. |
| 32. | Zhou Q, Lui VW, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol. 2011;7:1149-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 33. | Alexia C, Fallot G, Lasfer M, Schweizer-Groyer G, Groyer A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochem Pharmacol. 2004;68:1003-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoël MJ, Bertrand F, Cherqui G, Perret C, Capeau J. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene. 2001;20:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 248] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | Hu TH, Huang CC, Lin PR, Chang HW, Ger LP, Lin YW, Changchien CS, Lee CM, Tai MH. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer. 2003;97:1929-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 224] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 36. | Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972-183, 1972-183. [PubMed] |
| 37. | Zhu AX, Abrams TA, Miksad R, Blaszkowsky LS, Meyerhardt JA, Zheng H, Muzikansky A, Clark JW, Kwak EL, Schrag D. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094-5102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 38. | Decaens T, Luciani A, Itti E, Hulin A, Roudot-Thoraval F, Laurent A, Zafrani ES, Mallat A, Duvoux C. Phase II study of sirolimus in treatment-naive patients with advanced hepatocellular carcinoma. Dig Liver Dis. 2012;44:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 280] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 40. | Mas VR, Maluf DG, Archer KJ, Yanek KC, Fisher RA. Angiogenesis soluble factors as hepatocellular carcinoma noninvasive markers for monitoring hepatitis C virus cirrhotic patients awaiting liver transplantation. Transplantation. 2007;84:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 438] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 42. | El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 242] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Chao Y, Li CP, Chau GY, Chen CP, King KL, Lui WY, Yen SH, Chang FY, Chan WK, Lee SD. Prognostic significance of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin in patients with resectable hepatocellular carcinoma after surgery. Ann Surg Oncol. 2003;10:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Li XM, Tang ZY, Zhou G, Lui YK, Ye SL. Significance of vascular endothelial growth factor mRNA expression in invasion and metastasis of hepatocellular carcinoma. J Exp Clin Cancer Res. 1998;17:13-17. [PubMed] |
| 45. | Poon RT, Lau C, Yu WC, Fan ST, Wong J. High serum levels of vascular endothelial growth factor predict poor response to transarterial chemoembolization in hepatocellular carcinoma: a prospective study. Oncol Rep. 2004;11:1077-1084. [PubMed] |
| 46. | Yamaguchi R, Yano H, Iemura A, Ogasawara S, Haramaki M, Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 270] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 47. | Uematsu S, Higashi T, Nouso K, Kariyama K, Nakamura S, Suzuki M, Nakatsukasa H, Kobayashi Y, Hanafusa T, Tsuji T. Altered expression of vascular endothelial growth factor, fibroblast growth factor-2 and endostatin in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2005;20:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | El-Assal ON, Yamanoi A, Ono T, Kohno H, Nagasue N. The clinicopathological significance of heparanase and basic fibroblast growth factor expressions in hepatocellular carcinoma. Clin Cancer Res. 2001;7:1299-1305. [PubMed] |
| 49. | Poon RT, Ng IO, Lau C, Yu WC, Fan ST, Wong J. Correlation of serum basic fibroblast growth factor levels with clinicopathologic features and postoperative recurrence in hepatocellular carcinoma. Am J Surg. 2001;182:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Hisai H, Kato J, Kobune M, Murakami T, Miyanishi K, Takahashi M, Yoshizaki N, Takimoto R, Terui T, Niitsu Y. Increased expression of angiogenin in hepatocellular carcinoma in correlation with tumor vascularity. Clin Cancer Res. 2003;9:4852-4859. [PubMed] |
| 51. | Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593-4599. [PubMed] |
| 52. | Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli J. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992-2998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 395] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 53. | Hsu CH, Yang TS, Hsu C, Toh HC, Epstein RJ, Hsiao LT, Chen PJ, Lin ZZ, Chao TY, Cheng AL. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;102:981-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Sun W, Sohal D, Haller DG, Mykulowycz K, Rosen M, Soulen MC, Caparro M, Teitelbaum UR, Giantonio B, O’Dwyer PJ. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer. 2011;117:3187-3192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Thomas MB, Morris JS, Chadha R, Iwasaki M, Kaur H, Lin E, Kaseb A, Glover K, Davila M, Abbruzzese J. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 56. | Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be? Oncologist. 2006;11:790-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 57. | Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293-4300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 908] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 58. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10266] [Article Influence: 603.9] [Reference Citation Analysis (2)] |
| 59. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4649] [Article Influence: 273.5] [Reference Citation Analysis (0)] |
| 60. | Lee JH, Chung YH, Kim JA, Shim JH, Lee D, Lee HC, Shin ES, Yoon JH, Kim BI, Bae SH. Genetic predisposition of hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer. 2013;119:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471-478. [PubMed] |
| 62. | Abrams TJ, Murray LJ, Pesenti E, Holway VW, Colombo T, Lee LB, Cherrington JM, Pryer NK. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with “standard of care” therapeutic agents for the treatment of breast cancer. Mol Cancer Ther. 2003;2:1011-1021. [PubMed] |
| 63. | Murray LJ, Abrams TJ, Long KR, Ngai TJ, Olson LM, Hong W, Keast PK, Brassard JA, O’Farrell AM, Cherrington JM. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20:757-766. [PubMed] |
| 64. | Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 367] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 65. | Faivre S, Raymond E, Boucher E, Douillard J, Lim HY, Kim JS, Zappa M, Lanzalone S, Lin X, Deprimo S. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 66. | Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2319] [Cited by in RCA: 2266] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 67. | Tille JC, Wood J, Mandriota SJ, Schnell C, Ferrari S, Mestan J, Zhu Z, Witte L, Pepper MS. Vascular endothelial growth factor (VEGF) receptor-2 antagonists inhibit VEGF- and basic fibroblast growth factor-induced angiogenesis in vivo and in vitro. J Pharmacol Exp Ther. 2001;299:1073-1085. [PubMed] |
| 68. | Park JW, Finn RS, Kim JS, Karwal M, Li RK, Ismail F, Thomas M, Harris R, Baudelet C, Walters I. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2011;17:1973-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Finn RS, Kang YK, Mulcahy M, Polite BN, Lim HY, Walters I, Baudelet C, Manekas D, Park JW. Phase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2090-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 70. | Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V. Brivanib in Patients With Advanced Hepatocellular Carcinoma Who Were Intolerant to Sorafenib or for Whom Sorafenib Failed: Results From the Randomized Phase III BRISK-PS Study. J Clin Oncol. 2013;Aug 26; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Johnson P, Qin S, Park JW. Brivanib (BRI) versus Sorafenib (SOR) as First-line Therapy in Patients with Unresectable, Advanced Hepatocellular Carcinoma (HCC): Results from the Phase 3 BRISK-FL Study. Boston: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD 2012) 2012; Abstract LB-6. |
| 72. | Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178-2189. [PubMed] |
| 73. | Yau T, Chan P, Pang R, Ng K, Fan ST, Poon RT. Phase 1-2 trial of PTK787/ZK222584 combined with intravenous doxorubicin for treatment of patients with advanced hepatocellular carcinoma: implication for antiangiogenic approach to hepatocellular carcinoma. Cancer. 2010;116:5022-5029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Albert DH, Tapang P, Magoc TJ, Pease LJ, Reuter DR, Wei RQ, Li J, Guo J, Bousquet PF, Ghoreishi-Haack NS. Preclinical activity of ABT-869, a multitargeted receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2006;5:995-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 75. | Toh HC, Chen PJ, Carr BI, Knox JJ, Gill S, Ansell P, McKeegan EM, Dowell B, Pedersen M, Qin Q. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer. 2013;119:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 76. | Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, Kudo M, Kang YK, Chen PJ, Toh HC. Phase III trial of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2012;30:A249. |
| 77. | Kanai F, Yoshida H, Tateishi R, Sato S, Kawabe T, Obi S, Kondo Y, Taniguchi M, Tagawa K, Ikeda M. A phase I/II trial of the oral antiangiogenic agent TSU-68 in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2011;67:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 78. | Alberts SR, Fitch TR, Kim GP, Morlan BW, Dakhil SR, Gross HM, Nair S. Cediranib (AZD2171) in patients with advanced hepatocellular carcinoma: a phase II North Central Cancer Treatment Group Clinical Trial. Am J Clin Oncol. 2012;35:329-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 79. | Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1515] [Cited by in RCA: 1567] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 80. | Buckley AF, Burgart LJ, Sahai V, Kakar S. Epidermal growth factor receptor expression and gene copy number in conventional hepatocellular carcinoma. Am J Clin Pathol. 2008;129:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 81. | Zhu AX, Stuart K, Blaszkowsky LS, Muzikansky A, Reitberg DP, Clark JW, Enzinger PC, Bhargava P, Meyerhardt JA, Horgan K. Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 82. | Huether A, Hopfner M, Sutter AP, Baradari V, Schuppan D, Scherubl H. Signaling pathways involved in the inhibition of epidermal growth factor receptor by erlotinib in hepatocellular cancer. World J Gastroenterol. 2006;12:5160-5167. [PubMed] |
| 83. | Zhu AX, Rosmorduc O, Evans J, Ross P, Santoro A, Carrilho FJ, Leberre M, Jensen M, Meinhardt G, Kang YK. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with hepatocellular carcinoma (HCC). Ann Oncol. 2012;23:Abstract LBA2. |
| 84. | Burris HA, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O’Neil B, Marcom PK, Ellis MJ, Overmoyer B, Jones SF. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305-5313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 85. | Ramanathan RK, Belani CP, Singh DA, Tanaka M, Lenz HJ, Yen Y, Kindler HL, Iqbal S, Longmate J, Mack PC. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol. 2009;64:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 86. | Golan T, Javle M. Targeting the insulin growth factor pathway in gastrointestinal cancers. Oncology (Williston Park). 2011;25:518-526, 529. [PubMed] |
| 87. | Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1043] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 88. | Cariani E, Lasserre C, Seurin D, Hamelin B, Kemeny F, Franco D, Czech MP, Ullrich A, Brechot C. Differential expression of insulin-like growth factor II mRNA in human primary liver cancers, benign liver tumors, and liver cirrhosis. Cancer Res. 1988;48:6844-6849. [PubMed] |
| 89. | De Souza AT, Hankins GR, Washington MK, Fine RL, Orton TC, Jirtle RL. Frequent loss of heterozygosity on 6q at the mannose 6-phosphate/insulin-like growth factor II receptor locus in human hepatocellular tumors. Oncogene. 1995;10:1725-1729. [PubMed] |
| 90. | Chen YW, Boyartchuk V, Lewis BC. Differential roles of insulin-like growth factor receptor- and insulin receptor-mediated signaling in the phenotypes of hepatocellular carcinoma cells. Neoplasia. 2009;11:835-845. [PubMed] |
| 91. | Scharf JG, Braulke T. The role of the IGF axis in hepatocarcinogenesis. Horm Metab Res. 2003;35:685-693. [PubMed] |
| 92. | Higano CS, Yu EY, Whiting SH, Gordon MS, LoRusso P, Fox F, Katz TL, Roecker JM, Schwartz JD. A phase I, first in man study of weekly IMC-A12, a fully human insulin like growth factor-I receptor IgG1 monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol. 2007;25:s3505. |
| 93. | Abou-Alfa GK, Gansukh B, Chou JF, Shia J, Capanu M, Kalin M, Chen HX, Zojwalla NJ, Katz S, Reidy DL. Phase II Study of Cixutumumab (IMC-A12, NSC742460; C) in Hepatocellular Carcinoma. J Clin Oncol. 2011;29:Abstract 4043. |
| 94. | Faivre S, Fartoux L, Bouattour M, Bumsel F, Dreyer C, Raymond E, Rosmorduc O. A phase I study of AVE1642, a human monoclonal antibody-blocking insulin-like growth factor-1 receptor (IGF-1R), given as a single agent and in combination with sorafenib as first-line therapy in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2011;29:Abstract 270. |
| 95. | You WK, McDonald DM. The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Rep. 2008;41:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 96. | Yap TA, de Bono JS. Targeting the HGF/c-Met axis: state of play. Mol Cancer Ther. 2010;9:1077-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 97. | Boix L, Rosa JL, Ventura F, Castells A, Bruix J, Rodés J, Bartrons R. c-met mRNA overexpression in human hepatocellular carcinoma. Hepatology. 1994;19:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Tavian D, De Petro G, Benetti A, Portolani N, Giulini SM, Barlati S. u-PA and c-MET mRNA expression is co-ordinately enhanced while hepatocyte growth factor mRNA is down-regulated in human hepatocellular carcinoma. Int J Cancer. 2000;87:644-649. [PubMed] |
| 99. | Ueki T, Fujimoto J, Suzuki T, Yamamoto H, Okamoto E. Expression of hepatocyte growth factor and its receptor, the c-met proto-oncogene, in hepatocellular carcinoma. Hepatology. 1997;25:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 100. | Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlierberghe H, Trojan J, Kolligs FT, Weiss A. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 463] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 101. | Herencia C, Martínez-Moreno JM, Herrera C, Corrales F, Santiago-Mora R, Espejo I, Barco M, Almadén Y, de la Mata M, Rodríguez-Ariza A. Nuclear translocation of β-catenin during mesenchymal stem cells differentiation into hepatocytes is associated with a tumoral phenotype. PLoS One. 2012;7:e34656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 102. | Zhang Y, Wei W, Cheng N, Wang K, Li B, Jiang X, Sun S. Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology. 2012;56:1631-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 103. | Srisuttee R, Koh SS, Kim SJ, Malilas W, Boonying W, Cho IR, Jhun BH, Ito M, Horio Y, Seto E. Hepatitis B virus X (HBX) protein upregulates β-catenin in a human hepatic cell line by sequestering SIRT1 deacetylase. Oncol Rep. 2012;28:276-282. [PubMed] |
| 104. | Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 814] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 105. | Bromberg JF. Activation of STAT proteins and growth control. Bioessays. 2001;23:161-169. [PubMed] |
| 106. | Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, Factor VM, Thorgeirsson SS. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 552] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 107. | Jin YJ, Chung YH, Kim JA, Park WH, Lee D, Seo DD, Ryu SH, Jang MK, Yu E, Lee YJ. Factors predisposing metastatic tumor antigen 1 overexpression in hepatitis B virus associated hepatocellular carcinoma. Dig Dis Sci. 2012;57:2917-2923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Jung SW, Park NH, Shin JW, Park BR, Kim CJ, Lee JE, Shin ES, Kim JA, Chung YH. Polymorphisms of DNA repair genes in Korean hepatocellular carcinoma patients with chronic hepatitis B: possible implications on survival. J Hepatol. 2012;57:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |