Published online Sep 14, 2013. doi: 10.3748/wjg.v19.i34.5727
Revised: July 24, 2013
Accepted: August 4, 2013
Published online: September 14, 2013
Processing time: 118 Days and 14.5 Hours
AIM: To study the diagnostic value of immunoglobulin heavy chain (IgH) and T-cell receptor γ (TCR-γ) gene monoclonal rearrangements in primary gastric lymphoma (PGL).
METHODS: A total of 48 patients with suspected PGL at our hospital were prospectively enrolled in this study from January 2009 to December 2011. The patients were divided into three groups (a PGL group, a gastric linitis plastica group, and a benign gastric ulcer group) based on the pathological results (gastric mucosal specimens obtained by endoscopy or surgery) and follow-up. Endoscopic ultrasonography (EUS) and EUS-guided biopsy were performed in all the patients. The tissue specimens were used for histopathological examination and for IgH and TCR-γ gene rearrangement polymerase chain reaction analyses.
RESULTS: EUS and EUS-guided biopsy were successfully performed in all 48 patients. In the PGL group (n = 21), monoclonal IgH gene rearrangements were detected in 14 (66.7%) patients. A positive result for each set of primers was found in 12 (57.1%), 8 (38.1%), and 4 (19.0%) cases using FR1/JH, FR2/JH, and FR3/JH primers, respectively. Overall, 12 (75%) patients with mucosal-associated lymphoid tissue lymphoma (n = 16) and 2 (40%) patients with diffuse large B-cell lymphoma (n = 5) were positive for monoclonal IgH gene rearrangements. No patients in the gastric linitis plastica group (n = 17) and only one (10%) patient in the benign gastric ulcer group (n = 10) were positive for a monoclonal IgH gene rearrangement. No TCR-γ gene monoclonal rearrangements were detected. The sensitivity of monoclonal IgH gene rearrangements was 66.7% for a PGL diagnosis, and the specificity was 96.4%. In the PGL group, 8 (100%) patients with stage IIE PGL (n = 8) and 6 (46.1%) patients with stage IE PGL (n = 13) were positive for monoclonal IgH gene rearrangements.
CONCLUSION: IgH gene rearrangements may be associated with PGL staging and may be useful for the diagnosis of PGL and for differentiating between PGL and gastric linitis plastica.
Core tip: In 2003, a new primer system was successfully developed and standardized for the detection of clonally rearranged immunoglobulin (Ig) and T-cell receptor (TCR) genes. This study was a prospective analysis of Ig heavy chain (IgH) and TCR-γ gene rearrangements using the new primer system and endoscopic biopsy specimens from patients with suspected primary gastric lymphoma (PGL). Our study revealed that the detection of monoclonal IgH gene rearrangements is useful for the diagnosis of PGL and for differentiating between PGL and gastric linitis plastica. Monoclonal IgH gene rearrangements may be associated with PGL staging. The sensitivity and the specificity of IgH gene rearrangements for the diagnosis of PGL were 66.7% and 96.4%, respectively.
- Citation: Shan GD, Hu FL, Yang M, Chen HT, Chen WG, Wang YG, Chen LH, Li YM, Xu GQ. Clonal immunoglobulin heavy chain and T-cell receptor γ gene rearrangements in primary gastric lymphoma. World J Gastroenterol 2013; 19(34): 5727-5731
- URL: https://www.wjgnet.com/1007-9327/full/v19/i34/5727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i34.5727
Primary gastric lymphoma (PGL) is a relatively rare tumor type that accounts for 5% of all gastric tumors[1]. Gastroscopy and biopsy are the primary methods for diagnosis. However, most PGLs arise in the submucosa, and the diagnosis of PGL by gastroscopy and biopsy is often difficult. An endoscopic presentation of polypoid lesions, flat lesions, enlarged gastric folds, ulcers, erosions, and negative or inconclusive histology may lead clinicians to suspect gastric lymphoma[2-8]. It is recommended that biopsy specimens undergo histomorphological, immunohistochemical, and immunophenotypic analyses for a diagnosis of gastric lymphoma. However, these methods may not lead to a diagnosis, especially in the early stages of the disease.
Immunoglobulin (Ig) and T cell receptors (TCRs) are the molecules responsible for B- and T-cell immune responses. The analysis of antigen receptor gene rearrangements by polymerase chain reaction (PCR) is a routine diagnostic tool for lymphoproliferative disorders[9].
Previous studies have demonstrated that Ig gene rearrangements in endoscopic biopsy samples were an additional tool for the diagnosis of gastric mucosal-associated lymphoid tissue (MALT) lymphoma[10-15]. In previous studies, the primers and PCR conditions were not standardized. Multiplex PCR assays have been available since 2003, and these assays have been standardized for the detection of clonally rearranged Ig and TCR genes[16]. However, multiplex PCR assays for the detection of Ig heavy chain (IgH) and TCR-γ gene rearrangements in PGL have not been previously reported. The aim of this study was to investigate the detection rate and the diagnostic value of IgH and TCR-γ gene monoclonal rearrangements in PGL endoscopic biopsy specimens.
A total of 48 patients with suspected PGL at our hospital were prospectively enrolled in this study from January 2009 to December 2011. The patients were divided into three groups based on the pathological results (gastric mucosal specimens obtained by endoscopy or surgery) and follow-up. The PGL group consisted of 21 patients (14 males, 7 females, a mean age of 51 years, range 20-81 years). The gastric linitis plastica group consisted of 17 patients (11 males, 6 females, a mean age of 53 years, range 17-79 years). The benign gastric ulcer group consisted of 10 patients (7 males, 3 females, a mean age of 47 years, range 22-76 years).
Patients who met the criteria for suspected gastric lymphoma (an endoscopic presentation of polypoid lesions, flat lesions, enlarged gastric folds, ulcers, erosions, and negative or inconclusive histology) were included in the study. Patients with palpable superficial lymphadenopathy, obvious mediastinal lymphadenopathy, abnormal total and differential white blood cell counts, and the involvement of other organs in the abdomen were excluded from the study.
Informed consent was obtained from all the patients and this study was approved by the hospital before endoscopic ultrasonography (EUS) and the medical records analysis. EUS and EUS-guided biopsy were performed in all the patients. Overall, 8-10 biopsies were obtained from each patient. The specimens were submitted for histopathological examination. A portion of each specimen was stored at -80 °C for the gene rearrangement analysis by PCR.
DNA was isolated from frozen tissue by cell lysis, phenol extraction, and ethanol precipitation according to standard procedures. Alternatively, reactive DNAzol (Songon Biotech, Shanghai, China) was used according to the manufacturer’s specifications. In each experiment, polyclonal DNA (reactive lymphoid tissue) and negative (sterile water) and positive controls were systematically included. To analyze the IgH gene, three sets of VH primers and one JH consensus primer were combined in three multiplex tubes. To analyze the TCR-γ gene, four Vγ primers and two Jγ primers were divided into two tubes (Table 1).
| Gene | Sequence |
| IgH tube A | |
| VH1-FR1 | 5’GGCCTCAGTGAAGGTCTCCTGCAAG3’ |
| VH2-FR1 | 5’GTCTGGTCCTACGCTGGTGAAACCC3’ |
| VH3-FR1 | 5’CTGGGGGGTCCCTGAGACTCTCCTG3’ |
| VH4-FR1 | 5’CTTCGGAGACCCTGCCCTCACCTG3’ |
| VH5-FR1 | 5’CGGGGAGTCTCTGAAGATCTCCTGT3’ |
| VH6-FR1 | 5’TCGCAGACCCTCTCACTCACCTGTG3’ |
| JH consensus | 5'CTTACCTGAGGAGACGGTGACC3' |
| IgH tube B | |
| VH1-FR2 | 5’CTGGG TGCGA CAGGC CCCTG GACAA3’ |
| VH2-FR2 | 5’TGGAT CCGTC AGCCC CCAGG GAAGG3’ |
| VH3-FR2 | 5’GGTCC GCCAG GCTCC AGGGA A3’ |
| VH4-FR2 | 5’TGGAT CCGCC AGCCC CCAGG GAAGG3’ |
| VH5-FR2 | 5’GGGTG CGCCA GATGC CCGGG AAAGG3’ |
| VH6-FR2 | 5’TGGAT CAGGC AGTCC CCATC GAGAG3’ |
| VH7-FR2 | 5’TTGGG TGCGA CAGGC CCCTG GACAA3’ |
| JH consensus | 5'CTTACCTGAGGAGACGGTGACC3' |
| IgH tube C | |
| VH1-FR3 | 5’TGGAG CTGAG CAGCC TGAGA TCTGA3’ |
| VH2-FR3 | 5’CAATG ACCAA CATGG ACCCT GTGGA3’ |
| VH3-FR3 | 5’TCTGC AAATG AACAG CCTGA GAGCC3’ |
| VH4-FR3 | 5’GAGCT CTGTG ACCGC CGCGG ACACG3’ |
| VH5-FR3 | 5’CAGCA CCGCC TACCT GCAGT GGAGC3’ |
| VH6-FR3 | 5’GTTCT CCCTG CAGCT GAACT GTGTG3’ |
| VH7-FR3 | 5’CAGCA CGGCA TATCT GCAGA TCAG3’ |
| JH consensus | 5'CTTACCTGAGGAGACGGTGACC3' |
| TCR-γ tube A | |
| Vγ1f | 5’GGAAG GCCCC ACAGC RTCTT3’ |
| vγ10 | 5’AGCATGGGTAAGACAAGCAA3’ |
| Jγ1.1/2.1 | 5’TTACCAGGCGAAGTTACTATGAGC3’ |
| Jγ:1.3/2.3 | 5’GTGTTGTTCCACTGCCAAAGAG3’ |
| TCR-γ tube B | |
| Vγ9 | 5’CGGCA CTGTC AGAAA GGAATC3’ |
| Vγ11 | 5’CTTCC ACTTC CACTT TGAAA3’ |
| Jγ1.1/2.1 | 5’TTACCAGGCGAAGTTACTATGAGC3’ |
| Jγ:1.3/2.3 | 5’GTGTTGTTCCACTGCCAAAGAG3’ |
The PCR conditions were × 1 PCR buffer [50 mmol/L KCl, 10 mmol/L Tris (pH, 8.3), 1.5 mmol/L MgCl2], 200 µmol/L of each deoxynucleotide, 10 pmol of each primer, and 2 U of Taq polymerase (Songon Biotech, Shanghai, China). The total PCR reaction volume was 50 μL. The thermal cycling conditions were pre-activation for 7 min at 95 °C, followed by annealing at 60 °C. Each reaction consisted of 35 cycles. The cycles were preceded by an initial denaturation step for 45 s, followed by a terminal extension for 10 min.
Patients with PGL were staged at baseline using computed tomography scans of the neck, the thorax, and the abdomen, followed by EUS and bone marrow biopsy. EUS staging was performed according to the Ann Arbor staging system[10].
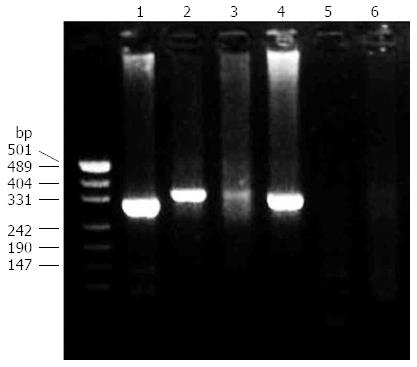
EUS and EUS-guided biopsy were successfully performed in all 48 patients. In the PGL group, monoclonal IgH gene rearrangements were detected in 14 (66.7%) patients. Positive results for each set of primers were obtained in 12 (57.1%), 8 (38.1%), and 4 (19.0%) cases using FR1/JH, FR2/JH, and FR3/JH primers, respectively (Figure 1). No patients in the gastric linitis plastica group were positive for a monoclonal IgH gene rearrangement. In the benign gastric ulcer group, a monoclonal IgH gene rearrangement was detected in one (10%) patient. Overall, no TCR-γ gene monoclonal rearrangements were detected. For the diagnosis of primary gastric lymphomas, the sensitivity of the monoclonal IgH gene rearrangements was 66.7% and the specificity was 96.4%.
In the PGL group, the clinical stage distribution was as follows: IE in 13 patients and IIE in 8 patients. All 8 (100%) patients with stage IIE PGL were positive for monoclonal IgH gene rearrangements. Additionally, 6 (46.1%) patients with stage IE PGL were positive for monoclonal IgH gene rearrangements. The PGL group consisted of 16 patients with MALT lymphoma and 5 patients with diffuse large B-cell lymphoma (DLBCL). In the patients with MALT lymphomas, 12 (75%) were positive for monoclonal IgH gene rearrangements. In the patients with DLBCL, 2 (40%) were positive for monoclonal IgH gene rearrangements.
In the benign gastric ulcer group, patients were treated with a proton-pump inhibitor (esomeprazole 20 mg daily) for 8-16 wk. Repeated endoscopies revealed that the gastric ulcers were completely healed in all the patients. All the patients were followed up for 12-18 mo, and no malignant gastric lesions were found.
Antigen receptor gene rearrangement in lymphocytes is a physiological process. Tumor cells that originate from lymphocytes often carry the same Ig and TCR gene rearrangements (monoclonal), whereas T and B cells have a unique type of rearrangement in benign lymphoid disorders (polyclonal). Antigen receptor gene rearrangement analysis is useful in differentiating between malignant lymphoproliferative disorders and non-neoplastic lymphoid disorders. There are several PCR targets for the detection of Ig and TCR rearrangements. Three multiplex PCR assays are available for the detection of clonal IgH (VH-JH) rearrangements, and these assays can reliably identify clonal B-cell proliferation and TCR-γ gene monoclonal rearrangements that occur in most T-cell lymphoid neoplasms[16]. IgH (VH-JH) and TCR-γ are the most common PCR targets for detecting Ig and TCR rearrangements.
In the PGL group, the positive rate of monoclonal IgH gene rearrangement in patients with stage IIE PGL was 100% but only 46.1% in patients with stage IE PGL. Previous studies have demonstrated that the positive rate of monoclonal IgH gene rearrangements was associated with histological grading (the histological grading of lymphoid infiltrates in the stomach according to the Wotherspoon-Isaacson histological scoring system). Aiello et al[10] reported that monoclonal IgH gene rearrangements were detected in 64.2%, 41.6%, and 3.1% of samples with histological grading scores of 5, 4, and 0-3, respectively. Additionally, the results of this study suggest that the positive rate of monoclonal IgH gene rearrangements may be associated with PGL staging.
In the PGL group, 75% (12/16) of MALT lymphoma patients were positive for monoclonal IgH gene rearrangements. However, only 40% (2/5) of DLBCL patients were positive for monoclonal IgH gene rearrangements. A previous study reported that the positive rate of monoclonal IgH gene rearrangements in MALT lymphoma patients ranged from 62.5%-98.5%[10-13]. In the series in this study, a similar rate was observed for monoclonal IgH gene rearrangements in MALT lymphoma patients. Thériault et al[17] reported that the positive rate of monoclonal IgH gene rearrangements in DLBCL patients was 78.9% (30/38). However, the specimens in the study included lymph nodes, tonsils, spleens, bone marrow, skin biopsies, and gastrointestinal tract samples. In this study, the positive rate for DLBCL patients was lower than that in the previous study. In addition, recent studies have demonstrated that the detection rate of monoclonal IgH gene rearrangement was closely associated with the cell origin of lymphomas[9].
The differential diagnosis between gastric linitis plastica and PGL is not easy for a physician to determine. PGL and gastric linitis plastica usually result in low rates of positive endoscopic biopsies[18]. In this series, the first endoscopic biopsies from PGL and gastric linitis plastica patients were all negative. The distinction between PGL and gastric linitis plastica is important because of the different prognoses of these diseases. In this study, 14 (66.7%) patients in the PGL group were positive for a monoclonal IgH gene rearrangement; however, no IgH gene rearrangements were detected in the patients in the gastric linitis plastica group. One case of gastric cancer was positive for a monoclonal IgH gene rearrangement. The patient was diagnosed with carcinoma accompanied by lymphoma[12]. This result suggests that the detection of IgH gene rearrangements may be helpful in differentiating between PGL and gastric linitis plastica.
According to previous studies, monoclonal rearrangements involving IgH genes were detected in 3% of lymphoid disorders that were benign based on clinical and immunohistological evaluations, which is consistent with the findings of this study[19]. The majority of these patients suffered from autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematous, and Sjögren’s syndrome. These diseases are characterized by polyclonal B-cell activation and autoantibodies[20]. In the series in this study, a monoclonal IgH gene rearrangement was detected in one patient who was suffering from a benign gastric ulcer. Additionally, this patient had suffered from Sjögren’s syndrome for several years.
Gene rearrangement studies can be informative; however, false-positive and false-negative PCR results are problematic. According to previous studies, there are two main problems with PCR techniques: improper primer annealing and difficulties in discriminating between monoclonal and polyclonal Ig/TCR gene rearrangements[16,21,22]. Single-strand conformation polymorphism analysis, denaturing gradient gel electrophoresis, heteroduplex analysis, or gene scanning may be performed to reduce false-positive and false-negative rates[16,23-26].
In this study, there was one patient with false-negative results in the PGL group. The three endoscopic biopsies for this patient were negative, but the PCR results for a monoclonal IgH gene rearrangement were always positive. Autoimmune diseases were excluded. The patient was followed up for 7 mo and was diagnosed with PGL based on the fourth endoscopic biopsy. In addition, Fend et al[14] reported that the detection of clonal rearrangements in the biopsy specimens from two patients preceded the histological diagnosis of lymphoma by several mo. Because the detection of clonal rearrangements can precede a histological diagnosis, we suggest that patients with suspected PGL and positive results for IgH rearrangements need close follow-up.
In conclusion, the presence of an IgH gene rearrangement is useful for the diagnosis of PGL and for differentiating between PGL and gastric linitis plastica. Additionally, IgH gene rearrangements may be associated with PGL staging.
Primary gastric lymphoma (PGL) is a relatively rare tumor type. The diagnosis of PGL by gastroscopy and biopsy is often difficult. The analysis of antigen receptor gene rearrangements by polymerase chain reaction is a routine diagnostic tool for lymphoproliferative disorders. Previous studies have demonstrated that the positive rate of gene rearrangement was relatively high in PGL patients; however, no prospective studies of gene rearrangement in PGL patients have been reported.
Studies are being performed to assess the diagnostic value of immunoglobulin heavy chain (IgH) and T-cell receptor γ (TCR-γ) gene monoclonal rearrangements in PGL patients.
This study was a prospective analysis of IgH and TCR-γ gene rearrangements in endoscopic biopsy specimens from patients with suspected PGL. According to the pathological results, 48 patients with suspected PGL were divided into three groups, including a PGL group, a gastric linitis plastica group, and a benign gastric ulcer group. The study revealed that the detection of monoclonal IgH gene rearrangements is useful for the diagnosis of PGL and for differentiating between PGL and gastric linitis plastica. Additionally, these gene rearrangements may be associated with PGL staging.
The results of this study may encourage the detection of IgH gene rearrangements for a diagnosis of PGL and for differentiating between PGL and gastric linitis plastica.
Antigen receptor gene rearrangement is a physiological process. Tumor cells that originate from lymphocytes often carry the same IgH and TCR gene rearrangements (monoclonal), whereas T and B cells have a unique type of rearrangement in benign lymphoid disorders (polyclonal). Antigen receptor gene rearrangement analysis is useful in differentiating between malignant lymphoproliferative disorders and non-neoplastic lymphoid disorders.
This paper includes interesting results and presents an acceptable case for publication because few reports have been published on this subject in China.
P- Reviewers Arcaini L, Alshehabi Z, de Re V S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | al Mofleh IA. Endoscopic features of primary upper gastrointestinal lymphoma. J Clin Gastroenterol. 1994;19:69-73; discussion 73-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Kelessis NG, Vassilopoulos PP, Tsamakidis KG, Bai MG, Avital S, Rosenthal RJ. Is gastroscopy still a valid diagnostic tool in detecting gastric MALT lymphomas? A dilemma beyond the eye. Mucosa-associated lymphoid tissue. Surg Endosc. 2003;17:469-474. [PubMed] |
| 3. | Zullo A, Hassan C, Andriani A, Cristofari F, Cardinale V, Spinelli GP, Tomao S, Morini S. Primary low-grade and high-grade gastric MALT-lymphoma presentation. J Clin Gastroenterol. 2010;44:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Chandran RR, Raj EH, Chaturvedi HK. Primary gastrointestinal lymphoma: 30-year experience at the Cancer Institute, Madras, India. J Surg Oncol. 1995;60:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Kitamura K, Yamaguchi T, Okamoto K, Ichikawa D, Hoshima M, Taniguchi H, Takahashi T. Early gastric lymphoma: a clinicopathologic study of ten patients, literature review, and comparison with early gastric adenocarcinoma. Cancer. 1996;77:850-857. [PubMed] |
| 6. | Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267-1271. [PubMed] |
| 7. | Suekane H, Iida M, Kuwano Y, Kohrogi N, Yao T, Iwashita A, Fujishima M. Diagnosis of primary early gastric lymphoma. Usefulness of endoscopic mucosal resection for histologic evaluation. Cancer. 1993;71:1207-1213. [PubMed] |
| 8. | Al-Akwaa AM, Siddiqui N, Al-Mofleh IA. Primary gastric lymphoma. World J Gastroenterol. 2004;10:5-11. [PubMed] |
| 9. | Segal GH. Assessment of B-cell clonality by the polymerase chain reaction: a pragmatic overview. Adv Anat Pathol. 1996;3:195–203. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Aiello A, Giardini R, Tondini C, Balzarotti M, Diss T, Peng H, Delia D, Pilotti S. PCR-based clonality analysis: a reliable method for the diagnosis and follow-up monitoring of conservatively treated gastric B-cell MALT lymphomas? Histopathology. 1999;34:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Weston AP, Banerjee SK, Horvat RT, Cherian R, Campbell DR, Zoubine MN. Specificity of polymerase chain reaction monoclonality for diagnosis of gastric mucosa-associated lymphoid tissue (MALT) lymphoma: direct comparison to Southern blot gene rearrangement. Dig Dis Sci. 1998;43:290-299. [PubMed] |
| 12. | Algara P, Martinez P, Sanchez L, Villuendas R, Benitez J, Rivas C, Piris MA. The detection of B-cell monoclonal populations by polymerase chain reaction: accuracy of approach and application in gastric endoscopic biopsy specimens. Hum Pathol. 1993;24:1184-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Sukpanichnant S, Vnencak-Jones CL, McCurley TL. Determination of B-cell clonality in paraffin-embedded endoscopic biopsy specimens of abnormal lymphocytic infiltrates and gastrointestinal lymphoma by polymerase chain reaction. Am J Clin Pathol. 1994;102:299-305. [PubMed] |
| 14. | Fend F, Schwaiger A, Weyrer K, Propst A, Mairinger T, Umlauft F, Judmaier G, Grünewald K. Early diagnosis of gastric lymphoma: gene rearrangement analysis of endoscopic biopsy samples. Leukemia. 1994;8:35-39. [PubMed] |
| 15. | Zucca E, Bertoni F, Roggero E, Bosshard G, Cazzaniga G, Pedrinis E, Biondi A, Cavalli F. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid-tissue lymphoma of the stomach. N Engl J Med. 1998;338:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 157] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, García-Sanz R. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2284] [Cited by in RCA: 2378] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 17. | Thériault C, Galoin S, Valmary S, Selves J, Lamant L, Roda D, Rigal-Huguet F, Brousset P, Delsol G, Al Saati T. PCR analysis of immunoglobulin heavy chain (IgH) and TcR-gamma chain gene rearrangements in the diagnosis of lymphoproliferative disorders: results of a study of 525 cases. Mod Pathol. 2000;13:1269-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Caletti G, Fusaroli P, Togliani T, Bocus P, Roda E. Endosonography in gastric lymphoma and large gastric folds. Eur J Ultrasound. 2000;11:31-40. [PubMed] |
| 19. | Ferraccioli GF, De Vita S, Casatta L, Damato R, Pegoraro I, Bartoli E. Autoimmune connective tissue disease, chronic polyarthritides and B cell expansion: risks and perspectives with immunosuppressive drugs. Clin Exp Rheumatol. 1996;14 Suppl 14:S71-S80. [PubMed] |
| 20. | Frizzera G. Immunosuppression, autoimmunity, and lymphoproliferative disorders. Hum Pathol. 1994;25:627-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Davis TH, Yockey CE, Balk SP. Detection of clonal immunoglobulin gene rearrangements by polymerase chain reaction amplification and single-strand conformational polymorphism analysis. Am J Pathol. 1993;142:1841-1847. [PubMed] |
| 22. | Bourguin A, Tung R, Galili N, Sklar J. Rapid, nonradioactive detection of clonal T-cell receptor gene rearrangements in lymphoid neoplasms. Proc Natl Acad Sci USA. 1990;87:8536-8540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Bottaro M, Berti E, Biondi A, Migone N, Crosti L. Heteroduplex analysis of T-cell receptor gamma gene rearrangements for diagnosis and monitoring of cutaneous T-cell lymphomas. Blood. 1994;83:3271-3278. [PubMed] |
| 24. | Langerak AW, Szczepański T, van der Burg M, Wolvers-Tettero IL, van Dongen JJ. Heteroduplex PCR analysis of rearranged T cell receptor genes for clonality assessment in suspect T cell proliferations. Leukemia. 1997;11:2192-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 156] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Kneba M, Bolz I, Linke B, Hiddemann W. Analysis of rearranged T-cell receptor beta-chain genes by polymerase chain reaction (PCR) DNA sequencing and automated high resolution PCR fragment analysis. Blood. 1995;86:3930-3937. [PubMed] |
| 26. | Linke B, Bolz I, Fayyazi A, von Hofen M, Pott C, Bertram J, Hiddemann W, Kneba M. Automated high resolution PCR fragment analysis for identification of clonally rearranged immunoglobulin heavy chain genes. Leukemia. 1997;11:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |