Published online Sep 14, 2013. doi: 10.3748/wjg.v19.i34.5658
Revised: May 8, 2013
Accepted: June 18, 2013
Published online: September 14, 2013
Processing time: 192 Days and 17.2 Hours
AIM: To describe the cardiovascular disease (CVD) risk factors in a population of children with celiac disease (CD) on a gluten-free diet (GFD).
METHODS: This cross-sectional multicenter study was performed at Schneider Children’s Medical Center of Israel (Petach Tiqva, Israel), and San Paolo Hospital (Milan, Italy). We enrolled 114 CD children in serologic remission, who were on a GFD for at least one year. At enrollment, anthropometric measurements, blood lipids and glucose were assessed, and compared to values at diagnosis. The homeostasis model assessment-estimated insulin resistance was calculated as a measure of insulin resistance.
RESULTS: Three or more concomitant CVD risk factors [body mass index, waist circumference, low density lipoprotein (LDL) cholesterol, triglycerides, blood pressure and insulin resistance] were identified in 14% of CD subjects on a GFD. The most common CVD risk factors were high fasting triglycerides (34.8%), elevated blood pressure (29.4%), and high concentrations of calculated LDL cholesterol (24.1%). On a GFD, four children (3.5%) had insulin resistance. Fasting insulin and HOMA-IR were significantly higher in the Italian cohort compared to the Israeli cohort (P < 0.001). Children on a GFD had an increased prevalence of borderline LDL cholesterol (24%) when compared to values (10%) at diagnosis (P = 0.090). Trends towards increases in overweight (from 8.8% to 11.5%) and obesity (from 5.3% to 8.8%) were seen on a GFD.
CONCLUSION: This report of insulin resistance and CVD risk factors in celiac children highlights the importance of CVD screening, and the need for dietary counseling targeting CVD prevention.
Core tip: In our study we demonstrate a relatively high proportion of children with celiac disease (CD) adherent to a gluten-free diet (GFD) with one or more cardiovascular disease (CVD) risk factors. Furthermore, this is the first report of insulin resistance in celiac patients either in adults or children. These findings suggest that screening for CVD risk factors in celiac children both at diagnosis and during follow-up is important. Furthermore, dietary counseling over time, targeting obesity and CVD risk factors in addition to monitoring adherence to a GFD in children and adolescents diagnosed with CD, may be warranted.
- Citation: Norsa L, Shamir R, Zevit N, Verduci E, Hartman C, Ghisleni D, Riva E, Giovannini M. Cardiovascular disease risk factor profiles in children with celiac disease on gluten-free diets. World J Gastroenterol 2013; 19(34): 5658-5664
- URL: https://www.wjgnet.com/1007-9327/full/v19/i34/5658.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i34.5658
Celiac disease (CD) is a common gastrointestinal autoimmune disorder characterized by inflammation of the small bowel mucosa triggered and sustained by ingestion of gluten in genetically predisposed individuals[1]. The prevalence of CD worldwide ranges between 0.5% and 3% of the general population[2-4]. Although CD has traditionally been considered a malabsorptive disorder associated with diarrhea and weight loss, these symptoms are now seen less frequently[5]. Several recent studies have reported that only a minority of newly diagnosed patients were underweight. Instead, many patients, both children and adults were overweight or even obese[6,7].
A definitive diagnosis of CD is currently made using IgA anti-tissue transglutaminase (tTG) antibody screening followed, in most cases, by confirmatory biopsies of the small intestine with compatible histopathological findings[8]. Currently, the only treatment for CD is a strict, life-long gluten-free diet (GFD) which leads to rapid clinical improvement, especially in children. The normalization of serological tests usually occurs 6 to 12 mo after initiation of a GFD[2].
Deranged adiposity, blood lipid profile abnormalities and other risk factors for cardiovascular disease (CVD) in CD patients are still debated, and clear conclusions have yet to be reached[9]. Several studies have demonstrated that CVD risk factors, namely obesity, abnormal blood lipid profile, hypertension and insulin resistance have their roots in childhood and tend to track into adulthood[10-12]. The primary aim of this study was to describe CVD risk factors in a population of celiac children adherent to a GFD for at least one year, in two Mediterranean countries.
This cross-sectional multicenter study was performed at Schneider Children’s Medical Center of Israel (Petach Tiqva, Israel), and San Paolo Hospital (Milan, Italy) between June 2010 and December 2011.
The study population included individuals less than 18 years old, previously diagnosed with CD, without known co-morbidities, who were referred for follow-up to the pediatric gastroenterology clinics of the participating centers. Patients were included if they had a definitive diagnosis of CD, ascertained by both positive serology and confirmed by compatible duodenal biopsies, and who had been on a GFD for at least one year with complete normalization of their CD serology (tTG antibodies) at the time of enrollment.
At enrollment, all patients underwent physical examination including measurement of weight using a digital scale, and height using a stadiometer. Measurement of waist circumference was performed with a tape measured to the nearest 0.5 cm at the midpoint between the bottom of the rib cage and the top of the iliac crest. Blood pressure was measured in the sitting position using an appropriately sized cuff. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. To evaluate BMI values across different age and gender groups we used the BMI standard deviation score percentiles that were calculated according to the Center of Disease Control and Prevention growth charts of 2000[13]. Children with BMI values lower than the 5th percentile for age and gender were classified as “underweight”, those in the 5th to 85th percentile were classified as “normal weight”, those in the 85th and 95th percentile were classified as “overweight” and those greater than the 95th percentile were classified as “obese”[13]. Pre-hypertension was defined as an average systolic or diastolic blood pressure between the 90th and 95th percentile for sex, age, and height-percentile-specific, and hypertension if the values were above the 95th percentile[14]. The same pediatrician performed the Tanner stage of puberty.
After an overnight fast of at least 8 h, blood samples were drawn for fasting glucose and insulin, triglycerides, total cholesterol, high density lipoprotein (HDL) cholesterol and tTG antibodies. The samples were analyzed in local laboratories. tTG antibody levels were quantified by enzyme linked immunosorbent assay. Serum glucose level was measured by the enzymatic UV test method using an automated analyzer and total cholesterol, triglycerides, and HDL cholesterol concentrations were measured by an enzymatic colorimetric method on an automated analyzer. LDL cholesterol was calculated using the Fridewald equation: LDL cholesterol = total cholesterol - [HDL cholesterol + (triglyceride/5)]. Serum insulin concentrations were measured by an immunometric assay with the Immulite 2000 Analyzer. According to the American Academy of Pediatrics (AAP) criteria, borderline levels of cholesterol were defined by values between the 75th and 95th percentile of LDL cholesterol, while values greater than the 95th percentile were considered elevated[15]. Insulin resistance was estimated by the homeostatic model assessment (HOMA-IR), as follows: HOMA-IR = [fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5). Although the hyperinsulinemic euglycemic clamp is the only validated method to evaluate insulin sensitivity in the pediatric population, HOMA has been widely used to estimate insulin resistance in the screening of large populations of euglycemic children[16]. In this study, insulin resistance was defined as HOMA > 3.16 according to the most recent cut-off for the pediatric population[17]. Based on the Bogalosa Heart Study[11], we analyzed six risk factors for CVD. The CVD risk factors considered were BMI Z-scores greater than the 85th percentile, waist circumference over the 90th percentile[18], fasting LDL cholesterol or triglycerides higher than the 75th percentile, systolic or diastolic blood pressure greater than the 90th percentile and the state of insulin resistance[19].
In addition, we retrieved all the available data on anthropometry, blood lipids and glucose profiles at the time of diagnosis of CD from patient files.
This study was approved by the institutional review boards at each of the participating centers. Signed informed consent was provided by a legal guardian of each participant.
Descriptive data are shown as mean ± SD or number of observations (percentage). Symmetry of distribution of the variables was tested by the Kolmogorov-Smirnov test (P > 0.05). Triglycerides were not symmetrically distributed and were therefore log-transformed for analysis. Comparisons between the groups for continuous variables were performed by the t test for unpaired data or the Wilcoxon-Mann-Whitney test as appropriate. The χ2 test for unpaired discrete variables and the Wilcoxon-Mann-Whitney test for paired discrete variables were used in this study. Additionally, a multiple logistic regression analysis was performed to assess the independent association of the center with HOMA-IR, adjusted for age, gender, BMI Z-score, Tanner stage and duration of diet. All P-values less than 0.05 were considered to indicate statistical significance (two tailed test). The SPSS software, version 18.0 (SPSS Inc, Chicago, IL, United States) was used for the statistical analysis.
During the study period, 114 children with a mean age of 10.4 (± 4.1) years (70 from Israel and 44 from Italy) were enrolled. The two populations of children had comparable demographic data and anthropometry both at diagnosis and after at least 1 year of a GFD (Table 1). The only significant difference was a longer follow-up period in the Italian children. Complete fasting lipid profiles prior to initiation of the GFD were available for 52/114 children, 36/70 from Israel and 16/44 from Italy, and insulin levels were not available from CD diagnosis as prior to our study the screening of lipid and glucose profiles was not routinely performed in patients with suspected CD.
| Variable | Israel | Italy | P-value |
| n | 70 | 44 | |
| Sex (female) | 71.40% | 59.10% | 0.176 |
| Age at diagnosis (mo) | 77.0 ± 43.5 | 68.7 ± 48.5 | 0.185 |
| Duration of GFD (mo) | 38.9 ± 30.4 | 69.7 ± 55.6 | < 0.00011 |
| Weight Z-score | |||
| T0 | -0.405 ± 1.21 | -0.931 ± 1.36 | 0.357 |
| T1 | -0.172 ± 1.25 | -0.240 ± 1.19 | 0.881 |
| Height Z-score | |||
| T0 | -0.397 ± 1.14 | -0.682 ± 1.40 | 0.455 |
| T1 | -0.192 ± 1.78 | -0.310 ± 1.02 | 0.227 |
| BMI Z-score | |||
| T0 | -0.103 ± 1.1 | -0.369 ± 1.0 | 0.489 |
| T1 | -0.025 ± 1.2 | -0.162 ± 1.2 | 0.760 |
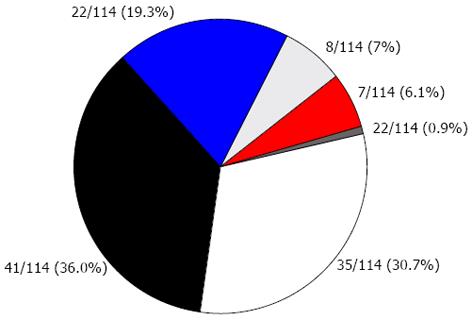
Overall, 14% of the cohort had 3 or more concomitant risk factors (Figure 1). Only 30.7% of the cohort did not have any risk factors (Figure 1). No significant difference was seen in the prevalence of CVD risk factors between the two countries in the cohort. The most common CVD risk factors were high fasting triglycerides (34.8%), elevated blood pressure (29.4%), and high concentrations of calculated LDL cholesterol (24.1%).
We did not find any significant difference in the anthropometrics data between the Israeli and Italian CD children (Table 1). Anthropometrics in the whole cohort prior to and following the introduction of a GFD revealed significant increases in both height and weight Z-scores with an increase in BMI Z-scores which did not reach significance (Table 2). When scores were pooled into the CDC BMI categories, we found that both the prevalence of overweight and obesity increased from 8.8% and 5.3%, respectively, at the time of diagnosis to 11.4% and 8.8%, respectively, after the introduction of a GFD. This trend did not attain statistical significance (P = 0.105).
| Variable | T0 | T1 | P-value |
| Z-score height | -0.447 ± 1.1 | -0.238 ± 1.1 | 0.0011 |
| Z-score weight | -0.567 ± 1.3 | -0.198 ± 1.2 | < 0.0011 |
| BMI Z-score | -0.207 ± 1.1 | -0.078 ± 1.2 | 0.103 |
| BMI categories2 | |||
| Underweight | 11/114 (9.6) | 12/114 (10.5) | |
| Normal weight | 87/114 (76.3) | 79/114 (69.3) | |
| Overweight | 10/114 (8.8) | 13/114 (11.4) | |
| Obese | 6/114 (5.3) | 10/114 (8.8) | 0.105 |
There were no significant differences in the lipid profiles between the Israeli and Italian cohorts, except for higher levels of HDL cholesterol in the Italian patients. According to AAP criteria, 63% of the patients in our cohort had normal LDL cholesterol, 30% had borderline and 7% had hypercholesterolemia after at least one year of a GFD (Table 2). Although data on the lipid profile before CD diagnosis were available only in 50% of enrolled patients, we found significant increases in both total cholesterol and HDL cholesterol in patients on a GFD. The categorization of the LDL cholesterol values highlighted an increase in the prevalence of borderline cholesterol levels (from 9.6% to 23.1%), which did not reach statistical significance (P = 0.090).
The Italian children were found to have both higher fasting insulin and HOMA-IR levels while on a GFD when compared to the Israeli cohort (Table 3). Four patients (3.5%) were identified with frank insulin resistance, three from Italy, and one from Israel (Table 3). Two of these had normal weight and the remaining patients were overweight.
| Variable | Israel | Italy | P-value |
| Total cholesterol (mg/dL) | 158.3 ± 27.6 | 162.5 ± 24.9 | 0.570 |
| Cholesterol LDL (mg/dL) | 95.4 ± 21 | 89.9 ± 22.3 | 0.116 |
| Cholesterol HDL (mg/dL) | 49.9 ± 10.6 | 59.4 ± 12.4 | < 0.0011 |
| Triglycerides (mg/dL) | 71.1 ± 25.2 | 62.7 ± 20.9 | 0.055 |
| LDL cholesterol classes2 | |||
| Normal | 44/70 (62.9) | 28/44 (63.6) | |
| Borderline | 22/70 (31.4) | 12/44 (27.3) | |
| Hypercholesterolemia | 4/70 (5.7) | 4/44 (9.1) | 0.742 |
| Glucose (mg/dL) | 83.4 ± 6.9 | 80.3 ± 8.8 | 0.04613 |
| Insulin μU/mL | 3.3 ± 2.7 | 7.5 ± 4.3 | < 0.00113 |
| HOMA index | 0.69 ± 0.6 | 1.55 ± 1.0 | 0.00113 |
| Insulin resistance | |||
| HOMA-IR < 3.16 | 69/70 (98.6) | 41/44 (93.2) | |
| HOMA-IR > 3.16 | 1/70 (1.4) | 3/44 (6.8) | 0.108 |
This cross-sectional study is the first to describe the profile of CVD risk factors in a cohort of children with CD in serologic remission on a GFD. Furthermore, this is the first report of insulin resistance in children with CD on a GFD.
Less than one third of our cohort did not have any CVD risk factors, while 14% had three or more risk factors. This finding suggests that CVD screening may be important in pediatric CD patients both at diagnosis and during follow-up. Studies have demonstrated that an earlier onset and greater number of CVD risk factors increase the chance of atheromatous plaque formation[10,11].
Our study design, which did not include a healthy control group, did not intend to determine whether children with CD have a higher risk than the general population for the development of CVD. Further prospective studies are needed to evaluate if changes in lifestyle and environment are responsible for a higher cardiovascular risk in celiac patients compared with the normal population. Nevertheless, although this study is limited by the lack of data prior to initiation of a GFD, it may suggest that the clinical and dietary follow-up should target adiposity, lipid profile and other CVD risk factors in addition to the common practice of dietary monitoring of adherence to a GFD.
The introduction of a GFD in CD patients increases the intestinal absorption of both macro and micro-nutrients. This leads to improved weight and height in celiac children presenting with malabsorption (weight loss, failure to thrive, poor weight gain)[20]. In our cohort, the majority of patients were of normal weight at the time of diagnosis and the percentage of overweight or obese patients was higher than those who were underweight. This drift in clinical presentation is concordant with previous reports[7] and may be attributed to increased awareness and early diagnosis. Alternatively, it may be explained by the radical change in diet and lifestyle in developed countries in recent decades, in line with the increasing prevalence of overweight and obesity in the general population. The increased prevalence of overweight and obesity after the introduction of a GFD in this study, although not significant (P = 0.10), may suggest the potential of a GFD to increase weight even in children presenting as normal or overweight at the time of CD diagnosis. The influence of a GFD on BMI remains unclear both in adults and children[9]. In adults, the debate is mainly based on two discordant theories. Dickey et al[21] demonstrated further weight gain in patients already overweight at the time of CD diagnosis after the introduction of a GFD, while Cheng et al[22] showed a positive effect of a GFD by demonstrating weight gain in previously underweight patients and weight loss in those previously overweight. Furthermore a recent study[23] recruiting a very large cohort of adult patients found that strict GFD adherence could increase the prevalence of overweight and obesity in CD patients. Contrasting studies have also recently appeared in the pediatric literature. Valletta et al[24] reported an increase in the fraction of overweight children following the introduction of a GFD, while Brambilla et al[25] demonstrated a beneficial effect of GFD on BMI in the majority of CD children. Reilly et al[26] found a beneficial effect of GFD on the BMI of overweight celiac children. Our data, demonstrating that a GFD increases the prevalence of overweight and obesity in children with CD, is in agreement with studies reporting increased weight as a potential adverse effect of GFD.
The data concerning LDL cholesterol after at least one year of a GFD suggests an important role for cholesterol as a CVD risk factor in our cohort. In this study, LDL cholesterol was the third most prevalent CVD risk factor in celiac children on a GFD.
The increase in total and HDL cholesterol after GFD introduction in comparison to levels prior to initiation of a GFD (available from a subset of patients), is concordant with some studies which theorized that derangement of intestinal absorption, chylomicron production and lipoprotein metabolism may underlie the finding of lower levels of total and HDL cholesterol in untreated CD, which can revert to normal after treatment[27-30]. In contrast, we found that the rate of borderline LDL cholesterol concentrations more than doubled (from 9.6% to 23.1%) following adherence to a GFD. This may be the result of a tendency in adult and adolescent patients to consume gluten-free products with high fat contents[31-33] in order to compensate for the withdrawal of common gluten-containing carbohydrates from the diet.
Our data seem to suggest that although the increase in the rate of borderline LDL cholesterol could raise the cardiovascular risk, the concomitant increase in HDL may be cardioprotective, and thus future studies looking at surrogate markers of atherosclerosis are needed to determine whether a GFD is harmful in this regard.
Four children (3.5%) on a GFD had insulin resistance. As far as we are aware, the only studies reporting HOMA-IR in CD were performed in patients with concomitant insulin-dependent diabetes mellitus (IDDM) 1[34]. It is not known whether insulin resistance was present on CD diagnosis. As such, this is the first description of the presence of insulin resistance in CD children.
Due to the lack of insulin levels before CD diagnosis, we were unable to assess whether such insulin resistance is directly related to the introduction of a GFD. Previous publications have reported that available gluten-free products (e.g., gluten-free bread, pasta, pizza etc.) have much higher glycemic indices than their gluten-containing equivalents, ingestion of which may lead to increased secretion of insulin[35-37]. Our findings, along with the previously mentioned change in the pattern of CD presentation, may suggest that future assessment of fasting glucose and insulin in children diagnosed with CD before and during the introduction of GFD should be performed. This is especially true in light of the role of insulin resistance as a CVD risk factor, and a predisposing condition for the development of type 2 diabetes[19]. The significantly higher fasting insulin levels and HOMA-IR in our Italian cohort may be explained by genetic and dietary differences between the two groups[37]. Our findings suggest that despite the classical consideration of CD as a malabsorptive condition, metabolic derangements, generally not attributed to this condition, should be actively sought even in patients who are non-obese. Although our data may hint to insulin resistance as a new complication of CD, a word of caution is due, as this study was performed on a cohort of CD patients and data is lacking in the literature regarding the prevalence of glucose intolerance in the healthy, non-overweight/obese children and adolescents.
This study has a number of limitations such as the relatively small number of patients, the cross-sectional design which did not allow for pre-GFD levels of all measured parameters, and the lack of familial history for CVD risk factors which may have further impacted our findings. However, despite these limitations, we have described the presence of insulin resistance in pediatric CD for the first time, and specifically addressed other CVD risk factors in the pediatric CD population on a GFD in serological remission.
Prior to initiation of the study, the relationship between CD and CVD risk factors was not clear, and therefore screening of lipid and glucose profiles was not routinely performed in patients with suspected CD. Additionally, the similarity in most findings between patients from two different countries, suggest that these findings are neither geographically nor ethnically specific. Prospective studies are needed to delineate the role of the GFD in the development of CVD risk factors in celiac children.
In conclusion, this cross-sectional study demonstrates a relatively high proportion of children with CD adherent to a GFD with one or more CVD risk factors including insulin resistance. These findings suggest the importance of screening for CVD risk factors in celiac children both at diagnosis and during follow-up. Furthermore, dietary counseling over time, targeting obesity and CVD risk factors in addition to monitoring adherence to a GFD in children and adolescents diagnosed with CD, may be warranted.
Celiac disease has traditionally been considered a malabsorptive disorder associated with diarrhea and weight loss, however, these symptoms are now seen less frequently. Several recent studies have reported that only a minority of newly diagnosed patients were underweight. Instead, many patients, both children and adults were overweight or even obese.
Several studies have demonstrated that cardiovascular disease (CVD) risk factors, namely obesity, abnormal blood lipid profile, hypertension and insulin resistance have their roots in childhood and tend to track into adulthood.
Deranged adiposity, blood lipid profile and other risk factors for CVD in celiac disease (CD) are still debated, and clear conclusions have yet to be reached. The authors’ study aimed to describe CVD risk factors in a population of celiac children adhering to a gluten-free diet (GFD) for at least one year, in two Mediterranean countries.
The authors’ results suggest the importance of screening for CVD risk factors in celiac children both at diagnosis and during follow-up. Furthermore, they highlight that dietary counseling over time should target obesity and CVD risk factors in addition to monitoring adherence to a GFD in children and adolescents diagnosed with CD.
The homeostatic model assessment is a method used to quantify insulin resistance and beta-cell function. This model correlated well with estimates using the euglycemic clamp method (r = 0.88).
The present study is an appreciable work in the sense that it explores a concept that was not given attention before. With changing lifestyle and environment are celiac children more exposed to cardiovascular risk factors than normal population it needs to be validated in further prospective studies. The results of study convince for metabolic screening in celiac disease children in follow up visits and hence initiate early intervention to prevent cardiovascular morbidity.
P- Reviewers Maggiore G, Reddy DN S- Editor Wen LL L- Editor Webster JR E- Editor Zhang DN
| 1. | Fasano A, Araya M, Bhatnagar S, Cameron D, Catassi C, Dirks M, Mearin ML, Ortigosa L, Phillips A. Federation of International Societies of Pediatric Gastroenterology, Hepatology, and Nutrition consensus report on celiac disease. J Pediatr Gastroenterol Nutr. 2008;47:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | NIH Consensus Development Conference on Celiac Disease. NIH Consens State Sci Statements. 2004;21:1-23. [PubMed] |
| 3. | Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1272] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 4. | Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology. 2005;128:S74-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Dickey W, Bodkin S. Prospective study of body mass index in patients with coeliac disease. BMJ. 1998;317:1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Tucker E, Rostami K, Prabhakaran S, Al Dulaimi D. Patients with coeliac disease are increasingly overweight or obese on presentation. J Gastrointestin Liver Dis. 2012;21:11-15. [PubMed] |
| 8. | Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1837] [Article Influence: 141.3] [Reference Citation Analysis (5)] |
| 9. | Norsa L, Shamir R, Zevit N. Gluten free-diet in celiac disease: protective or providing additive risk factors for the development of cardiovascular disease? Nutr Ther Metabol. 2012;30:1-9. |
| 10. | Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. JAMA. 1990;264:3018-3024. [PubMed] [DOI] [Full Text] |
| 11. | Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2610] [Cited by in RCA: 2526] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 12. | Guo SS, Huang C, Maynard LM, Demerath E, Towne B, Chumlea WC, Siervogel RM. Body mass index during childhood, adolescence and young adulthood in relation to adult overweight and adiposity: the Fels Longitudinal Study. Int J Obes Relat Metab Disord. 2000;24:1628-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 227] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC Growth Charts: United States. Advance data from vital and health statistics; no 314. Hyattsville, Maryland: National Center for Health Statistics 2000; Available from: http://www.cdc.gov/growthcharts. |
| 14. | National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4058] [Cited by in RCA: 3835] [Article Influence: 182.6] [Reference Citation Analysis (0)] |
| 15. | Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 780] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 16. | Marcovecchio ML, Mohn A, Chiarelli F. Obesity and insulin resistance in children. J Pediatr Gastroenterol Nutr. 2010;51 Suppl 3:S149-S150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115:e500-e503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 727] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 18. | Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics. 2004;114:e198-e205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 262] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Nguyen QM, Srinivasan SR, Xu JH, Chen W, Kieltyka L, Berenson GS. Utility of childhood glucose homeostasis variables in predicting adult diabetes and related cardiometabolic risk factors: the Bogalusa Heart Study. Diabetes Care. 2010;33:670-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | United European Gastroenterology. When is a coeliac a coeliac? Report of a working group of the United European Gastroenterology Week in Amsterdam, 2001. Eur J Gastroenterol Hepatol. 2001;13:1123-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Dickey W, Kearney N. Overweight in celiac disease: prevalence, clinical characteristics, and effect of a gluten-free diet. Am J Gastroenterol. 2006;101:2356-2359. [PubMed] |
| 22. | Cheng J, Brar PS, Lee AR, Green PH. Body mass index in celiac disease: beneficial effect of a gluten-free diet. J Clin Gastroenterol. 2010;44:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Kabbani TA, Goldberg A, Kelly CP, Pallav K, Tariq S, Peer A, Hansen J, Dennis M, Leffler DA. Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment Pharmacol Ther. 2012;35:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Valletta E, Fornaro M, Cipolli M, Conte S, Bissolo F, Danchielli C. Celiac disease and obesity: need for nutritional follow-up after diagnosis. Eur J Clin Nutr. 2010;64:1371-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Brambilla P, Picca M, Dilillo D, Meneghin F, Cravidi C, Tischer MC, Vivaldo T, Bedogni G, Zuccotti GV. Changes of body mass index in celiac children on a gluten-free diet. Nutr Metab Cardiovasc Dis. 2013;23:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Reilly NR, Aguilar K, Hassid BG, Cheng J, Defelice AR, Kazlow P, Bhagat G, Green PH. Celiac disease in normal-weight and overweight children: clinical features and growth outcomes following a gluten-free diet. J Pediatr Gastroenterol Nutr. 2011;53:528-531. [PubMed] |
| 27. | Rosenthal E, Hoffman R, Aviram M, Benderly A, Erde P, Brook JG. Serum lipoprotein profile in children with celiac disease. J Pediatr Gastroenterol Nutr. 1990;11:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Brar P, Kwon GY, Holleran S, Bai D, Tall AR, Ramakrishnan R, Green PH. Change in lipid profile in celiac disease: beneficial effect of gluten-free diet. Am J Med. 2006;119:786-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Capristo E, Malandrino N, Farnetti S, Mingrone G, Leggio L, Addolorato G, Gasbarrini G. Increased serum high-density lipoprotein-cholesterol concentration in celiac disease after gluten-free diet treatment correlates with body fat stores. J Clin Gastroenterol. 2009;43:946-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Lewis NR, Sanders DS, Logan RF, Fleming KM, Hubbard RB, West J. Cholesterol profile in people with newly diagnosed coeliac disease: a comparison with the general population and changes following treatment. Br J Nutr. 2009;102:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Mariani P, Viti MG, Montuori M, La Vecchia A, Cipolletta E, Calvani L, Bonamico M. The gluten-free diet: a nutritional risk factor for adolescents with celiac disease? J Pediatr Gastroenterol Nutr. 1998;27:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 165] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Bardella MT, Fredella C, Prampolini L, Molteni N, Giunta AM, Bianchi PA. Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten-free diet. Am J Clin Nutr. 2000;72:937-939. [PubMed] |
| 33. | Ferrara P, Cicala M, Tiberi E, Spadaccio C, Marcella L, Gatto A, Calzolari P, Castellucci G. High fat consumption in children with celiac disease. Acta Gastroenterol Belg. 2009;72:296-300. [PubMed] |
| 34. | Saadah OI, Zacharin M, O’Callaghan A, Oliver MR, Catto-Smith AG. Effect of gluten-free diet and adherence on growth and diabetic control in diabetics with coeliac disease. Arch Dis Child. 2004;89:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5-56. [PubMed] |
| 36. | Berti C, Riso P, Monti LD, Porrini M. In vitro starch digestibility and in vivo glucose response of gluten-free foods and their gluten counterparts. Eur J Nutr. 2004;43:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Zuccotti G, Fabiano V, Dilillo D, Picca M, Cravidi C, Brambilla P. Intakes of nutrients in Italian children with celiac disease and the role of commercially available gluten-free products. J Hum Nutr Diet. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |