Published online Sep 21, 2013. doi: 10.3748/wjg.v19.i35.5863
Revised: May 7, 2013
Accepted: June 5, 2013
Published online: September 21, 2013
Processing time: 253 Days and 20.3 Hours
AIM: To evaluate individual components of the antro-pyloro-duodenal (APD) motor response to graded small intestinal glucose infusions in healthy humans.
METHODS: APD manometry was performed in 15 healthy subjects (12 male; 40 ± 5 years, body mass index 26.5 ± 1.6 kg/m2) during four 20-min intraduodenal infusions of glucose at 0, 0.5, 1.0 and 1.5 kcal/min, in a randomised double-blinded fashion. Glucose solutions were infused at a rate of 1 mL/min and separated by 40-min “wash-out” period. Data are mean ± SE. Inferential analyses are repeated measure analysis of variance with Bonferroni post-hoc testing.
RESULTS: At 0 kcal/min frequency of pressure waves were: antrum (7.5 ± 1.8 waves/20 min) and isolated pyloric pressure waves (IPPWs) (8.0 ± 2.3 waves/20 min) with pyloric tone (0.0 ± 0.9 mmHg). Intraduodenal glucose infusion acutely increased IPPW frequency (P < 0.001) and pyloric tone (P = 0.015), and decreased antral wave frequency (P = 0.007) in a dose-dependent fashion. A threshold for stimulation was observed at 1.0 kcal/min for pyloric phasic pressure waves (P = 0.002) and 1.5 kcal/min for pyloric tone and antral contractility.
CONCLUSION: There is hierarchy for the activation of gastrointestinal motor responses to duodenal glucose infusion. An increase in IPPWs is the first response observed.
Core tip: Antro-pyloro-duodenal manometry was performed in 15 healthy subjects. Subjects were randomly given 20 min intraduodenal infusions of glucose at 0, 0.5, 1.0 and 1.5 kcal/min. Intraduodenal glucose infusion acutely increased isolated pyloric pressure wave frequency and pyloric tone and decreased antral wave frequency in a dose-dependent fashion. A threshold for stimulation was observed at 1.0 kcal/min for pyloric phasic pressure waves and 1.5 kcal/min for pyloric tone and antral contractility. These data suggest that there is hierarchy for the activation of gastrointestinal motor responses to small intestinal glucose stimulation.
- Citation: Deane AM, Besanko LK, Burgstad CM, Chapman MJ, Horowitz M, Fraser RJ. Modulation of individual components of gastric motor response to duodenal glucose. World J Gastroenterol 2013; 19(35): 5863-5869
- URL: https://www.wjgnet.com/1007-9327/full/v19/i35/5863.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i35.5863
Gastric emptying of liquid nutrient is regulated at approximately 2-3 kcal/min by antro-pyloro-duodenal (APD) motor activity[1,2]. Nutrient in the small intestine stimulates receptors and initiates a feedback loop that affects motility of individual components of the APD area[3]. These motor changes include antral suppression[1], and stimulation of phasic and tonic pyloric contractions[4,5].
The precise APD motor response to nutrient probably depends in part on the macronutrient composition of the “meal”. Recent data suggest that the “doses” of lipid nutrient initiating these motor changes in health are much less than previously recognised[6]. Likewise small intestinal carbohydrate infusions at rates that are within normal gastric emptying rates have marked effects on the APD unit[7]. However, the specific threshold and/or hierarchy of the APD response to nutrient stimulation are unknown. The aim of this study was to assess the responses of the distal stomach to graded small intestinal nutrient stimulation in health.
Studies were performed in fifteen healthy volunteers [male:female, 12:4; age: 40 ± 5 years; body mass index (BMI): 26.5 ± 1.6 kg/m2]. Subjects were screened and excluded if they were diabetic, pregnant or breast feeding, had previous gastrointestinal surgery, a history of gastrointestinal disease or taking medications known to alter gastrointestinal motility. None of the subjects regularly smoked tobacco or drank more than 20 g of alcohol per day.
The protocol was approved by the research ethics committee of the Royal Adelaide Hospital, and each subject gave written informed consent prior to the commencement of the study.
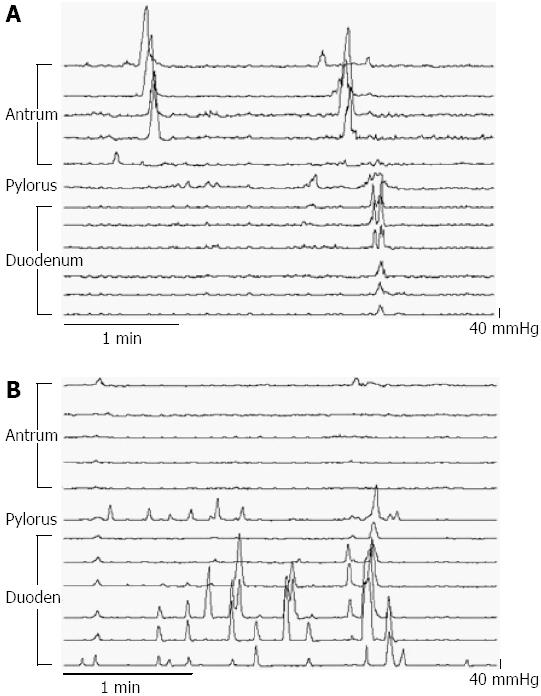
Multi-lumen perfusion manometry: APD motility was assessed by a 100-cm multi-lumen perfusion manometric assembly (outer diameter 3.5 mm; Mui Scientific, Ontario, Canada). The assembly incorporated 15 pressure recording channels (side-holes spaced 1.5 cm apart), with a 4.5 cm sleeve-sensor, and an infusion port. Correct placement of the sleeve across the pylorus was determined using continuous measurement of the antro-duodenal transmucosal potential difference (TMPD) gradient[8]. The assembly was positioned so that five side holes (A1-A5) were located in the gastric antrum and seven in the proximal duodenum (Figure 1). The infusion port was located at the catheter tip to enable the delivery of enteral feed directly into the duodenum 9 cm distal to the pylorus. Thirteen manometric lumina were perfused with degassed water at a rate of 0.04 mL/min except for the sleeve perfused at a rate of 0.15 mL/min. To monitor TMPD two channels on either end of the sleeve were perfused with degassed 0.9% saline. Pressure and TMPD data were recorded on a computer using purpose written software program (Medical Measurement Systems, Enschede, The Netherlands)[8].
Blood glucose concentration: As hyperglycaemia has a major impact on gastric motility[9], blood glucose concentrations were measured using a portable glucometer (Precision Plus, Abbott Laboratories, Bedford, United States) every 20 min throughout the study.
Subjects were studied in the gastrointestinal motility laboratory of the Royal Adelaide Hospital after an overnight fast. The manometric catheter was inserted into an anaesthetised nostril and passed into the stomach. The catheter passed into the duodenum assisted by spontaneous peristalsis. A cannula was inserted into an antecubital vein for blood sampling.
Each subject received intraduodenal infusions of 50% glucose solution (Pharmalab NSW Australia) diluted in water at: (1) 0.5 kcal/min; (2) 1 kcal/min; (3) 1.5 kcal; and (4) 0 kcal/min (0.9% saline only). Each solution was prepared in separate 20 mL syringes by a study investigator who was not involved in the data analysis and infused at a rate of 1 mL/min. Randomisation of glucose load was computer generated. Each syringe was then covered by the investigator preparing the syringes and labelled according to the randomisation schedule. The syringe was connected to the manometric catheter using opaque minimal volume extension tubing to ensure blinding of the research staff.
Following correct positioning of the catheter sleeve across the pylorus, a 20 min fasting period commenced. At the end of the fasting period the scheduled load was infused directly into the duodenum, via a volumetric syringe driver [Terumo Syringe Pump (STC-523), Medtel Australia], followed by a 40 min “washout” period of 0.9% saline (1 mL/min). A similar schedule was followed for all glucose loads. Blood samples were taken every 20 min throughout the study period to measure blood glucose concentrations.
Manometric data were imported into Acqknowledge 3.2.7 and were analysed manually. The frequencies of APD pressure waves were determined as previously described[8]. In brief, pressure waves were included in the analysis when a rise in intraluminal pressure was greater than the minimum amplitude over the appropriate time-period and when the assembly was positioned correctly according to established TMPD criteria. Migrating motor complex (MMC) phase III activity associated pressure waves were considered to be representative of fasting motor patterns and were counted as zero for the period of phase III activity. Antral phase III MMC activity was defined as rhythmic pressure wave activity occurring at a maximum frequency (three pressure waves per minute) for at least one minute with a temporal relationship with duodenal activity. Duodenal phase III MMC was defined as a maximum frequency of 10-12 pressure waves/min for at least 2 min[8].
A pressure wave in the antrum and pylorus was defined as a pressure rise of 10 mmHg or more from baseline and lasting between 6.1 and 20 s[10]. Isolated pyloric pressure waves (IPPWs) were defined as pressure waves at least 10 mmHg amplitude recorded only in the sleeve channel[5]. A duodenal PW was defined as a pressure rise of 6 mmHg or more from baseline and lasting between 0.8 and 7 s[10]. Change in pyloric tone (basal pyloric pressure) was calculated as the difference in baseline pressure in the sleeve sensor from the duodenum[11] at 4-min intervals and presented as mean over 20 min.
Data are presented as mean ± SE. Repeated-measures analysis of variance (RM-ANOVA) were used to test for effects on pressure wave activity and pyloric tone of different caloric loads. Residuals were normally distributed and, furthermore, analyses using the equivalent non-parametric test (Friedman) remained significant. On testing there was no order effect apparent. Differences at the level of P < 0.05 were considered significant and allowed post-hoc comparison between loads which were corrected according to Bonferroni adjustment.
All subjects tolerated the study without adverse symptoms or effects.
An example of a manometric trace at two different loads is shown in Figure 1.
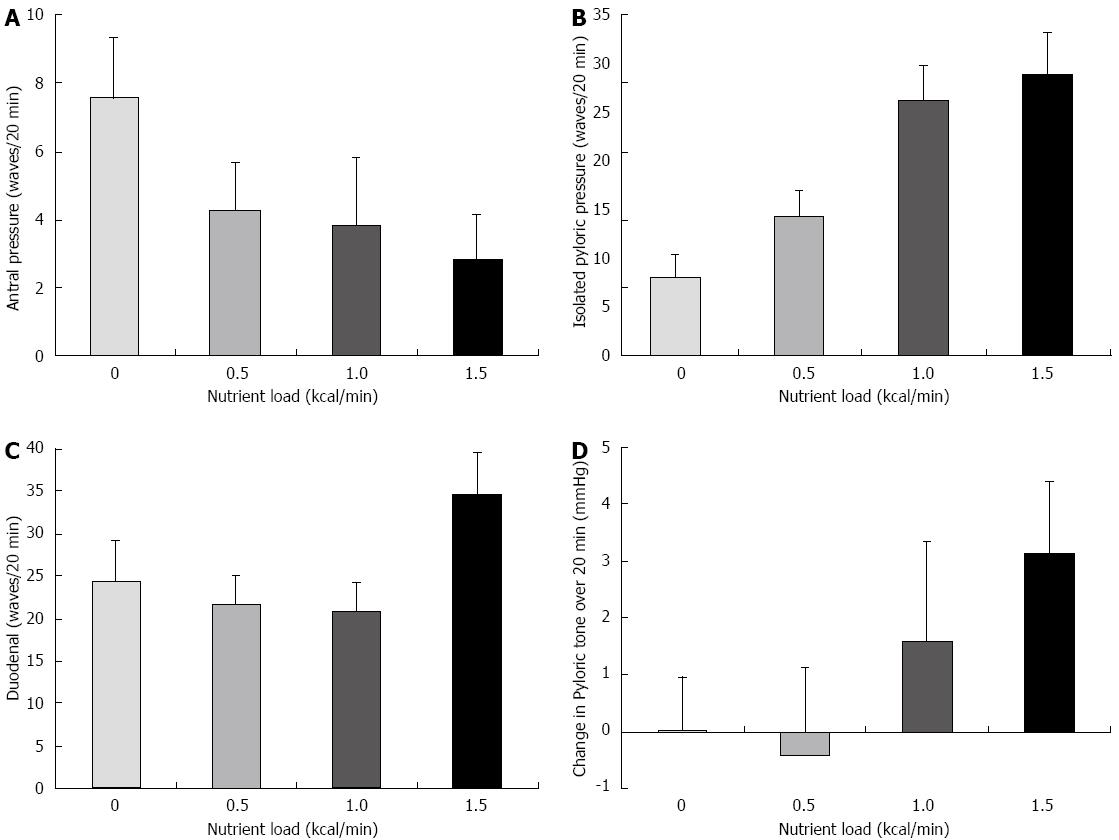
Antral pressure waves: The effect of glucose loads on antral pressure wave activity is shown in Figure 2A. Increasing the caloric load had an effect on antral wave frequency (P = 0.007) with marked attenuation of antral pressure wave activity at 1.5 kcal/min, when compared to 0 kcal/min (0 kcal/min: 7.5 ± 1.8 waves/20 min vs 1.5 kcal: 2.8 ± 1.3 waves/20 min; P = 0.007).
IPPWs: The effects of glucose loads on IPPW activity are shown in Figure 2B. The frequency of IPPWs were affected by caloric load (P < 0.001) with a substantial increase in pressure waves occurring with increasing nutrient (0 kcal/min: 8.0 ± 2.3 waves/20 min vs 1.0 kcal: 25.9 ± 3.7 waves/20 min; P = 0.002). The increasing frequency of IPPW during glucose infusion occurred in a dose dependent fashion with 1.0 kcal/min the observed threshold to stimulate the pylorus (0 kcal/min: 8.0 ± 2.3 waves/20 min vs 0.5 kcal/min: 14.2 ± 2.7 waves/20 min; P = 0.294; but 0.5 kcal/min 14.2 ± 2.7 waves/20 min vs 1.0 kcal: 25.9 ± 3.7 waves/20 min; P = 0.037).
Duodenal pressure waves: The effects of glucose loads on duodenal wave activity are shown in Figure 2C. There was a difference between treatments over time in duodenal motor wave activity with different caloric loads (P = 0.012). However, post-hoc testing did not reveal a difference between the individual loads (0 kcal/min: 24.4 ± 4.7 waves/20 min vs 34.7 ± 4.9 waves/20 min, P = 0.22; and 0.5 kcal/min 21.7 ± 3.3 waves/20 min vs 34.7 ± 4.9 waves/20 min, P = 0.058).
Pyloric tone: The effects of glucose loads on pyloric tone are shown in Figure 2D. There was an observed difference between treatments in pyloric tone (P = 0.015). The difference between 0 and 1.5 kcal/min was significant prior (P = 0.035), but not following Bonferroni adjustment (0.0 ± 0.9 vs 3.12 ± 1.3; P = 0.207). However, the difference remained significant between 0.5 and 1.5 kcal/min (-0.4 ± 1.1 vs 3.1 ± 1.3; P = 0.008).
Blood glucose concentrations were similar prior to commencing each infusion (0, 0.5, 1.0 and 1.5 kcal/min: 6.5 ± 0.3 mmol/L, 5.9 ± 0.2 mmol/L, 5.8 ± 0.3 mmol/L vs 5.7 ± 0.2 mmol/L; P = 0.625) and at the completion of the infusion (5.9 ± 0.2, 5.9 ± 0.2, 6.4 ± 0.3 vs 6.2 ± 0.2; P = 0.079).
The major finding of this study is that, in health, there is a hierarchical response in the APD motor area to increasing glucose loads. The hierarchical response is graded, with initial stimulation of IPPWs and then inhibition of antral activity and an increase in pyloric tone. An increase in duodenal pressure wave frequency also occurred with a caloric load of 1.5 kcal/min.
In health, gastric emptying of nutrient liquid is regulated by the distal stomach which is affected by nutrient stimulating receptors in the small intestine[2]. Pilichiewicz et al[6] showed that intraduodenal infusions of as little as 0.25 kcal/min of lipid emulsion (10% intralipid) attenuated antral motility and increased pyloric phasic pressure waves. The same authors also showed that glucose at 1 kcal/min for 120 min reduced antral wave frequency but an increase in IPPWs only occurred at 4 kcal/min[7]. Pressure waves isolated to the pylorus are an integral component of the APD response to duodenal nutrient, and may be the most important mechanism to slow gastric emptying[4]. In addition, pyloric pressure waves assist with the mixing of chyme[12] and initial stimulation of IPPWs prior to effects on other components of the APD unit should assist with trituration. Accordingly, we hypothesised that APD motor function would be affected by carbohydrate load in a hierarchical fashion and, given their substantial importance, IPPWs would occur early. It was anticipated that the magnitude of effect may be, relatively, small and the protocol was designed to detect small differences. The carbohydrate loads chosen were around 1 kcal/min[7], which were considered physiologically relevant, the sample size was increased compared to previous studies[6,7] and the study was undertaken on a single day to minimise intrasubject variability. Lastly, the infusion periods were limited to 20 min as “adaptation” to small intestinal caloric loads has been reported during prolonged infusions[13] and if this occurred, it would have reduced the likelihood of detecting a true difference. This study shows that modulation of each component of the APD unit is hierarchical and dependent on caloric load; initially resistance to trans-pyloric flow occurs with IPPWs and, subsequently, antral propulsive force decreases. The implication of this finding is that small intestinal delivery of nutrient, even within so-called “normal” gastric emptying rates has a substantial effect on APD motor patterns.
Duodenal phasic activity is characterised by irregular motor patterns with both antegrade and retrograde pressure wave sequences[14]. These contraction wave sequences commonly propagate only over a short distance causing intermittent and bidirectional flow of chyme (to aid mixing of chyme and exposure of chyme to luminal receptors). However, more prolonged antegrade wave activity is required for aboral movement of chyme and it has been previously reported that increasing nutrient load decreases the frequency of the sequences[7,15]. In contrast, we detected a strong trend to increased duodenal activity with increasing loads. This may reflect a chance finding or the relationship between nutrient load and duodenal activity is non-linear, with initial small increments in load increasing frequency of contractions and above a certain threshold (perhaps 1.5 kcal/min) a reduction in duodenal wave frequency occurs.
The proposed mechanisms underlying modulation of the APD unit are neural and hormonal. Fone and colleagues showed that stimulation of phasic pyloric pressure waves during intraduodenal glucose at 2.4 kcal/min are mediated via ascending enteric nerves and ACh-stimulation of muscarinic receptors[16]. Both cholecystokinin and glucagon-like peptide-1 are secreted, albeit temporarily, in response to 1 kcal/min of intraduodenal glucose[17] and endogenous secretion of these hormones are known to have substantial effects on APD motility and, thereby, gastric emptying[18-21].
While this study was undertaken in health, implications of these data for pathological conditions can be speculated upon. Gastric emptying is frequently slowed with healthy aging and in conditions such as diabetes and critical illness[22-24]. This slowing has been attributed in part to “hypersensitivity” to small intestinal nutrient, which appears to be, at least in part, hormonally-mediated via hormones such as cholecystokinin[25]. These data in health suggest the hypothesis that the process of aging or the effects of critical illness exacerbates this hierarchical sensitivity. Further study in this area would be of interest.
The limitations of this study should be recognised. Only occlusive pressure waves are detected by manometry. Non-occlusive antral pressure waves and/or non-peristaltic flow may also have been affected[26]. However pyloric closure prevents transpyloric flow. It would be of interest to repeat this study using measurement techniques that detect non-occlusive antral pressure waves. In addition, the duodenal nutrient infusion was non-pulsatile and short term (20 min). However, the feedback mechanism is the same whether the intra-duodenal nutrient is infused in a pulsatile or non-pulsatile fashion[27]. The aim of this study was only to measure the acute response to duodenal nutrient infusion and as motor responses adapt[13], particularly at lower loads[17], the response during more prolonged stimulation at these loads could be different. It should also be recognized that blood glucose concentrations were not clamped and hyperglycemia, even within the physiological range, affects APD motor responses and gastric emptying[28-30]. However glucose concentrations were similar despite the differing loads, suggesting that this is unlikely to explain the adaptive response observed.
In summary, glucose loads as low as 1 kcal/min infused into the duodenum initiate APD motor responses that will slow gastric emptying. The acute APD motor response to an intra-duodenal carbohydrate load is hierarchical and “dose” dependant, with an increased frequency of IPPW, followed by a decrease in antral wave frequency.
The authors acknowledge the technical assistance of Mr Matthew Summers and Mr Antony Zaknic.
Emptying of liquid nutrient from the stomach is regulated by antro-pyloro-duodenal (APD) motor activity. Nutrient within the small intestine initiates a feedback loop that affects motility of the APD area. Recent data suggest that the “doses” of lipid nutrient initiating these motor changes in health are much less than previously recognised. Likewise small intestinal carbohydrate infusions at rates that are within normal gastric emptying rates have marked effects on the APD unit. However, the specific threshold and/or hierarchy of the APD response to nutrient stimulation are unknown. The aim of this study was to assess the responses of the distal stomach to graded small intestinal nutrient stimulation in health.
The specific threshold and/or hierarchy of the APD response to nutrient stimulation are unknown.
This study shows that modulation of each component of the APD unit is hierarchical and dependent on caloric load; initially resistance to trans-pyloric flow occurs with isolated pyloric pressure waves and, subsequently, antral propulsive force decreases.
The implication of this finding is that small intestinal delivery of nutrient, even within so-called “normal” gastric emptying rates has a substantial effect on APD motor patterns.
APD manometry is a technique used to measure luminal occlusive contractions, which result in peristaltic flow.
This is a continuum of authors’ previous works and tested a role of diet in the duodenum in the stimulation of the APD motor response. It’s worth to publish on this journal as a brief report or communication.
P- Reviewers Luzza F, Yin DP S- Editor Huang XZ L- Editor A E- Editor Ma S
| 1. | Collins PJ, Houghton LA, Read NW, Horowitz M, Chatterton BE, Heddle R, Dent J. Role of the proximal and distal stomach in mixed solid and liquid meal emptying. Gut. 1991;32:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia. 1993;36:857-862. [PubMed] |
| 3. | Brener W, Hendrix TR, McHugh PR. Regulation of the gastric emptying of glucose. Gastroenterology. 1983;85:76-82. [PubMed] |
| 4. | Tougas G, Anvari M, Dent J, Somers S, Richards D, Stevenson GW. Relation of pyloric motility to pyloric opening and closure in healthy subjects. Gut. 1992;33:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Heddle R, Dent J, Read NW, Houghton LA, Toouli J, Horowitz M, Maddern GJ, Downton J. Antropyloroduodenal motor responses to intraduodenal lipid infusion in healthy volunteers. Am J Physiol. 1988;254:G671-G679. [PubMed] |
| 6. | Pilichiewicz AN, Papadopoulos P, Brennan IM, Little TJ, Meyer JH, Wishart JM, Horowitz M, Feinle-Bisset C. Load-dependent effects of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2170-R2178. [PubMed] |
| 7. | Pilichiewicz AN, Chaikomin R, Brennan IM, Wishart JM, Rayner CK, Jones KL, Smout AJ, Horowitz M, Feinle-Bisset C. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab. 2007;293:E743-E753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 8. | Chapman M, Fraser R, Vozzo R, Bryant L, Tam W, Nguyen N, Zacharakis B, Butler R, Davidson G, Horowitz M. Antro-pyloro-duodenal motor responses to gastric and duodenal nutrient in critically ill patients. Gut. 2005;54:1384-1390. [PubMed] |
| 9. | Fraser R, Horowitz M, Dent J. Hyperglycaemia stimulates pyloric motility in normal subjects. Gut. 1991;32:475-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Houghton LA, Read NW, Heddle R, Maddern GJ, Downton J, Toouli J, Dent J. Motor activity of the gastric antrum, pylorus, and duodenum under fasted conditions and after a liquid meal. Gastroenterology. 1988;94:1276-1284. [PubMed] |
| 11. | Defilippi CC, Gomez E. Continuous recording of pyloric sphincter pressure in dogs. Relationship to migratory motor complex. Dig Dis Sci. 1985;30:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Yuan SY, Costa M, Brookes SJ. Neuronal control of the pyloric sphincter of the guinea-pig. Neurogastroenterol Motil. 2001;13:187-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Edelbroek M, Horowitz M, Fraser R, Wishart J, Morris H, Dent J, Akkermans L. Adaptive changes in the pyloric motor response to intraduodenal dextrose in normal subjects. Gastroenterology. 1992;103:1754-1761. [PubMed] |
| 14. | Thompson DG, Wingate DL. Effects of osmoreceptor stimulation on human duodenal motor activity. Gut. 1988;29:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Andrews JM, Doran SM, Hebbard GS, Malbert CH, Horowitz M, Dent J. Nutrient-induced spatial patterning of human duodenal motor function. Am J Physiol Gastrointest Liver Physiol. 2001;280:G501-G509. [PubMed] |
| 16. | Fone DR, Horowitz M, Dent J, Read NW, Heddle R. Pyloric motor response to intraduodenal dextrose involves muscarinic mechanisms. Gastroenterology. 1989;97:83-90. [PubMed] |
| 17. | Pilichiewicz AN, Little TJ, Brennan IM, Meyer JH, Wishart JM, Otto B, Horowitz M, Feinle-Bisset C. Effects of load, and duration, of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol. 2006;290:R668-R677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 18. | Katschinski M, Schirra J, Begliner C, Langbein S, Wank U, D’Amato M, Arnold R. Intestinal phase of human antro-pyloro-duodenal motility: cholinergic and CCK-mediated regulation. Eur J Clin Invest. 1996;26:574-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Schwizer W, Borovicka J, Kunz P, Fraser R, Kreiss C, D’Amato M, Crelier G, Boesiger P, Fried M. Role of cholecystokinin in the regulation of liquid gastric emptying and gastric motility in humans: studies with the CCK antagonist loxiglumide. Gut. 1997;41:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Göke B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut. 2006;55:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Deane AM, Nguyen NQ, Stevens JE, Fraser RJ, Holloway RH, Besanko LK, Burgstad C, Jones KL, Chapman MJ, Rayner CK. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab. 2010;95:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 22. | Kuo P, Rayner CK, Horowitz M. Gastric emptying, diabetes, and aging. Clin Geriatr Med. 2007;23:785-808, vi. [PubMed] |
| 23. | Bhutto A, Morley JE. The clinical significance of gastrointestinal changes with aging. Curr Opin Clin Nutr Metab Care. 2008;11:651-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Deane A, Chapman MJ, Fraser RJ, Bryant LK, Burgstad C, Nguyen NQ. Mechanisms underlying feed intolerance in the critically ill: implications for treatment. World J Gastroenterol. 2007;13:3909-3917. [PubMed] |
| 25. | Deane A, Chapman MJ, Fraser RJ, Horowitz M. Bench-to-bedside review: the gut as an endocrine organ in the critically ill. Crit Care. 2010;14:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Hausken T, Mundt M, Samsom M. Low antroduodenal pressure gradients are responsible for gastric emptying of a low-caloric liquid meal in humans. Neurogastroenterol Motil. 2002;14:97-105. [PubMed] |
| 27. | Vozzo R, Su YC, Fraser RJ, Wittert GA, Horowitz M, Malbert CH, Shulkes A, Volombello T, Chapman IM. Antropyloroduodenal, cholecystokinin and feeding responses to pulsatile and non-pulsatile intraduodenal lipid infusion. Neurogastroenterol Motil. 2002;14:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Schvarcz E, Palmér M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113:60-66. [PubMed] |
| 29. | Samsom M, Smout AJ. Abnormal gastric and small intestinal motor function in diabetes mellitus. Dig Dis. 1997;15:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Kuo P, Wishart JM, Bellon M, Smout AJ, Holloway RH, Fraser RJ, Horowitz M, Jones KL, Rayner CK. Effects of physiological hyperglycemia on duodenal motility and flow events, glucose absorption, and incretin secretion in healthy humans. J Clin Endocrinol Metab. 2010;95:3893-3900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |