Published online Sep 7, 2013. doi: 10.3748/wjg.v19.i33.5534
Revised: June 3, 2013
Accepted: July 18, 2013
Published online: September 7, 2013
Processing time: 186 Days and 16.5 Hours
AIM: To propose an appropriate staging system for hepatocellular carcinoma (HCC) classification.
METHODS: Here, 288 in-patients with HCC were studied and divided into three groups: those with expansive growth, invasive growth (including satellite nodules, nodule fusions and direct tumor invasion of adjacent organs), or disseminative growth (including vascular involvement, regional lymph node metastasis and distant metastasis). A survival analysis was performed using a Kaplan-Meier analysis, and prognostic factors for overall survival were determined by the Cox proportional hazards regression model.
RESULTS: The overall survival (OS) of patients with invasive tumor growth was shorter than that of patients with expansive tumor growth (27.796 ± 3.730 and 57.398 ± 4.873 mo, respectively, P < 0.001). No significant difference in survival was observed between patients with vascular involvement and patients with regional lymph node metastasis (21.667 ± 4.773 and 14.619 ± 2.456 mo, respectively, P = 0.801). The OS of patients with distant metastasis (6.417 ± 1.395 mo) was shorter than that of the other groups (P < 0.001). No significant difference in survival was observed between patients with expansive tumor growth and vascular and/or regional lymph node involvement and patients with invasive tumor growth and no vascular and/or lymph node involvement (25.762 ± 7.024, 21.200 ± 7.794 and 39.533 ± 5.840 mo, respectively; P = 0.871, 0.307 and 0.563, respectively).
CONCLUSION: These data led to the proposal of a new staging system: the Expansive-Invasive-Disseminative growth staging classification.
Core tip: A number of staging systems were designed for all of hepatocellular carcinoma (HCC) patients based on some character of tumor, such as tumor size, vascular invasion, regional lymph node metastasis and extrahepatic spread. But those systems failed to adequately stratify HCC patients with respect to prognosis. In our study, we explore an appropriate staging system for resectable patients with HCC based on tumor’s growth characteristics, the Expansive-Invasive-Disseminative growth staging classification, which is a simple and efficacious prognostic model for postoperative patients with HCC.
- Citation: Zhu CH, Liu XH, Cao R, Wu XZ. Proposal of new classification for postoperative patients with hepatocellular carcinoma based on tumor growth characteristics. World J Gastroenterol 2013; 19(33): 5534-5541
- URL: https://www.wjgnet.com/1007-9327/full/v19/i33/5534.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i33.5534
A staging system has been widely used for malignant diseases to stratify patients into comparable groups to predict patients’ long-term outcomes. The American Joint Committee on Cancer (AJCC) uses the tumor-node-metastasis (TNM) system as a staging system for many malignant diseases to predict prognosis. Nevertheless, the TNM system fails to adequately stratify hepatocellular carcinoma (HCC) patients with respect to prognosis. In fact, prognosis of patients with cirrhosis and HCC depends on both residual liver function and tumor characteristics. Staging systems that include liver function status were first proposed by Okuda et al[1]. In 1998, investigators from the Cancer of the Liver Italian Program (CLIP) proposed the CLIP score that is based on Child-Pugh stage, tumor morphology and extension, alpha-fetoprotein (AFP) level, and portal vein thrombosis[2,3]. Although the CLIP score has good prognostic value in HCC patients, this score has some limitations when applied to patients with resectable HCC[4]. The Chinese University Prognostic Index for HCC was identified in 2002[5]. It combines the conventional TNM system with liver function and AFP. In 2003, the Japan Integrated Staging score was proposed by Kudo et al[4]. It is based on new adapted TNM system and Child-Pugh grading.
Some of the tumor characteristics of HCC include the tumor size, tumor number, invasive growth, vascular invasion, regional lymph node metastasis, and extrahepatic spread[6-17]. The TNM staging system is based on tumor characteristics, such as tumor size, vascular invasion, regional lymph node metastasis and extrahepatic spread. In 1999, the Barcelona Clinic Liver Cancer (BCLC) staging classification for HCC was proposed by Llovet et al[18] based on certain tumor characteristics. Cammà et al[19] reported that the overall predictive ability of BCLC, CLIP and French classification staging systems was unsatisfactory for patients with both cirrhosis and HCC and did not have uniform predictive results for treated patients and untreated patients. None of the scoring systems provided confident prediction of survival in individual patients. However, because the liver function of the most resectable patients is either an A or B score by Child-Pugh analysis, the TNM staging system provides an effective means of assessing the prognosis of patients following curative resection of HCC[11]. Unfortunately, the TNM system fails to include comprehensive characteristics of the tumor, especially the tumor’s growth pattern. Thus, it is crucial to design a system to evaluate the effects of tumor characteristics on the clinical outcome of resectable patients with HCC.
In this study, 288 postoperative patients with HCC were studied and followed until August 2012. Patients with a C score from the Child-Pugh analysis were excluded to eliminate the effect of poor liver function on the long-term outcome. The purpose of the study was to explore an appropriate staging system for resectable patients with HCC based on the patient’s tumor growth characteristics.
Two hundred and eighty-eight in-patients who were diagnosed with HCC and underwent curative resection of HCC at Tianjin Medical University Cancer Institute and Hospital from March 1999 to July 2007 were included in this study and were followed until August 2012. Pathological testing for all patients was performed to confirm HCC. Contrast-enhanced computed tomography (CT), magnetic resonance imaging or positron emission tomography-CT was performed to confirm patients without metastatic disease. The patients’ medical records were reviewed, and demographic, clinical and histological variables were derived from the medical records. The pT and pN status were identified based on the 7th edition of AJCC TNM classification. This study was approved by our institutional research review board.
Overall survival (OS) curves were plotted by the Kaplan-Meier method and compared using the Log-rank test. The prognostic factors which showed the potential associations with OS were analyzed using a univariate analysis. They Cox proportional hazard model was used to find independent characteristic factors for survival time for the multivariate analysis from the univariate analyses. Statistical calculations were performed using SPSS (Version: 16.0, Chicago, United States).
All of the patients with HCC had an A and B score by Child-Pugh analysis. The patients and tumor characteristics are summarized in Table 1. The median age of patients in this study was 54 years. In total, 119 patients had stage I, 22 patients had stage II, 24 patients had stage IIIA, 25 patients had stage IIIB, 66 patients had stage IIIC, and 21 patients had stage IVA HCC. Although most patients with stage IVB HCC were excluded from tumor resection, 11 patients at Tianjin Medical University Cancer Institute and Hospital in stage IVB had a resected primary tumor from March 1999 to July 2007. The OS and median survival time were 45.704 ± 3.380 and 20.000 ± 2.314 mo, respectively, for postoperative patients with HCC. Tumor size, tumor status, regional lymph node metastasis, distant metastasis, Child-Pugh score, AFP and tumor growth pattern were the factors affecting survival (Table 2).
| Characteristics | n = 288 |
| Age (yr) | |
| ≤ 60 | 208 (72.2) |
| > 60 | 80 (27.8) |
| Gender | |
| Female | 49 (17.0) |
| Male | 239 (83.0) |
| HBV infection | 211 (75.36) |
| TNM stage | |
| I | 119 (41.3) |
| II | 22 (7.6) |
| IIIA | 24 (8.3) |
| IIIB | 25 (8.7) |
| IIIC | 66 (22.9) |
| IVA | 21 (7.3) |
| IVB | 11 (3.8) |
| Tumor size (cm) | |
| ≤ 5 | 105 (36.5) |
| 5 < size ≤ 10 | 125 (43.4) |
| > 10 | 58 (20.1) |
| Child-Pugh score | |
| A | 260 (90.3) |
| B | 28 (9.7) |
| AFP (ng/mL) | |
| ≤ 200 | 161 (57.7) |
| > 200 | 118 (42.3) |
| Death/all cases | Median survival time (95%CI) | P value | |
| Age (yr) | 0.612 | ||
| ≤ 60 | 165/208 | 19 (13.470-24.530) | |
| > 60 | 63/80 | 23 (15.988-30.012) | |
| Gender | 0.605 | ||
| Male | 36/49 | 20 (6.283-33.717) | |
| Female | 193/239 | 20 (15.131-24.869) | |
| Tumor size (cm) | < 0.001 | ||
| ≤ 5 | 67/105 | 34 (17.568-50.432) | |
| 5 < size ≤ 10 | 107/125 | 17 (8.965-25.035) | |
| > 10 | 55/58 | 8 (4.890-11.110) | |
| Tumor status | < 0.001 | ||
| T1 | 90/126 | 33 (24.933-41.067) | |
| T2 | 14/22 | 25 (0.000-73.264) | |
| T3a | 26/31 | 15 (5.184-24.816) | |
| T3b | 24/27 | 8 (5.470-10.530) | |
| T4 | 75/82 | 12 (7.905-16.095) | |
| Regional lymph node metastasis | < 0.001 | ||
| No | 205/264 | 22 (16.882-27.118) | |
| Yes | 24/24 | 8 (3.999-12.001) | |
| Distant metastasis | < 0.001 | ||
| No | 217/276 | 22 (17.691-26.309) | |
| Yes | 12/12 | 4 (2.303-5.697) | |
| Child-Pugh score | < 0.001 | ||
| A | 203/260 | 23 (18.485-27.515) | |
| B | 26/28 | 7 (2.851-11.149) | |
| AFP (ng/mL) | < 0.001 | ||
| ≤ 200 | 118/161 | 28 (21.162-34.838) | |
| > 200 | 102/118 | 12 (8.198-15.802) | |
| Expansive growth and invasive growth | < 0.001 | ||
| Single tumor | 103/143 | 33 (24.408-41.592) | |
| Multiple tumors | 18/25 | 25 (8.680-41.320) | |
| Satellite nodules (including perforation tumor encapsulation) | 18/22 | 13 (10.242-15.758) | |
| Nodules fusion (including diffuse growth which lack tumor encapsulation) | 16/18 | 12 (3.684-20.316) | |
| Direct invasion of adjacent organs other than the gallbladder or with perforation of visceral peritoneum | 74/80 | 12 (7.956-16.044) | |
| Disseminative growth | < 0.001 | ||
| Without vascular or regional lymph node involvement | 155/210 | 28 (21.928-34.072) | |
| Vascular involvement | 42/46 | 8 (4.979-11.021) | |
| Regional lymph node involvement | 21/21 | 10 (4.019-15.981) | |
| Distant metastasis | 12/12 | 4 (2.303-5.697) | |
| EID stage | < 0.001 | ||
| I | 96/140 | 36 (27.626-44.374) | |
| II | 84/99 | 16 (11.450-20.550) | |
| III | 38/38 | 8 (4.375-11.625) | |
| IV | 11/11 | 5 (3.436-6.564) | |
| TNM stage | < 0.001 | ||
| I | 84/119 | 35 (26.448-43.552) | |
| II | 14/22 | 25 (0.000-73.264) | |
| IIIA | 19/24 | 16 (2.557-29.443) | |
| IIIB | 22/25 | 8 (1.880-14.120) | |
| IIIC | 58/66 | 15 (10.033-19.967) | |
| IVA | 21/21 | 10 (4.019-15.981) | |
| IVB | 11/11 | 5 (3.436-6.564) |
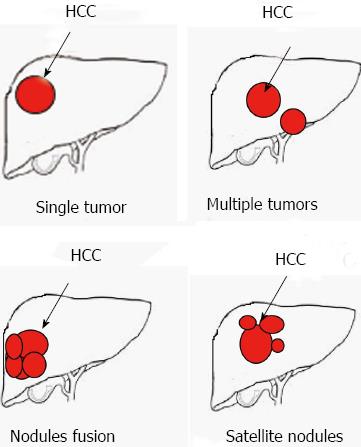
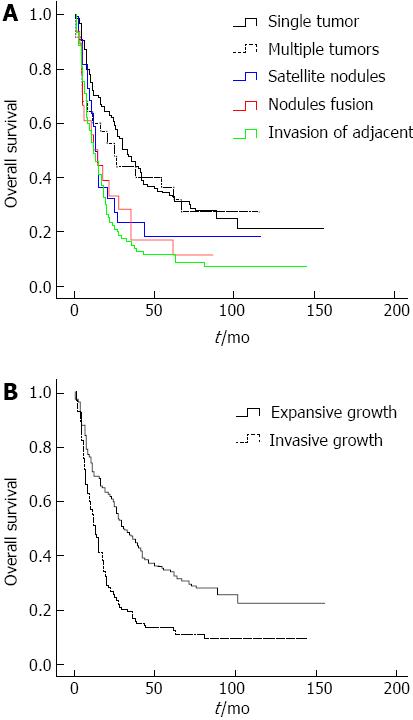
Patients were divided into five groups based on tumor number and invasive growth characteristics as follows: single tumor, multiple tumors, satellite nodules (including perforation the tumor encapsulation), nodule fusion (including diffuse growth lack tumor encapsulation) and invasion of adjacent organs (tumor with direct invasion of adjacent organs other than the gallbladder or with perforation of visceral peritoneum) (Figure 1). No significant difference in survival was observed between patients with a single tumor and patients with multiple tumors (Figure 2A). Moreover, no significant difference in survival was observed among patients with satellite nodules, patients with nodule fusion or patients with tumor invasion of adjacent organs (Figure 2A). Based on the data, patients were divided into two groups: expansive tumor growth (single tumor and multiple tumors) and invasive tumor growth (satellite nodules, nodules fusion, and tumor with direct invasion of adjacent organs). The OS of patients with invasive tumor growth was shorter than that of patients with expansive tumor growth (P < 0.001, Figure 2B). The OS was 57.398 ± 4.873 mo for patients with expansive tumor growth, while it was 27.796 ± 3.730 mo for patients with invasive tumor growth. The median survival time of patients with expansive tumor growth and patients with invasive tumor growth were 30 and 13 mo, respectively.
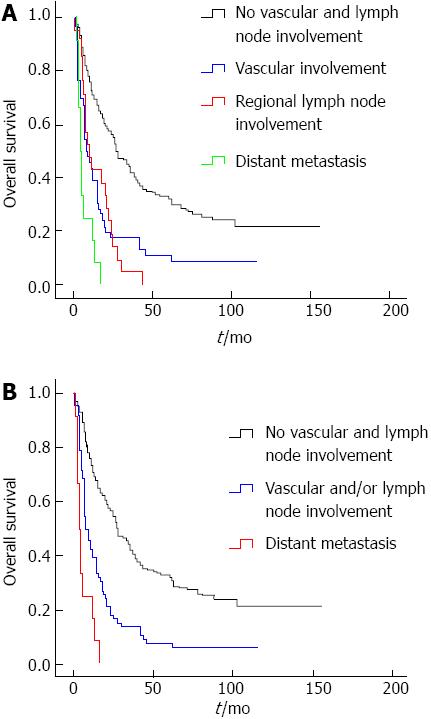
Based on the extrahepatic metastatic tendency of tumor, the patients were divided to four groups: tumor without vascular and regional lymph node involvement; tumor with vascular involvement; regional lymph node metastasis; and distant metastasis. No significant differences in survival were observed between patients with vascular involvement of the tumor and patients with regional lymph node metastasis (Figure 3A). The OS of patients who lacked vascular and regional lymph node involvement was better than other groups, while the OS of patients with distant metastasis was shorter than those of other groups (Figure 3A). The OS of patients who lacked vascular and regional lymph node involvement was 55.532 ± 4.237 mo, while the OS of patients with distant metastasis was 6.417 ± 1.395 mo. The OS of patients with vascular and regional lymph node tumor involvement was 21.667 ± 4.773 and 14.619 ± 2.456 mo, respectively. The median survival times for each group were 28, 8, 10 and 4 mo, respectively. Based on these data, the patients were divided into three groups: tumor without vascular and regional lymph node involvement; tumor with vascular and/or regional lymph node involvement; and distant metastasis (Figure 3B).
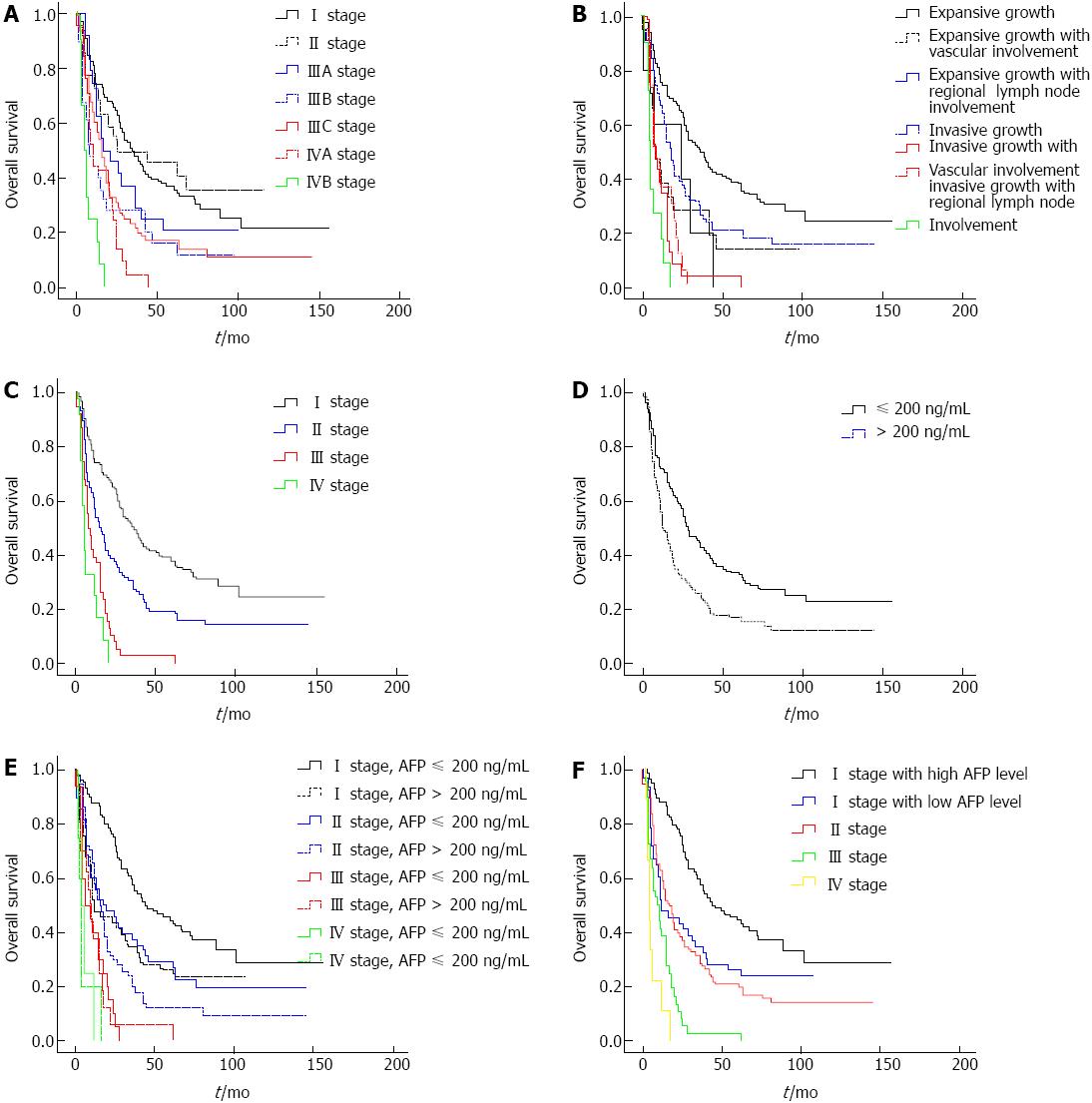
According to the 7th edition TNM classification, no significant difference in survival was observed between the groups with stage I or II HCC (Figure 4A). The OS of patients with stage I was 59.460 ± 5.806 and 55.585 ± 10.289 mo for patients with stage II. Moreover, no significant differences in survival were observed among the different stage III groups (Figure 4A). The OS of patients with stage IIIA, IIIB and IIIC were 35.917 ± 7.138, 24.760 ± 6.250 and 31.996 ± 5.457 mo, respectively.
Patients were divided to seven groups based on the growth characteristics of the tumor: expansive growth, expansive growth with vascular involvement, expansive growth with regional lymph node involvement, invasive growth, invasive growth with vascular involvement, invasive growth with regional lymph node involvement, and distant metastasis. The OS of each group was 62.632 ± 5.415, 25.762 ± 7.024, 21.200 ± 7.794, 39.533 ± 5.840, 11.478 ± 2.635, 12.653 ± 2.076 and 6.727 ± 1.490 mo, respectively. There were significant differences in survival among patients with expansive tumor growth, invasive tumor growth and disseminative tumor growth. No significant difference in survival was observed between patients with expansive tumor growth along with vascular and/or regional lymph node involvement and patients with invasive tumor growth who lacked vascular and regional lymph node involvement (Figure 4B).
These data enable the proposal of a new staging system: the Expansive-Invasive-Disseminative growth (EID) staging classification, which comprises four stages that select the best candidates for the best therapies currently available. Stage I includes patients with expansive tumor growth. Stage II has two subgroups: the first group consists of patients with expansive tumor growth along with vascular and/or regional lymph node involvement, and the second group includes patients with invasive tumor growth. Stage III includes patients with invasive tumor growth along with vascular/regional lymph node involvement. Stage IV includes patients with distant metastasis (Figure 4C). The OS values of patients at each stage were 62.637 ± 5.453, 36.880 ± 7.779, 12.053 ± 1.796 and 6.727 ± 1.490 mo, respectively. The overall median survival times of patients at each stage were 36, 16, 8 and 5 mo, respectively.
Table 2 showed the univariate analysis of the factors measured association with survival. The tumor characteristics that were statistically significant were tumor size, AFP, Child Pugh score, growth pattern, TNM stage and EID stage. Multivariate analyses identified EID stage and AFP as independent factors associated with OS (P < 0.001, P = 0.008, respectively). There was no significant difference between the TNM stage and Child Pugh score. The OS of patients with high levels of AFP (AFP > 200 ng/mL; 56.229 ± 4.849 mo) was shorter than that of patients with low levels of AFP (33.208 ± 4.212 mo; P < 0.001, Figure 4D). Moreover, the OS of stage I patients with high levels of AFP was shorter than that of patients with low levels of AFP (72.240 ± 6.793 mo, 37.804 ± 6.054 mo, respectively, P < 0.001). No significant difference in survival was observed between I stage patients with high levels of AFP and II stage patients. Dramatically, for stage II, III and IV patients, no significant difference in survival was observed between patients with high levels of AFP and patients with low levels of AFP (Figure 4E). Thus, AFP levels only affect the OS of stage I patients (Figure 4F).
There are four main factors affecting the prognosis of HCC: (1) the stage, aggressiveness and growth rate of the tumor; (2) the general health of the patient; (3) the liver function of the patient; and (4) the specific intervention[20]. A number of staging systems have been devised for patients with HCC. Each staging system includes variables which evaluate one or more of the first 3 factors listed above. For example, the TNM staging system evaluates only the tumor characteristics, whereas the Child Pugh score provides information regarding liver function.
Some of the characteristics of HCC tumors include the tumor size, tumor number, aggressiveness of growth, vascular involvement, regional lymph node metastasis, and extrahepatic spread. Although the TNM and BCLC staging classification for HCC were proposed based on certain tumor characteristics, no staging system systematically evaluates the effect of tumor growth patterns on the clinical outcome of patients with HCC.
In this study, 288 postoperative patients with HCC were studied, and the tumor growth patterns were divided into three types: (1) expansive growth (single tumor and multiple tumors without invasive and disseminative growth); (2) invasive growth (satellite nodules including perforation of the tumor encapsulation, nodule fusion including diffuse growth that lacks tumor encapsulation, and tumors with direct invasion of adjacent organs); and (3) disseminative growth (vascular involvement, regional lymph node metastasis and distant metastasis). Cheng et al[6] reported that the lack of tumor encapsulation was an independent factor for HCC. Other research showed that the presence of satellite nodules was an independent factor for the long-term survival of patients with HCC after curative resection[9,21]. The OS of patients with invasive tumor growth was shorter than that of patients with expansive tumor growth. No significant difference in survival was observed between patients with vascular involvement and patients with regional lymph node metastasis. The OS of patients with expansive tumor growth was longer than those of other groups, while the OS of patients with distant metastasis was shorter than those of other groups. No significant differences in survival were observed between patients with expansive tumor growth with vascular and/or regional lymph node involvement and patients with invasive tumor growth that lacked vascular and regional lymph node involvement.
These data enable the proposal of a new staging system to select the best candidates for the best therapies currently available: the four-stage EID staging classification (Table 3). Stage I includes patients with expansive tumor growths. Stage II has two subgroups: the first group contains patients with expansive tumor growth along with vascular and/or regional lymph node involvement, and the second group consists of patients with invasive tumor growth. Stage III includes patients with invasive tumor growth along with vascular/regional lymph node involvement. Stage IV comprises patients with distant tumor metastasis. The OS values of each stage were 62.637 ± 5.453, 36.880 ± 7.779, 12.053 ± 1.796 and 6.727 ± 1.490 mo, respectively. The median survival times of patients at each stage were 36, 16, 8 and 5 mo, respectively.
| Phase | Growth characteristics |
| I | Expansive tumor growth (single tumor and multiple tumors) |
| II | Expansive tumor growth along with vascular and/or regional lymph node involvement |
| Invasive tumor growth (satellite nodules, nodule fusion and tumor direct invasion of adjacent organs) | |
| III | Invasive tumor growth along with vascular/regional lymph node involvement |
| IV | Distant metastasis of tumor |
Univariate analysis showed that the statistically significant factors were EID staging classification and AFP (Table 4). There were no statistically significant correlative differences between OS and the Child-Pugh score. There is a substantial amount of research showing that the Child-Pugh score and AFP values > 200 ng/mL are independent factors for HCC[6-8,15,22]. In fact, patients with a C Child-Pugh score were excluded for this study to eliminate the effects of poor liver function on long-term outcome. The OS of stage I patients with high levels of AFP was shorter than that of patients with low levels of AFP. There is research showing that the Japan Integrated Staging Score and BCLC staging system combined with AFP levels may serve as a better staging system for early-stage HCC patients[22,23]. Dramatically, for patients in stages II, III and IV, no significant difference in survival was observed between patients with high levels of AFP and patients with low levels of AFP. Thus, AFP levels likely only affect the OS of stage I patients.
| Characteristics | I | II | III | IV |
| Age (yr) | ||||
| ≤ 60 | 96 (68.6) | 78 (78.8) | 26 (68.4) | 8 (72.9) |
| > 60 | 44 (31.4) | 21 (21.2) | 12 (31.6) | 3 (27.3) |
| Gender | ||||
| Female | 27 (19.3) | 15 (15.2) | 5 (13.2) | 2 (18.2) |
| Male | 113 (80.7) | 84 (84.8) | 33 (86.8) | 9 (81.8) |
| HBV infection | 105 (75.0) | 75 (75.8) | 23 (60.5) | 8 (72.7) |
| TNM stage | ||||
| I | 117 (83.6) | 2 (2.0) | 0 | 0 |
| II | 12 (8.6) | 10 (10.1) | 0 | 0 |
| IIIA | 9 (6.4) | 15 (15.2) | 0 | 0 |
| IIIB | 0 | 20 (20.2) | 5 (13.2) | 0 |
| IIIC | 2 (1.4) | 46 (44.5) | 18 (47.4) | 0 |
| IVA | 0 | 6 (6.1) | 15 (39.5) | 0 |
| IVB | 0 | 0 | 0 | 11 (100) |
| Tumor size (cm) | ||||
| ≤ 5 | 71 (50.7) | 27 (27.3) | 5 (13.2) | 2 (18.2) |
| 5 < size ≤ 10 | 52 (37.1) | 50 (50.5) | 18 (47.4) | 5 (45.5) |
| > 10 | 17 (12.1) | 22 (22.2) | 15 (39.5) | 4 (36.4) |
| Child-Pugh score | ||||
| A | 128 (91.4) | 92 (92.9) | 30 (78.9) | 10 (90.9) |
| B | 12 (8.6) | 7 (7.1) | 8 (21.1) | 1 (9.1) |
| AFP (ng/mL) | ||||
| ≤ 200 | 91 (66.4) | 46 (47.4) | 20 (55.6) | 4 (44.4) |
| > 200 | 46 (33.6) | 51 (52.6) | 16 (44.4) | 5 (55.6) |
In conclusion, the EID staging classification is a simple and efficacious prognostic model for postoperative patients with HCC. Because the EID staging classification is easily obtained and objective, we propose it for widespread use in clinical practice as a staging system for postoperative patients with HCC.
The tumor-node-metastasis (TNM) system fails to include comprehensive characteristics of the tumor, especially the tumor’s growth pattern. Thus, it is crucial to design a system to evaluate the effects of tumor characteristics on the clinical outcome of resectable patients with hepatocellular carcinoma (HCC).
The characteristics of HCC tumors include the tumor size, tumor number, aggressiveness of growth, vascular involvement, regional lymph node metastasis, and extrahepatic spread. Although the TNM and Barcelona Clinic Liver Cancer staging classification for HCC were proposed based on certain tumor characteristics, no staging system systematically evaluates the effect of tumor growth patterns on the clinical outcome of patients with HCC.
These data enable the proposal of a new staging system to select the best candidates for the best therapies currently available: the four-stage Expansive-Invasive-Disseminative growth (EID) staging classification. Stage I includes patients with expansive tumor growths. Stage II has two subgroups: the first group contains patients with expansive tumor growth along with vascular and/or regional lymph node involvement, and the second group consists of patients with invasive tumor growth. Stage III includes patients with invasive tumor growth along with vascular/regional lymph node involvement.
The EID staging classification is a simple and efficacious prognostic model for postoperative patients with HCC. Because the EID staging classification is obtained easily, authors propose it for postoperative patients with HCC.
Expansive growth includes single tumor and multiple tumors without invasive and disseminative growth; invasive growth consists of satellite nodules including perforation of the tumor encapsulation, nodule fusion including diffuse growth that lacks tumor encapsulation and tumors with direct invasion of adjacent organs; disseminative growth contains vascular involvement, regional lymph node metastasis and distant metastasis
The prognostic significance of the new staging method was confirmed by the detailed statistical analysis of 288 patients treated in a single facility, being compared with other staging systems previously proposed. This clinical study is interesting and novel. The author found a simply and efficacy prognostic model for postoperative patients with HCC.
P- Reviewers Francis H, Li ZF, Su C, UedaY S- Editor Zhai HH L- Editor A E- Editor Li JY
| 1. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [PubMed] |
| 2. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [PubMed] |
| 3. | Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840-845. [PubMed] |
| 4. | Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207-215. [PubMed] |
| 5. | Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 452] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Cheng CH, Lee CF, Wu TH, Chan KM, Chou HS, Wu TJ, Yu MC, Chen TC, Lee WC, Chen MF. Evaluation of the new AJCC staging system for resectable hepatocellular carcinoma. World J Surg Oncol. 2011;9:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Sterling RK, Wright EC, Morgan TR, Seeff LB, Hoefs JC, Di Bisceglie AM, Dienstag JL, Lok AS. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am J Gastroenterol. 2012;107:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Benz-Bohm G, Gross-Fengels W, Widemann B, Linden A. Bone marrow metastases of neuroblastoma: MRT in comparison with bone marrow cytology and mIBG scintigraphy. Rofo. 1990;152:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Zhou L, Rui JA, Wang SB, Chen SG, Qu Q, Chi TY, Wei X, Han K, Zhang N, Zhao HT. Factors predictive for long-term survival of male patients with hepatocellular carcinoma after curative resection. J Surg Oncol. 2007;95:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Yang T, Lin C, Zhai J, Shi S, Zhu M, Zhu N, Lu JH, Yang GS, Wu MC. Surgical resection for advanced hepatocellular carcinoma according to Barcelona Clinic Liver Cancer (BCLC) staging. J Cancer Res Clin Oncol. 2012;138:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Choi SB, Lee JG, Kim KS, Yoon DS, Choi JS, Lee WJ, Kim BR. The prognosis and survival analysis according to seven staging systems of hepatocellular carcinoma following curative resection. Hepatogastroenterology. 2008;55:2140-2145. [PubMed] |
| 12. | Chen YL, Ko CJ, Chien SY, Chen LS, Chen ML, Chi CW, Lai HW. Tumor size as a prognostic factor in resected small hepatocellular carcinoma: a controversy revisited. J Gastroenterol Hepatol. 2011;26:851-857. [PubMed] |
| 13. | Hosaka T, Ikeda K, Kobayashi M, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Akuta N, Suzuki F, Suzuki Y. Predictive factors of advanced recurrence after curative resection of small hepatocellular carcinoma. Liver Int. 2009;29:736-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 15. | Toyama T, Hiramatsu N, Yakushijin T, Oze T, Nakanishi F, Yasumaru M, Mochizuki K, Kanto T, Takehara T, Kasahara A. A new prognostic system for hepatocellular carcinoma including recurrent cases: a study of 861 patients in a single institution. J Clin Gastroenterol. 2008;42:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Dupont-Bierre E, Compagnon P, Raoul JL, Fayet G, de Lajarte-Thirouard AS, Boudjema K. Resection of hepatocellular carcinoma in noncirrhotic liver: analysis of risk factors for survival. J Am Coll Surg. 2005;201:663-670. [PubMed] |
| 17. | Shimada K, Sano T, Sakamoto Y, Kosuge T. A long-term follow-up and management study of hepatocellular carcinoma patients surviving for 10 years or longer after curative hepatectomy. Cancer. 2005;104:1939-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2866] [Article Influence: 110.2] [Reference Citation Analysis (1)] |
| 19. | Cammà C, Di Marco V, Cabibbo G, Latteri F, Sandonato L, Parisi P, Enea M, Attanasio M, Galia M, Alessi N. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther. 2008;28:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 21. | Capussotti L, Muratore A, Amisano M, Massucco P, Polastri R, Bouzari H. Liver resection for large-size hepatocellular carcinomas in 47 non-cirrhotic patients--no mortality and long-term survival. Hepatogastroenterology. 2006;53:768-772. [PubMed] |
| 22. | Santambrogio R, Opocher E, Costa M, Barabino M, Zuin M, Bertolini E, De Filippi F, Bruno S. Hepatic resection for “BCLC stage A” hepatocellular carcinoma. The prognostic role of alpha-fetoprotein. Ann Surg Oncol. 2012;19:426-434. [PubMed] |
| 23. | Yen YH, Changchien CS, Wang JH, Kee KM, Hung CH, Hu TH, Lee CM, Lin CY, Wang CC, Chen TY. A modified TNM-based Japan Integrated Score combined with AFP level may serve as a better staging system for early-stage predominant hepatocellular carcinoma patients. Dig Liver Dis. 2009;41:431-441. [PubMed] |