Published online Sep 14, 2013. doi: 10.3748/wjg.v19.i34.5706
Revised: July 25, 2013
Accepted: August 8, 2013
Published online: September 14, 2013
Processing time: 135 Days and 20.7 Hours
AIM: To prospectively compare the healing rates of endoscopic submucosal dissection (ESD)-induced ulcers treated with either a proton-pump inhibitor (PPI) or rebamipide.
METHODS: We examined 90 patients with early gastric cancer who had undergone ESD. All patients were administered an intravenous infusion of the PPI lansoprazole (20 mg) every 12 h for 2 d, followed by oral administration of lansoprazole (30 mg/d, 5 d). After 7-d treatment, the patients were randomly assigned to 2 groups and received either lansoprazole (30 mg/d orally, n = 45; PPI group) or rebamipide (300 mg orally, three times a day; n = 45; rebamipide group). At 4 and 8 wk after ESD, the ulcer outcomes in the 2 groups were compared.
RESULTS: No significant differences were noted in patient age, underlying disease, tumor location, Helicobacter pylori infection rate, or ESD-induced ulcer size between the 2 groups. At both 4 and 8 wk, the healing rates of ESD-induced ulcers were similar in the PPI-treated and the rebamipide-treated patients (4 wk: PPI, 27.2%; rebamipide, 33.3%; P = 0.5341; 8 wk: PPI, 90.9%; rebamipide, 93.3%; P = 0.6710). At 8 wk, the rates of granulation lesions following ulcer healing were significantly higher in the PPI-treated group (13.6%) than in the rebamipide-treated group (0.0%; P = 0.0103). Ulcer-related symptoms were similar in the 2 treatment groups at 8 wk. The medication cost of 8-wk treatment with the PPI was 10945 yen vs 4889 yen for rebamipide. No ulcer bleeding or complications due to the drugs were observed in either treatment group.
CONCLUSION: The healing rate of ESD-induced ulcers was similar with rebamipide or PPI treatment; however, rebamipide treatment is more cost-effective and prevents granulation lesions following ulcer healing.
Core tip: In this prospective randomized, parallel-controlled study, we demonstrated that rebamipide monotherapy was as effective as proton-pump inhibitor (PPI) in the healing of endoscopic submucosal dissection-induced ulcers, regardless of the location of the resected cancer, the degree of atrophic gastritis, or the presence of Helicobacter pylori infection. In addition, rebamipide treatment is more cost-effective and results in a better quality of ulcer healing compared with the PPI lansoprazole.
- Citation: Takayama M, Matsui S, Kawasaki M, Asakuma Y, Sakurai T, Kashida H, Kudo M. Efficacy of treatment with rebamipide for endoscopic submucosal dissection-induced ulcers. World J Gastroenterol 2013; 19(34): 5706-5712
- URL: https://www.wjgnet.com/1007-9327/full/v19/i34/5706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i34.5706
Endoscopic mucosal resection (EMR) is a well-established curative treatment for gastric neoplasms, such as early gastric cancer confined to the mucosa. However, EMR, performed using conventional techniques such as strip biopsy or cap EMR, does not always achieve en bloc resection. Thus, endoscopic submucosal dissection (ESD) has become the preferred treatment method. Compared with EMR, ESD facilitates the collection of larger specimens, regardless of lesion size or location, resulting in a higher rate of en bloc and histologically complete resection. Moreover, the rate of local recurrence of tumors after ESD may be lower than that after conventional EMR[1]. However, the iatrogenic ulcer that develops as a result of ESD is large, and requires a considerably longer healing time compared to that resulting from conventional EMR.
Proton-pump inhibitors (PPIs) are the most effective medications for the treatment of ESD-induced ulcers. However, studies have shown that PPI monotherapy does not heal the ESD-induced ulcers sufficiently within 4 wk[2-6]. Increased understanding of the mucosal defense system has prompted the development of mucoprotective agents for clinical use in Japan. The efficacy of combination treatment involving PPIs and the mucoprotective agent rebamipide in the early treatment of ESD-induced ulcers and in the prevention of relapse of such disorders has been clearly indicated[2-5].
Rebamipide [2-(4-chlorobenzoylamino)-3-[2-(1H)-quinolinon-4-yl]-propionic acid), a novel mucosal-protective and ulcer-healing drug, is widely prescribed in East Asia. Previous studies have indicated that rebamipide is effective in the treatment of gastric ulcers as well as decreasing the recurrence rate, without affecting the Helicobacter pylori infection status of the patients[7-12]. In addition, previous randomized-controlled studies have also found that rebamipide can prevent the formation of peptic ulcers induced by the administration of nonsteroidal anti-inflammatory drugs (NSAIDs) and can suppress the mucosal inflammation associated with chronic erosive gastritis[13,14]. However, to our knowledge, no reports on the use of rebamipide for the treatment of ESD-induced ulcers have been published.
In the present study, we have prospectively evaluated the efficacy of rebamipide monotherapy in comparison to PPI monotherapy for the treatment of iatrogenic ulcers resulting from ESD for early gastric cancers.
We examined 90 consecutive patients with early gastric cancer who had been treated with ESD at Kinki University Hospital between February 2011 and January 2013. The study protocol was approved by the Kinki University Ethics Committee, and all participants provided written informed consent before undergoing ESD. In addition, the study was registered at the University Hospital Medical Information Network 000005134. All patients with early gastric cancer, including well-differentiated or moderately differentiated adenocarcinoma, were included in the study. The exclusion criteria were as follows: (1) current use of other anti-ulcer drugs, aspirin, NSAIDs, or prednisolone; (2) treatment with anti-coagulative agents; or (3) previous endoscopic treatment or surgery.
ESD was performed with an insulation-tipped knife (KD-610L; Olympus Medical Systems, Tokyo, Japan) and a flush knife (BTDK2618JB; Fujifilm Medical System, Tokyo, Japan). The electrosurgical unit used was A VIO-300D (ERBE). The injection solutions contained glycerin with 1% indigo carmine dye and, depending on the tumor location, hyaluronic acid sodium (0.4%) was also added. The ulcers that developed after ESD were carefully examined endoscopically, and any visible vessels were heat-coagulated by using hot biopsy forceps (KD-410LR; Olympus Medical Systems). Thereafter, the resected specimens were stretched, pinned flat on a rubber plate, and measured.
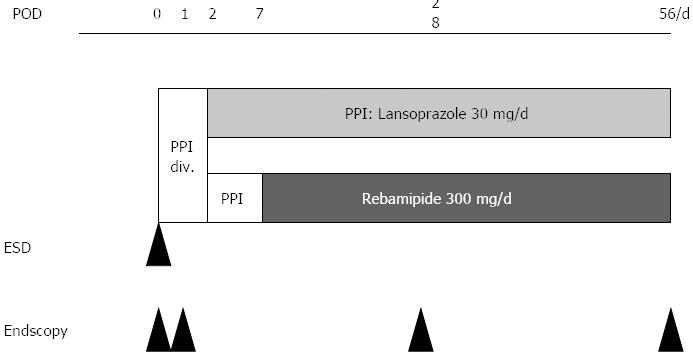
The design of this single-center, open-label, prospective, randomized, parallel-controlled study is illustrated in Figure 1.
After ESD, all patients were administered an intravenous infusion of lansoprazole (20 mg; Takepron; Takeda Pharmaceutical, Osaka, Japan) every 12 h for 2 d, and received oral lansoprazole (30 mg/d) for 5 d. On postoperative day 7, the patients were randomly assigned to 2 groups and received either lansoprazole at a dose of 30 mg/d orally (PPI group; n = 45), or rebamipide (Mucosta; Otsuka Pharmaceutical Co., Tokyo, Japan) at a dose of 300 mg orally, 3 times a day (n = 45) for 8 wk. The primary endpoint was endoscopically documented ulcer healing; complete healing was defined as regression to the S-stage on the Sakita and Miwa scale[23]. Moreover, we evaluated the healing rates of atrophic gastritis based on the Kimura and Takemoto classification[24], in the presence or absence of Helicobacter pylori (H. pylori) infection. We also compared the response of ulcers in relation to their locations in the stomach (lower, middle, or upper).
The secondary endpoint was the ulcer reduction ratio, which was compared according to the ulcer location. For calculation of the ratio, we determined the maximum diameters of the ulcers and the diameters perpendicular to the maximum diameters, which were measured using a bendable endoscopic measuring device (M2-3; Olympus Corp., Tokyo, Japan). Moreover, we determined the ulcer size (maximal diameter × diameter perpendicular to the maximal diameter). At 4 and 8 wk after ESD, the healing and reduction rates for the ulcers were compared between the 2 groups. In addition, at 8 wk after ESD, we evaluated the scar status of the ESD-induced ulcers according to the Quality of Ulcer Healing (QOUH).
Patient baseline characteristics and ulcer reduction ratios were compared using Pearson’s χ2 test or Student’s t-test. Pearson’s χ2 test was also used to compare the healing rates of the ESD-induced ulcers and for the evaluation of the scar status of the ESD-induced ulcers according to the QOUH. Statistical significance was defined as P < 0.05.
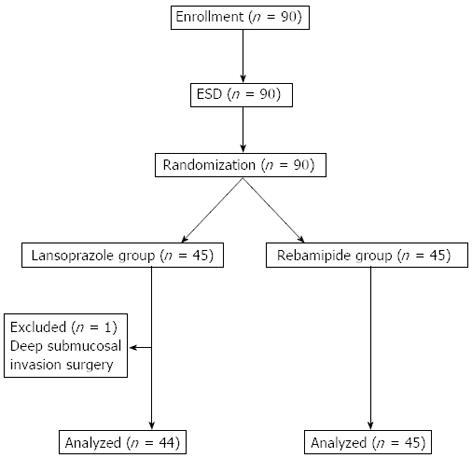
Table 1 shows the patient characteristics of the 2 treatment groups. No significant differences were noted between the groups with regard to age; gender; tumor location; tumor size; histologic classification; ESD-induced ulcer size; glandular atrophy; history of disease; and the rates of H. pylori infection, smoking, drinking alcohol, or the presence of complicated disease or intestinal metaplasia. One patient was excluded from the PPI group because histologic examination of the resected specimen indicated deep submucosal invasion (depth ≥ 500 μm; SM2 invasion). Hence, 44 patients in the PPI group and 45 in the rebamipide group constituted the final study cohort (Figure 2).
| Lansoprazole group (n = 45) | Rebamipide group (n = 45) | P-value | |
| Age (yr)(mean ± SD, median) | 70 ± 7.8, 72 | 67 ± 8.0, 67 | 0.0930 |
| Gender (M/F) | 36/9 | 31/14 | 0.2269 |
| H. pylori-infection | 86.7% | 84.4% | 0.7643 |
| Smoker | 31.1% | 33.3% | 0.8215 |
| Drinking alcohol | 46.7% | 42.2% | 0.6714 |
| History of disease | 46.7% | 31.1% | 0.1301 |
| Complicated disease | 71.1% | 71.1% | 1.0000 |
| Location of tumors | 0.1620 | ||
| Low | 23 | 16 | |
| Middle | 17 | 26 | |
| Upper | 5 | 3 | |
| Tumor size | 0.4986 | ||
| > 20 (mm) | 13 | 16 | |
| ≤ 20 (mm) | 32 | 29 | |
| Histologic classification | 0.6939 | ||
| Tub1 | 41 | 42 | |
| Tub2 | 4 | 3 | |
| Dissected size (mean ± SD, mm) | 30.5 ± 7.8 | 30.6 ± 6.4 | 0.9413 |
| Dissected area (mean ± SD, mm2) | 687.9 ± 393.1.7 | 712.3 ± 298.6 | 0.7417 |
| Glandular atrophy | 0.3167 | ||
| C-1 | 0 | 0 | |
| C-2 | 3 | 2 | |
| C-3 | 11 | 14 | |
| O-1 | 19 | 13 | |
| O-2 | 12 | 13 | |
| O-3 | 0 | 3 | |
| CandO | 0.6547 | ||
| C | 14 | 16 | |
| O | 31 | 29 | |
| Intestinal metaplasia | 71.1% | 68.9% | 0.8181 |
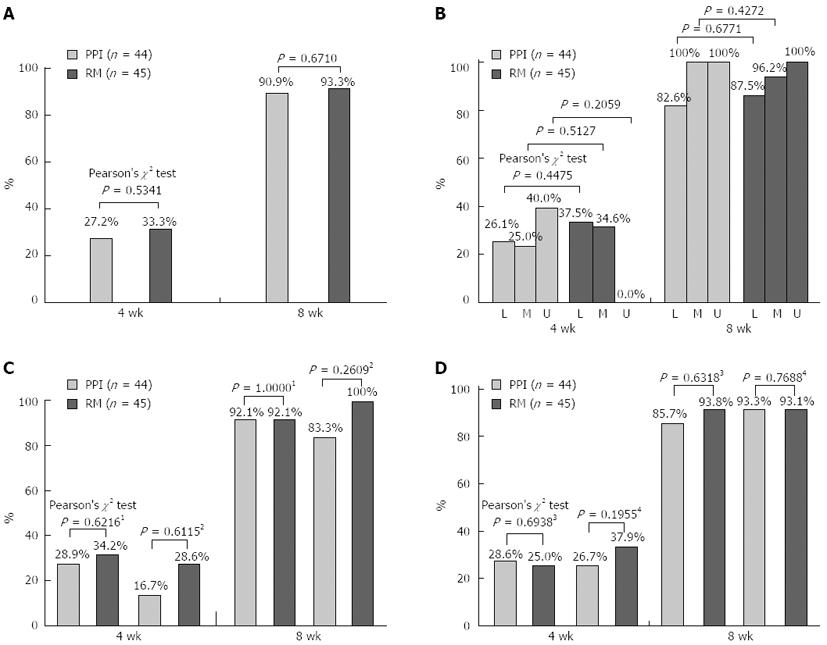
The rates of ulcer healing (regression to S-stage) were not significantly different between the PPI group (27.2%) and the rebamipide group (33.3%) at 4 wk (P = 0.5341) or at 8 wk (90.9% for the PPI group and 93.3% for the rebamipide group; P = 0.6710) (Figure 3A). Moreover, at 4 and 8 wk, the healing rates were not significantly different between the treatment groups with regard to ulcer location (low, middle, or upper stomach; Figure 3B) or with regard to the presence of absence of H. pylori infection (Figure 3C). In addition, at 4 and 8 wk, the healing rates of atrophic gastritis (closed or open type) were similar in the 2 treatment groups (Figure 3D).
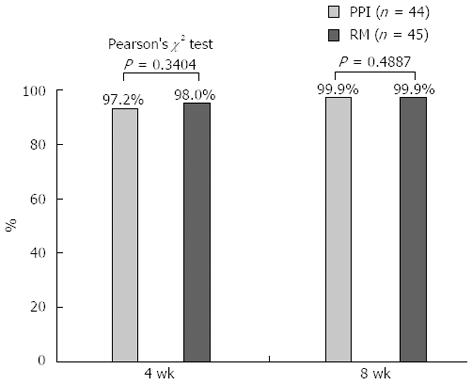
The reduction ratios of ESD-induced ulcers were similar at 4 and 8 wk in the rebamipide group (98.0% and 99.9%, respectively) and in the PPI group (97.2% and 99.9%, respectively) (Figure 4). These ratios were not influenced by the locations of the ulcers in the stomach (low, middle, and upper).
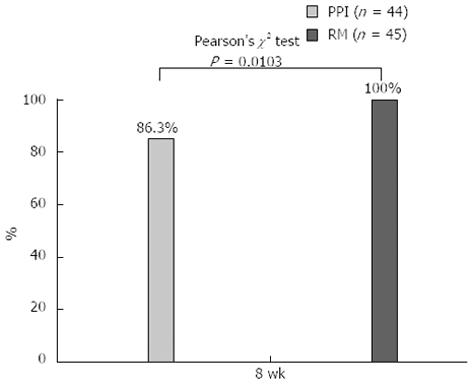
Six patients in the PPI group developed unusual gastric lesions, which comprised an overgrowth of granulation tissue at the ulcer site. At 8 wk, the proportion of patients who developed a flat scar in the rebamipide group (100%) was found to be significantly higher than that in the PPI group (86.3%; P = 0.0103) (Figure 5). No ulcer bleeding or complications related to the drugs used after ESD were observed in any of the study subjects.
Some authors have reported that the combination therapy involving PPI and rebamipide is superior to PPI monotherapy in the healing of ESD-induced ulcers[2-5]; however, to our knowledge, no reports on the efficacy of rebamipide for the treatment of ESD-induced ulcers have been published. In this prospective randomized, parallel-controlled study, we demonstrated that rebamipide monotherapy was as effective as PPI in the healing of ESD-induced ulcers, regardless of the location of the resected cancer, the degree of atrophic gastritis, or the presence of H. pylori infection.
Although the response of post-ESD ulcers to PPIs and rebamipide may be similar, the mechanisms of action of these drugs are different. PPIs decrease gastric acid production, whereas rebamipide stimulates the production of prostaglandins[19], epidermal growth factor[12,20], and nitric oxide[21], and decreases the level of oxygen-free radicals[22]. These mucosal protective actions of rebamipide appear to promote ulcer healing. Fujiwara et al[3] showed that 8 wk of PPI and rebamipide treatment was particularly effective for patients with severe atrophic gastritis, classified as O-3. Severe atrophic gastritis may result in the formation of a low-acid environment in the stomach; therefore, acid-suppressive agents such as PPI alone may have a limited effect. However, rebamipide can be effective in this environment because of its different mechanism of action. We believe that this is a contributing factor to the similar efficacies observed between PPIs and rebamipide regardless of the degree of atrophic gastritis.
Previous studies have reported that various mechanisms are involved in the effects of rebamipide on H. pylori-positive atrophic gastritis; these include prevention of adhesion of the bacteria to gastric epithelial cells, and inhibition of H. pylori-induced secretion of prostaglandin E2 from neutrophils and interleukin-8 expression in gastric epithelial cells[25-28]. Terano et al[15] indicated that the treatment of gastric ulcers with rebamipide promotes ulcer healing regardless of the success or failure of H. pylori eradication therapy, and Higuchi et al showed that rebamipide prevents the recurrence of gastric ulcers without affecting the H. pylori infection status[10]. In the present study, PPI and rebamipide appeared to aid in ulcer healing without affecting the H. pylori infection status.
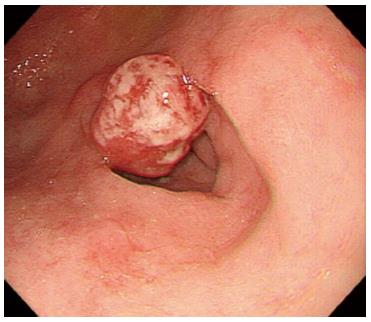
Moreover, we noted that the proportion of patients who developed a flat scar at the ulcer site was significantly higher in the rebamipide group than in the PPI group. Thus, rebamipide appears to be more effective than PPIs in improving the QOUH. In animal studies, rebamipide was found to improve the QOUH by increasing the level of prostaglandin E2 and decreasing the levels of malondialdehyde and interleukin-8 in the gastric mucosa[16]. In the present study, the unusual elevated gastric lesions that were observed following ulcer healing could not be easily characterized as benign granulation tissue or a malignant recurrence without performing a biopsy (Figure 6). Therefore, we believe that improvement in QOUH is essential for preventing the occurrence of mucosal protrusion due to the growth of granulation tissue.
The most frequent complication that occurs after endoscopic therapy is bleeding, and the rate of intraoperative bleeding is significantly higher with ESD than with EMR. Jeong et al[17] reported that PPIs may be more effective than histamine H2 inhibitors in preventing bleeding after ESD by promoting a more rapid healing of these large iatrogenic ulcers[17]. Moreover, Uedo et al[18] indicated that therapy with PPI was more effective than treatment with histamine H2 inhibitors in preventing delayed bleeding from ulcers induced by ESD. However, in the present study, no post-ESD bleeding or complications related to the drugs used were noted in the patients receiving rebamipide or PPI treatment; moreover, the ratio of ulcer reduction was at least 90% in both groups at 8 wk after initiation of therapy. Thus, our findings indicated that the rate of intraoperative bleeding was not significantly different between both the groups.
In addition, in the present study, we found that treatment with rebamipide was more cost-effective than treatment with the PPI lansoprazole. The cost of the 56-d treatment course was 4889 yen for rebamipide and 10945 yen for lansoprazole, which is a difference of 44.7%. This high difference in cost may be a factor in determining which medication to prescribe in the treatment of ESD-induced ulcers.
In conclusion, rebamipide monotherapy was equivalent to treatment with a PPI (lansoprazole) in the healing of ulcers induced by ESD for early gastric cancer. The similarity in the treatment efficacy was observed irrespective of the presence of H. pylori infection, the severity of atrophic gastritis, or the locations of the ulcers in the stomach. However, rebamipide therapy also resulted in a more favorable QOUH compared with that obtained by PPI treatment. Moreover, the treatment involving rebamipide was more cost-effective compared to the treatment with the PPI lansoprazole for the treatment of ESD-induced ulcers.
The authors wish to thank Otsuka Pharmaceutical Co., Ltd. for providing the drugs for the study.
Endoscopic submucosal dissection (ESD) is useful for treating early gastric cancer. The artificial ulcer that is generated after ESD is large, and needs a considerably longer healing time compared with conventional endoscopic mucosal resection (EMR). Rebamipide is one of the mucoprotective antiulcer drug, and is widely employed treatment of gastric ulcer in Japan.
Previous studies have shown that the combination therapy involving proton pump inhibitor (PPI) and rebamipide is superior to PPI monotherapy in the healing of ESD-induced ulcers; however, to people knowledge, no reports on the efficacy of rebamipide for the treatment of ESD-induced ulcers have been published. Therefore, the authors prospectively investigated differences in healing of ESD-induced ulcers according to treatment with PPI or rebamipide only.
In this prospective randomized, parallel-controlled study, the authors demonstrated that rebamipide monotherapy was as effective as PPI in the healing of endoscopic submucosal dissection-induced ulcers. In addition, rebamipide treatment was more cost-effective and resulted in a better quality of ulcer healing compared to the PPI lansoprazole treatment.
This article suggests that the healing rate of ESD-induced ulcers was similar with rebamipide or PPI treatment; however, rebamipide treatment is more cost-effective and prevents granulation lesions following ulcer healing. However, it is a small study, therefore, a prospective multicenter study with a large sample size should be performed to assess the efficacy of treatment with rebamipide for endoscopic submucosal dissection-induced ulcers.
ESD is a well-established curative treatment for early gastric cancer. ESD facilitates the collection of larger specimens, regardless of lesion size or location, resulting in a higher rate of en bloc and histologically complete resection. However, the iatrogenic ulcer that develops as a result of ESD is large, and requires a considerably longer healing time compared to that resulting from conventional EMR. Rebamipide is a novel mucosal-protective and ulcer-healing drug that has been widely prescribed in East Asia. Rebamipide stimulates the production of prostaglandins, epidermal growth factor, and nitric oxide, and decreases the level of oxygen-free radicals. These mucosal protective actions of rebamipide appear to promote ulcer healing.
This paper is well written. The clinical results are appropriately described.The authors present the Efficacy of treatment with rebamipide for endoscopic submucosal dissection-induced ulcers. The data indicate the healing rate of ESD-induced ulcers was similar with rebamipide or PPI treatment; however, rebamipide treatment is more cost-effective and prevents granulation lesions following ulcer healing.
P- Reviewers Mizukami K, Naito Y S- Editor Zhai HH L- Editor Cant MR E- Editor Zhang DN
| 1. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 526] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 2. | Kato T, Araki H, Onogi F, Ibuka T, Sugiyama A, Tomita E, Nagaki M, Moriwaki H. Clinical trial: rebamipide promotes gastric ulcer healing by proton pump inhibitor after endoscopic submucosal dissection--a randomized controlled study. J Gastroenterol. 2010;45:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Fujiwara S, Morita Y, Toyonaga T, Kawakami F, Itoh T, Yoshida M, Kutsumi H, Azuma T. A randomized controlled trial of rebamipide plus rabeprazole for the healing of artificial ulcers after endoscopic submucosal dissection. J Gastroenterol. 2011;46:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Kobayashi M, Takeuchi M, Hashimoto S, Mizuno K, Sato Y, Narisawa R, Aoyagi Y. Contributing factors to gastric ulcer healing after endoscopic submucosal dissection including the promoting effect of rebamipide. Dig Dis Sci. 2012;57:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Shin WG, Kim SJ, Choi MH, Kim KO, Jang HJ, Park CH, Baek IH, Kim KH, Baik GH, Kae SH. Can rebamipide and proton pump inhibitor combination therapy promote the healing of endoscopic submucosal dissection-induced ulcers? A randomized, prospective, multicenter study. Gastrointest Endosc. 2012;75:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Asakuma Y, Kudo M, Matsui S, Okada M, Kawasaki M, Umehara Y, Ichikawa T, Kitai S. Comparison of an ecabet sodium and proton pump inhibitor (PPI) combination therapy with PPI alone in the treatment of endoscopic submucosal dissection (ESD)--induced ulcers in early gastric cancer: prospective randomized study. Hepatogastroenterology. 2009;56:1270-1273. [PubMed] |
| 7. | Yamasaki K, Kanbe T, Chijiwa T, Ishiyama H, Morita S. Gastric mucosal protection by OPC-12759, a novel antiulcer compound, in the rat. Eur J Pharmacol. 1987;142:23-29. [PubMed] |
| 8. | Ogino K, Hobara T, Ishiyama H, Yamasaki K, Kobayashi H, Izumi Y, Oka S. Antiulcer mechanism of action of rebamipide, a novel antiulcer compound, on diethyldithiocarbamate-induced antral gastric ulcers in rats. Eur J Pharmacol. 1992;212:9-13. [PubMed] |
| 9. | Arakawa T, Watanabe T, Fukuda T, Yamasaki K, Kobayashi K. Rebamipide, novel prostaglandin-inducer accelerates healing and reduces relapse of acetic acid-induced rat gastric ulcer. Comparison with cimetidine. Dig Dis Sci. 1995;40:2469-2472. [PubMed] |
| 10. | Higuchi K, Arakawa T, Nebiki H, Uchida T, Fujiwara Y, Ando K, Yamasaki K, Takaishi O, Fukuda T, Kobayashi K. Rebamipide prevents recurrence of gastric ulcers without affecting Helicobacter pylori status. Dig Dis Sci. 1998;43:99S-106S. [PubMed] |
| 11. | Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A. Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci. 1998;43:5S-13S. [PubMed] |
| 12. | Tarnawski A, Arakawa T, Kobayashi K. Rebamipide treatment activates epidermal growth factor and its receptor expression in normal and ulcerated gastric mucosa in rats: one mechanism for its ulcer healing action? Dig Dis Sci. 1998;43:90S-98S. [PubMed] |
| 13. | Park SH, Cho CS, Lee OY, Jun JB, Lin SR, Zhou LY, Yuan YZ, Li ZS, Hou XH, Zhao HC. Comparison of Prevention of NSAID-Induced Gastrointestinal Complications by Rebamipide and Misoprostol: A Randomized, Multicenter, Controlled Trial-STORM STUDY. J Clin Biochem Nutr. 2007;40:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Du Y, Li Z, Zhan X, Chen J, Gao J, Gong Y, Ren J, He L, Zhang Z, Guo X. Anti-inflammatory effects of rebamipide according to Helicobacter pylori status in patients with chronic erosive gastritis: a randomized sucralfate-controlled multicenter trial in China-STARS study. Dig Dis Sci. 2008;53:2886-2895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Terano A, Arakawa T, Sugiyama T, Suzuki H, Joh T, Yoshikawa T, Higuchi K, Haruma K, Murakami K, Kobayashi K. Rebamipide, a gastro-protective and anti-inflammatory drug, promotes gastric ulcer healing following eradication therapy for Helicobacter pylori in a Japanese population: a randomized, double-blind, placebo-controlled trial. J Gastroenterol. 2007;42:690-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Qi Z, Jie L, Haixia C, Xiaoying Z. Effect of rebamipide on quality of peptic ulcer healing in rat. Dig Dis Sci. 2009;54:1876-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Jeong HK, Park CH, Jun CH, Lee GH, Kim HI, Kim HS, Choi SK, Rew JS. A prospective randomized trial of either famotidine or pantoprazole for the prevention of bleeding after endoscopic submucosal dissection. J Korean Med Sci. 2007;22:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Uedo N, Takeuchi Y, Yamada T, Ishihara R, Ogiyama H, Yamamoto S, Kato M, Tatsumi K, Masuda E, Tamai C. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Kleine A, Kluge S, Peskar BM. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of rebamipide in rats. Dig Dis Sci. 1993;38:1441-1449. [PubMed] |
| 20. | Tarnawski AS, Chai J, Pai R, Chiou SK. Rebamipide activates genes encoding angiogenic growth factors and Cox2 and stimulates angiogenesis: a key to its ulcer healing action? Dig Dis Sci. 2004;49:202-209. [PubMed] |
| 21. | Takaishi O, Arakawa T, Yamasaki K, Fujiwara Y, Uchida T, Tominaga K, Watanabe T, Higuchi K, Fukuda T, Kobayashi K. Protective effect of rebamipide against ammonia-induced gastric mucosal lesions. Dig Dis Sci. 1998;43:78S-82S. [PubMed] |
| 22. | Naito Y, Yoshikawa T, Tanigawa T, Sakurai K, Yamasaki K, Uchida M, Kondo M. Hydroxyl radical scavenging by rebamipide and related compounds: electron paramagnetic resonance study. Free Radic Biol Med. 1995;18:117-123. [PubMed] |
| 23. | Sakita T, Fukushima H. Endoscopic diagnosis. Ulcer of the stomach and duodenum. Tokyo: Nankodo 1971; 198-208. |
| 24. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87-97. |
| 25. | Hayashi S, Sugiyama T, Amano K, Isogai H, Isogai E, Aihara M, Kikuchi M, Asaka M, Yokota K, Oguma K. Effect of rebamipide, a novel antiulcer agent, on Helicobacter pylori adhesion to gastric epithelial cells. Antimicrob Agents Chemother. 1998;42:1895-1899. [PubMed] |
| 26. | Azuma T, Yamazaki S, Yamakawa A, Ito Y, Ohtani M, Dojo M, Yamazaki Y, Higashi H, Hatakeyama M. The effects of cure of Helicobacter pylori infection on the signal transduction of gastric epithelial cells. Aliment Pharmacol Ther. 2003;18 Suppl 1:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Yoshida N, Ishikawa T, Ichiishi E, Yoshida Y, Hanashiro K, Kuchide M, Uchiyama K, Kokura S, Ichikawa H, Naito Y. The effect of rebamipide on Helicobacter pylori extract-mediated changes of gene expression in gastric epithelial cells. Aliment Pharmacol Ther. 2003;18 Suppl 1:63-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Kim JS, Kim JM, Jung HC, Song IS. The effect of rebamipide on the expression of proinflammatory mediators and apoptosis in human neutrophils by Helicobacter pylori water-soluble surface proteins. Aliment Pharmacol Ther. 2003;18 Suppl 1:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |