Published online Sep 7, 2013. doi: 10.3748/wjg.v19.i33.5575
Revised: July 6, 2013
Accepted: July 30, 2013
Published online: September 7, 2013
Processing time: 131 Days and 22.8 Hours
All oral nucleoside analogues against hepatitis B virus, with an exception of telbivudine, have been reported causing lactic acidosis (LA). Here we report the first case of chronic hepatitis B developing severe refractory LA during telbivudine monotherapy. A 36-year-old man of Chinese origin received telbivudine antiviral treatment for chronic hepatitis B. After 11 mo of therapy, he developed anorexia, nausea, and vomiting with mild muscle weakness. The patient was found with elevated serum creatine phosphokinase up to 3683 U/L (upper limit of normal 170 U/L) and marked LA. LA did not resolve immediately following discontinuation of telbivudine. His condition began to improve after hemodialysis treatment for 16 times and usage of glucocorticosteroid. The patient fully recovered after 16 wk of treatment. This is the first documented case with severe LA caused by telbivudine monotherapy. Besides serum creatine phosphokinase, blood lactate level should also be closely monitored in patients receiving telbivudine.
Core tip: Myopathy is the most common side effect resulting from telbivudine. Lactic acidosis (LA) is rare but fatal, and LA caused by telbivudine has never been reported. Here, we report the first case of chronic hepatitis B developing severe refractory LA during telbivudine monotherapy. This case shows that telbivudine may cause muscle damage and even lead to fatal LA in chronic hepatitis B patients. Patients under telbivudine treatment should be closely monitored for muscular, blood lactate and other mitochondrial toxicity associated side effects.
- Citation: Jin JL, Hu P, Lu JH, Luo SS, Huang XY, Weng XH, Zhang JM. Lactic acidosis during telbivudine treatment for HBV: A case report and literature review. World J Gastroenterol 2013; 19(33): 5575-5580
- URL: https://www.wjgnet.com/1007-9327/full/v19/i33/5575.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i33.5575
Suppression of hepatitis B virus (HBV) DNA is a principal goal in treating chronic hepatitis B because this was shown to significantly improve liver histology as well as to decrease rates of hepatic complications and hepatocellular carcinoma. Current treatment options are pegylated interferon alpha and nucleoside analogues including lamivudine, telbivudine, entecavir, adefovir dipivoxil and tenofovir disoproxil. These agents have relatively fewer side effects than interferon alpha, and generally well tolerated[1]. Infrequent but serious adverse events have been reported in clinical trials and post-marketing surveillance in individual cases. Lactic acidosis (LA) is one of the severe adverse events and has been reported in the patients treated by all the other four nucleoside analogues except for telbivudine.
All of the five approved oral antiviral agents for HBV treatment can inhibit the polymerase activity of HBV, leading to a reduction in viral replication and serum HBV DNA levels. At the same time, some of these agents have a low level of activity against the human mitochondrial DNA (mtDNA) polymerase gamma and can lead to impaired mitochondrial replication with mitochondrial loss or dysfunction[1]. Clinical manifestations of mitochondrial toxicity vary based on the affected tissues, but may include myopathy, neuropathy, hepatic steatosis, pancreatitis, macrocytosis, nephrotoxicity, hyperlactatemia and LA. All nucleoside analogues have a “black box” warning regarding potential mitochondrial toxicity in their product labeling.
Telbivudine is a potent oral nucleoside analogue approved for the treatment of chronic hepatitis B in 2006 at a dose of 600 mg/d. A significantly higher incidence of grade 3-4 serum creatine phosphokinase (CPK) elevation (i.e., > 7 times upper limit of normal) was reported in a large, multinational registration clinical trial[2]. However, to date, there has been no published report of LA caused by telbivudine monotherapy. Here, we report a case of LA during telbivudine treatment, discuss the pathophysiology, clinical features and potential treatment of LA.
The patient is a 36-year-old, HIV-negative young male farmer. He was admitted to our hospital because of nausea and vomiting repeatedly for 40 d.
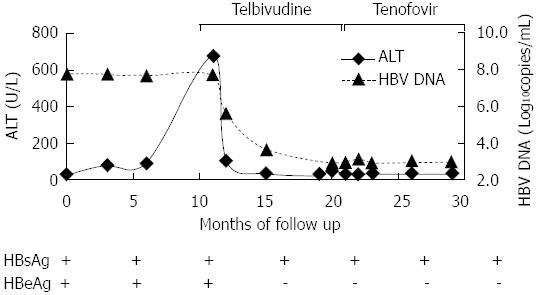
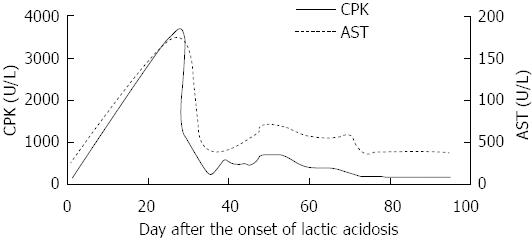
He had suffered from chronic hepatitis B for 13 years. His liver function test (LFT) revealed an intermittent elevation of alanine aminotransferase (ALT) levels between 1999 and 2011, and recovered to normal level after some symptomatic treatment. In September 2011, his LFT became abnormal again, the ALT was 704 U/L and HBV DNA was 7.0 × 107 copies/mL, HBV markers showed HBsAg, HBeAg and HBcAb were positive. Subsequently, he began to take telbivudine 600 mg/d regularly (Figure 1). In early September 2012 (47 d before admission), he began to develop anorexia, nausea and vomiting without apparent causes. There were no other concurrent symptoms, such as fever, headache, abdominal pain and altered level of consciousness. But he had mild muscle pain and weakness. The diagnostic workup including gastroscope, cranial CT and abdominal plain film revealed bilateral multiple renal calculi. CPK was significantly elevated at 3683 U/L (normal range: 25-170 U/L) 20 d before admission (Figure 2). The arterial blood gas analysis at that time showed pH 7.41, carbon dioxide partial pressure 37.2 mmHg, oxygen partial pressure 87.1 mmHg, actual bicarbonate 23.2 mmol/L, standard bicarbonate 23.6 mmol/L, base excess -1.4 mmol/L, and blood lactate level 4.4 mmol/L (upper limit of normal 2.5 mmol/L). It was considered that hyperlactatemia was caused by telbivudine at a local clinic. Subsequently telbivudine was discontinued.
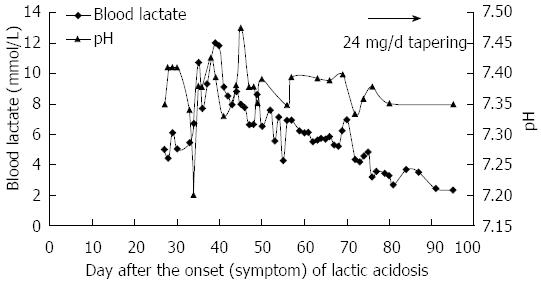
However, the patient’s condition continued to deteriorate despite alkalization treatment. Two weeks before admission, his CPK level decreased to 1183 U/L, but the arterial blood gas analysis demonstrated a worsening of metabolic acidosis: pH 7.2, actual bicarbonate 10.6 mmol/L, base excess 15.8 mmol/L, and blood lactate level elevated to 10.7 mmol/L (Figure 3). The clinical symptoms included persisting nausea and vomiting. The blood lactate level rose further to more than 12 mmol/L (the upper limit can be detected in the laboratory) (Figure 3). A week before admission, the patient received eight times of hemodialysis treatment at a local clinic. His blood lactate returned to a normal level each time after hemodialysis, however, it would rebound the next day. The patient was eventually transferred to our hospital because of refractory LA. On the day of admission, the blood lactate was 7.93 mmol/L, ALT was 42 U/L, aspartate aminotransferase was 66 U/L, LDH was 349 U/L and CPK was 632 U/L. Physical examination on admission revealed waddling gait and proximal muscular weakness in both lower limbs, quantitative value was 4 grade.
The patient was noticed to have a history of hypokalemic periodic paralysis for more than 10 years after a serious inquiry. His first attack was the most severe one, with paralysis affecting both of his legs but recovered after potassium supplement. There was no further event in the recent years.
The examination after admission also revealed hypothyroidism: TSH 12.39 mIU/L, T4 110.1 nmol/L, T3 1.31 nmol/L, and FT4 14.42 pmol/L. B-mode ultrasonography showed diffuse enlargement of thyroid. Endocrinologist consultation considered a subclinical hypothyroidism, and 25 μg euthyrox was prescribed daily.
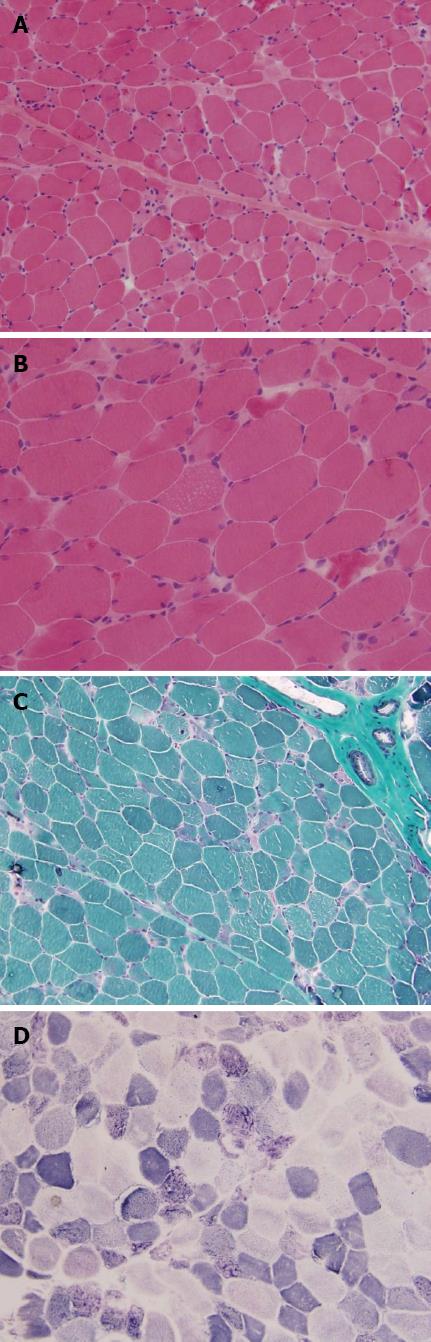
Electromyography revealed mild myopathic changes. Prolonged exercise test was normal. Muscle biopsy on left biceps revealed moderate variation in fiber size as well as increased muscle nucleus (Figure 4). A substantial number of degenerative muscle fibers occurred. Regeneration of muscle fiber could be seen, with no inflammatory cells infiltration. Mitochondrial damage was identified by modified Gomori trichrome stain and other histopathological studies. Modified Gomori trichrome staining revealed many ragged red fibers (RRF); reduced form of nicotinamide-adenine dinucleotid (NADH) and succinic dehydrogenase (SDH) staining showed disorganized enzyme activity in the fibers with RRF. ATP staining showed mosaic arrangement of type I and type II fibers. Oil Red O staining showed that several muscle fibers were filled with increased lipid droplets. Histo Immunochemical tests were Rod-Dystrophin (+), C-Dystrophin (+), N-Dystrophin (+), Dysferlin (+), Merosin (+), α-Sarcoglycan (+), β-Sarcoglycan (+), and γ-Sarcoglycan (+). The patient was diagnosed with LA (type B2), HBeAg negative chronic hepatitis B and drug-induced myopathy.
He was given hemodialysis for more than eight times after admission. The blood lactate level reduced to normal range (less than 2.5 mmol/L) after hemodialysis but slightly elevated the following day. The symptoms of nausea and vomiting completely recovered, so the hemodialysis was discontinued. He was given hydratation, alkalization and supplementation with Coenzyme Q 10 and Levocarnitine. Two weeks after hemodialysis, the blood lactate level still fluctuated between 5 and 7 mmol/L. As a result, methylprednisolone tablets (24 mg/d) was given. Meanwhile, HBV DNA was rechecked and showed a slight rebound at 1.59 × 103 copies/mL, consequently tenofovir (300 mg/d) was given to suppress the HBV.
In the following two weeks, his blood lactate level returned to a normal range, and the HBV DNA was undetectable (less than 1000 copies/mL), so methylprednisolone was tapered off within a ten-week period. The patient has remained very well and followed up regularly to date.
Our patient had marked LA without evidence of infection or organ hypoperfusion. It is very likely that his acidosis was secondary to the nucleoside analogue, telbivudine, during treatment of HBV.
In basic terms, lactic acid is the normal endpoint of the anaerobic breakdown of glucose in the tissues. In the setting of decreased tissue oxygenation, lactic acid is produced as the anaerobic cycle is utilized for energy production. The normal blood lactate concentration in unstressed patients is 0.5-1 mmol/L. Lactate concentration of less than 2 mmol/L can be considered to be normal in patients with critical illness. Hyperlactatemia is defined as a persistent, mild to moderate (2-4 mmol/L) increase in blood lactate concentration without metabolic acidosis; whereas LA is characterized by constant increased in blood lactate levels (usually > 5 mmol/L) in association with metabolic acidosis (usually present as pH < 7.3 and serum bicarbonate < 10 mmol/L)[1].
The LA syndrome linked to nucleoside analogue is associated with steatosis, abnormal mitochondrial appearance and function, pancreatitis, neuropathy, and myopathy. The onset may be abrupt or insidious, it generally begins with nausea, vomiting, and abdominal pain. It will progress to tachypnea, shortness of breath, and hypoxia. Patients with severe LA may subsequently develop renal failure, liver failure, coagulopathy, seizures, arrhythmias, and even death. The patient reported here was a severe LA case with a lactate level of more than 12 mmol/L and pH value of 7.2. His blood lactate level did not recover to normal even after hemodialysis treatment for 16 times.
The Food and Drug Administration approved oral nucleoside analogues for HBV treatment, including lamivudine, adefovir, telbivudine, entecavir, and tenofovir, are well tolerated. However, these still carry the “black box” warning for the potential development of mitochondrial damage with resultant LA based on the data from the human immunodeficiency virus (HIV) treatment literature[3-7] and the experience using fialuridine (FIAU) in HBV treatment[8].
Lamivudine[4,5] and tenofovir[3,7] associated LA was reported only in HIV patients treated with combination regimens (Table 1), while their mitochondrial toxicity is far less than those antiretroviral nucleoside analogues. The risk of LA with entecavir treatment in chronic hepatitis B patients remain controversial. However, it was reported to occur more often in patients with impaired liver function[1,9,10], especially in those with high MELD (model for end stage liver diseases) scores and multi-organ failure (Table 1). Report of LA caused by adefovir is rare, and all reported cases were present in a combination regimens[9].
| Patient ID | Age (yr) | Liver condition | Underlying disease | Child-Pugh | MELD score | Drug | LA therapy | Peak lactate (mmol/L) | Nadir pH | BE (mmol/L) | Peak CPK (U/L) | Prognosis | Ref. |
| 1 | 35 | CHB | HOKPP | A | 7 | LDT | 11 mo | > 12 | 7.2 | -15.8 | 3683 | Resolved | This paper |
| 2 | 36 | OLT, ITBL | - | C | 38 | ETV | 9 mo | 5.20 | 7.2 | -18 | Normal | Resolved | [7] |
| 3 | 79 | ALF | - | - | 29 | ETV | 6 d | 20.82 | 7.1 | -17 | Normal | Death | [7] |
| 4 | 60 | OLT, re-cirrhosis | - | C | 28 | ETV | 1 mo | 3.86 | 7.4 | -5 | Normal | Resolved | [7] |
| 5 | 60 | Cirrhosis HCC | - | B | 25 | ETV | 10 d | 6.77 | 7.3 | -12 | Normal | Resolved | [7] |
| 6 | 61 | Cirrhosis HCC | - | B | 22 | ETV | 4 d | 2.70 | 7.4 | -6 | Normal | Resolved | [7] |
| 7 | 63 | CHB, HCC | massive bilobar pneumonia | C | 30 | ETV | 10 d | 9.20 | 7.24 | - | Normal | Resolved | [8] |
| 8 | 54 | CHB, cirrhosis | CML | C | 24 | ETV + ADV | 10 d | 9.50 | 6.95 | - | Normal | Resolved | [9] |
| 9 | 42 | HIV | - | A | 7 | HARRT (stavudine + LAM) | 9 mo | 5.48 | 7.15 | - | Normal | Resolved | [6] |
| 10 | 51 | HIV | DM | A | 7 | HARRT (tenofovir) | 12 mo | 6.40 | 7.21 | - | Normal | Resolved | [7] |
Telbivudine, as with all the other approved nucleoside analoges, has a potential of mitochondrial toxicity which will lead to LA in theory. However, no single case has been reported to date. This will be the first documented case of type B LA in a chronic hepatitis B patient who received telbivudine monotherapy.
Among the five nucleoside analogues approved for the use in hepatitis B, the inhibitory strength of mtDNA polymerase gamma in an in vitro test system is actually far less than that seen in antiretroviral agents. In the registration trial of telbivudine for HBV, the side-effect profile of telbivudine was generally favorable[2] and similar to comparator arm of lamivudine throughout 2 years of treatment. There was no LA case reported, however, a significantly higher incidence of grade 3 to 4 serum CPK elevations (i.e., 7 times upper limit of normal) was noted in telbivudine-treated compared to lamivudine-treated patients at 2 years (12.9% vs 4.1%).
We noticed that our patient had a history of hypokalemic periodic paralysis. Hypokalemic periodic paralysis is an autosomal-dominant disorder characterized by episodic attacks of muscle weakness with hypokalemia. Whether there was pre-existence of myopathy in our patient prior to telbivudine treatment is uncertain, only transient CPK elevation was observed and most of time the CPK value was normal before LA occurred. The reason that LA and CPK elevation does not co-exist in most cases during monotherapy of nucleoside analogues in chronic hepatitis B patients is unclear. Interestingly, our case is a rare incident where CPK elevation and LA occurred simultaneously (Table 1). This case has suggested that besides CPK, serum lactate level should also be monitored closely during the treatment of telbivudine.
LA can be divided into 2 categories, type A and type B. Type A is LA occurring in association with clinical evidence of poor tissue perfusion or oxygenation of blood (e.g., hypotension, cyanosis, cool and mottled extremities). Type B is LA occurring when no clinical evidence of poor tissue perfusion or oxygenation exists. Type B can be divided into 3 subtypes based on underlying etiology. Type B1 occurs in relation to systemic disease, such as renal and hepatic failure, diabetes and malignancy. Type B3 is due to inborn errors of metabolism. Type B2 is caused by several classes of drugs and toxins, including biguanides, alcohols, iron, isoniazid, zidovudine, and salicylates.
Our patient had marked LA without evidence of infection or organ hypoperfusion. In view of the fact that no other underlying causes were identified, his acidosis may be due to telbivudine (Type B2 LA). The patient also had mild muscle pain and proximal muscle weakness consistent with a myopathy, as shown on the electromyography. It is likely LA and myopathy arise from the same pathological origin, i.e., mitochondrial dysfunction. Indeed, subsequent muscle biopsy showed RRF, lipid storage and mitochondrial dysfunction, which indicated the mitochondrial toxicity.
Management options for type B LA may include treatment for primary diseases, renal replacement therapy, bicarbonate alkalization and supplementation with thiamine, L-acetylcarnitine as well as Coenzyme Q 10[10]. In term of nucleoside analogues, discontinuation should be instantaneously. Most of the LA cases can resolve rapidly after discontinuation of the causative drug. Majority of the patients who developed LA secondary to nucleoside analogues had a good outcome. The recovery progression for our patient was slow with a total period of more than three months. The symptoms improved after hemodialysis therapy for 16 times, and blood lactate level normalized to the upper limit of normal, but halted for a period of time. No plausible reasons can be found for this phenomenon, but small dosage of glucocorticoid seems to be effective. The use of low-dose glucocorticoid for a short period of time may have an unusual effect. However, a larger controlled clinical trial is required for further clarification. It should be applied cautiously by an experienced clinical hepatologist.
This case shows that telbivudine may cause muscle damage and even lead to fatal LA in telbivudine-treated chronic hepatitis B patients. Thus patients receiving telbivudine should be closely monitored for muscular abnormalities, blood lactate level and other mitochondrial toxicity associated side effects.
P- Reviewer Vega J S- Editor Zhai HH L- Editor Ma JY E- Editor Ma S
| 1. | Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology. 2009;49:S185-S195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 2. | Chan HL, Chen YC, Gane EJ, Sarin SK, Suh DJ, Piratvisuth T, Prabhakar B, Hwang SG, Choudhuri G, Safadi R. Randomized clinical trial: efficacy and safety of telbivudine and lamivudine in treatment-naïve patients with HBV-related decompensated cirrhosis. J Viral Hepat. 2012;19:732-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Latzin P, Frey U, Roiha HL, Baldwin DN, Regamey N, Strippoli MP, Zwahlen M, Kuehni CE. Prospectively assessed incidence, severity, and determinants of respiratory symptoms in the first year of life. Pediatr Pulmonol. 2007;42:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Osler M, Stead D, Rebe K, Meintjes G, Boulle A. Risk factors for and clinical characteristics of severe hyperlactataemia in patients receiving antiretroviral therapy: a case-control study. HIV Med. 2010;11:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | ter Hofstede HJ, de Marie S, Foudraine NA, Danner SA, Brinkman K. Clinical features and risk factors of lactic acidosis following long-term antiretroviral therapy: 4 fatal cases. Int J STD AIDS. 2000;11:611-616. [PubMed] |
| 6. | Nelson M, Azwa A, Sokwala A, Harania RS, Stebbing J. Fanconi syndrome and lactic acidosis associated with stavudine and lamivudine therapy. AIDS. 2008;22:1374-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Patel V, Hedayati SS. Lactic acidosis in an HIV-infected patient receiving highly active antiretroviral therapy. Nat Clin Pract Nephrol. 2006;2:109-114; quiz 115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | McKenzie R, Fried MW, Sallie R, Conjeevaram H, Di Bisceglie AM, Park Y, Savarese B, Kleiner D, Tsokos M, Luciano C. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med. 1995;333:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 413] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Cohen SM, Levy RM, Jovanovich JF, Ahn J. Fatal lactic acidosis associated with the use of combination oral medications to treat reactivation of hepatitis B. J Clin Gastroenterol. 2009;43:1008-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Marzano A, Marengo A, Marietti M, Rizzetto M. Lactic acidosis during Entecavir treatment in decompensated hepatitis B virus-related cirrhosis. Dig Liver Dis. 2011;43:1027-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |