Published online Aug 21, 2013. doi: 10.3748/wjg.v19.i31.5094
Revised: April 24, 2013
Accepted: June 5, 2013
Published online: August 21, 2013
Processing time: 181 Days and 19.4 Hours
AIM: To investigate the effect of protein-energy malnutrition on intestinal barrier function during rotavirus enteritis in a piglet model.
METHODS: Newborn piglets were allotted at day 4 of age to the following treatments: (1) full-strength formula (FSF)/noninfected; (2) FSF/rotavirus infected; (3) half-strength formula (HSF)/noninfected; or (4) HSF/rotavirus infected. After one day of adjustment to the feeding rates, pigs were infected with rotavirus and acute effects on growth and diarrhea were monitored for 3 d and jejunal samples were collected for Ussing-chamber analyses.
RESULTS: Piglets that were malnourished or infected had lower body weights on days 2 and 3 post-infection (P < 0.05). Three days post-infection, marked diarrhea and weight loss were accompanied by sharp reductions in villus height (59%) and lactase activity (91%) and increased crypt depth (21%) in infected compared with non-infected pigs (P < 0.05). Malnutrition also increased crypt depth (21%) compared to full-fed piglets. Villus:crypt ratio was reduced (67%) with viral infection. There was a trend for reduction in transepithelial electrical resistance with rotavirus infection and malnutrition (P = 0.1). 3H-mannitol flux was significantly increased (50%; P < 0.001) in rotavirus-infected piglets compared to non-infected piglets, but there was no effect of nutritional status. Furthermore, rotavirus infection reduced localization of the tight junction protein, occludin, in the cell membrane and increased localization in the cytosol.
CONCLUSION: Overall, malnutrition had no additive effects to rotavirus infection on intestinal barrier function at day 3 post-infection in a neonatal piglet model.
Core tip: We are quite excited about these results which suggest involvement of intestinal tight-junction proteins in the pathology of rotaviral gastroenteritis. The work further examines the interplay of malnutrition superimposed on viral infection. 3H-mannitol flux was significantly increased in rotavirus infected piglets compared to non-infected piglets, but there was no effect of nutritional status. Furthermore, rotavirus infection reduced localization of the tight junction protein, occludin, in the cell membrane and increased localization in the cytosol. This extends work on the molecular mechanisms of rotavirus in the neonatal intestine that we previously published.
- Citation: Jacobi SK, Moeser AJ, Blikslager AT, Rhoads JM, Corl BA, Harrell RJ, Odle J. Acute effects of rotavirus and malnutrition on intestinal barrier function in neonatal piglets. World J Gastroenterol 2013; 19(31): 5094-5102
- URL: https://www.wjgnet.com/1007-9327/full/v19/i31/5094.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i31.5094
Pediatric diarrheal diseases are the second-leading cause of childhood mortality, and responsible for about 1.34 million deaths each year in children under 5 years of age[1]. Rotaviruses are the most common causes of acute, severe gastroenteritis and dehydrating diarrhea. Furthermore, rotavirus-associated enteritis represents a class of zoonotic diseases that cause major health concerns not only for humans, but also most domestic livestock species[2]. The food animal livestock industry estimated a multi-million dollar annual economic loss due to diarrheal diseases associated with a reduction in weight gain, treatment and death of young animals[2]. In addition to the mortality rates associated with diarrheal disease there is a about 60% increase in pediatric patient mortality rates when diarrheal disease is compounded with malnourishment[3]. In the neonatal piglet model, Zijlstra et al[4] demonstrated that malnutrition extends rotavirus infections up to a week longer in malnourished piglets compared with well nourished infected piglets.
Rotaviruses infect the differentiated epithelial cells of the mid- to upper-villus of the small intestine[5]. The infection is associated with cell death, reduced villus surface area, loss of absorptive capacity, osmotic deregulation, and infiltration of the lamina propria by mononuclear cells[6,7]. In pigs, acute viral injury to enterocytes leads to increased epithelial cell loss and intestinal lesions leaving the intestinal epithelial barrier compromised[6]. Rapid restoration of epithelial continuity is important following injury and depends on the migration of uninjured enterocytes from the crypts to cover the compromised barrier. Protein-energy malnutrition (PEM) also decreases intestinal barrier function and integrity, increasing bacterial translocation with subsequent enteritis and diarrhea[8]. Moreover, PEM also inhibits epithelial crypt cell proliferation which delays cellular migration along the crypt-villus axis and results in longer repair periods[9].
Intestinal barrier function is maintained in part by actual physical links between enterocytes by intercellular junction complexes. Tight junctions are located on the uppermost basolateral surface of polarized enterocytes and regulate diffusion between cells. They allow the epithelia to form a cellular barrier separating the luminal content of the intestine from the lamina propria. In cell culture models using Madin-Darby Canine Kidney and Caco-2 cells, studies have demonstrated dysregulation of the paracellular pathways[10,11]. In fact, rotavirus infection in these cells caused alterations in tight junction structure and function related to epithelial cell resistance and permeability. The authors determined that there was a time dependent disruption in localization of tight junction proteins claudin-1, occludin and zonula-occuluden when Caco-2 cells were infected with rotavirus[10,11]. Claudin-1 was the first tight-junction protein to become solubilized in the cytosol of the epithelial cells[11].
Nutritional factors have been shown to impact neonatal intestinal health[12]. In particular, our laboratory has investigated how dietary components impact intestinal health in neonatal piglets with rotavirus infection[13-15]. We have demonstrated supplemental dietary arginine activates mammalian target of rapamycin, mitogen-activate protein kinase, and ribosomal p70S6 kinase signaling in rotavirus infected enterocytes[15]. These cell signaling mechanisms lead to increase jejunal protein synthesis, cell migration and intestinal restitution in rotavirus infected piglets[13,14]. Moreover, we have demonstrated the value of dietary plasma protein because it maintained growth rates, reduced diarrhea, and maintained enzymatic activity in the small intestine of neonatal pigs with rotavirus infection[14]. Additionally, others have shown soy-based infant formula isoflavones are effective in reducing rotavirus infectivity in cell culture models of rotavirus infection[16]. These reports demonstrate the importance of nutritional factors involvement in modulation of host immune response and repair mechanisms associated with rotaviral infection. Therefore, the pathophysiological mechanisms of rotavirus infection and its diarrheal mechanism are the focus of much work toward developing effective vaccines and nutritional treatments for the virus. Understanding the viral interruption of paracellular pathways and the impact of nutritional status on these pathways is critical in the development of adequate medical treatment.
All protocols were approved by the Institutional Animal Care and Use Committee of North Carolina State University. The full experimental protocol was previously reported in Rhoads et al[15]. Briefly, 24 piglets were collected directly from the birth canal, colostrum deprived, cleaned with 70% ethanol and transported to a biosecure rearing facility. Piglets were individually housed and contained in two rooms with a temperature of 32 °C. Pigs were fed milk diet via a gravity flow feeding system, adapted from Oliver et al[17]. The formula composition was previously reported by Rhoads et al[15]. A liquid colostrum diet (LaBelle Associates, Inc., WA, United States) was fed for the first 24 h to provide passive immunity. Feedings (about 300 mL/kg body weight per day) were offered four times per day (8:00 am, 1:00 pm, 6:00 pm and 11:00 pm), and non-infected pigs were pair-fed to the level of their infected counterparts.
We compared well-nourished and malnourished piglets (n = 16) in a 2 × 2 factorial design examining effects of malnutrition and viral infection as follows: (1) full-strength formula (FSF) (180 g/L), non-infected (positive control); (2) FSF, rotavirus infected; (3) half-strength formula (HSF, 90 g/L), non-infected; or (4) HSF, rotavirus infected (negative control). Intestinal samples from this study were collected only on day 3 post-infection.
Rotavirus inoculation and clinical measures were previously described by Rhoads et al[15]. Briefly, the rotavirus inoculum, initially isolated by Lecce et al[18], was passaged through colostrum-deprived pigs and prepared as a bacteria-free intestinal supernatant. Approximately 107 particles of rotavirus or sham inoculants were suspended in full strength milk formula, and piglets were gastrically intubated at 10:00 am on day 0.
Piglet weights, feed intakes, and fecal consistency were recorded daily. Feces were given a diarrhea score of 0, 1, 2 or 3, corresponding with firm, soft but formed, runny, and severe watery diarrhea, respectively, by a single individual blinded to treatments. A rectal swab was collected daily from each piglet for the detection of rotavirus shedding (Virogen Rotatest; Wampole Laboratories, Cranbury, NJ, United States).
On day 3 post-infection, pigs were anesthetized with isoflurane and killed by the AVMA approved electrocution followed by exsanguination. Intestinal samples from the mid-jejunum area were collected, snap frozen and stored at -80 °C until analysis by Western blotting. Intestinal segments were collected and fixed for histological analysis of intestinal morphology[17]. Intestinal morphology and lactase measurements were performed as previously described[17].
Segments of mid-jejunum were harvested from the pigs and the mucosa was stripped from the seromuscular layer in oxygenated (95% O2/5% CO2) Ringer’s solution. Tissues were mounted in 1.14 cm2 aperture Ussing chambers, as described previously[19]. Tissues were bathed on the serosal and mucosal sides with 10 mL Ringer’s solution. The serosal bathing solution contained 10 mmol/L glucose, which was osmotically balanced on the mucosal side with 10 mmol/L mannitol. Bathing solutions were oxygenated (95% O2/5% CO2) and circulated in water-jacketed reservoirs maintained at 37 °C. The spontaneous potential difference (PD) was measured using Ringer-agar bridges connected to calomel electrodes, and the PD was short-circuited through Ag-AgCl electrodes using a voltage clamp that corrected for fluid resistance. Transepithelial electrical resistance (Ω∙cm2) was calculated from the spontaneous PD and the short-circuit current (Isc), as previously described[19].
Mucosal permeability was assessed following experimental treatments by adding 0.2 μCi/mL 3H-mannitol on the mucosal side of the Ussing chamber-mounted tissues and measuring the flux to the serosal compartment. Following a 15 min equilibration period samples were collected from the mucosal side of the chamber and following a 60 min flux period samples were collected from the serosal side of the chamber as previously described[20]. The concentration of 3H-mannitol was quantified using a liquid scintillation counter (LKB Wallac, model 1219 Rack Beta, Perkin Elmer Life and Analytical Sciences, Boston, MA, United States). The directional flux of 3H-mannitol from the mucosal to serosal chamber were determined by using the mannitol specific activity added to the mucosal bathing solution and calculating the net appearance of 3H-mannitol over time in the serosal bathing solution.
Intestinal mucosa scrapings from all animals were snap frozen and stored at -80 °C for SDS-PAGE analysis. Triton X-soluble and X-insoluble fractions were prepared as previously described[21]. Briefly, samples were homogenized in Triton X-soluble buffer and allowed to rest on ice for 30 min with intermittent vortexing. Thereafter, the homogenates were centrifuged at 400 g for 10 min at 4 °C to remove cell debris. The supernatant was removed to a new tube and centrifuged at 9000 g for 10 min at 4 °C to separate the soluble and insoluble protein fractions. The insoluble fraction pellet was dissolved in Triton X-insoluble fraction extraction buffer by heating at 95 °C for 5 min with intermittent vortexing. Protein concentrations of tissue extracts were determined using the DC protein assay (Bio-Rad; Hercules, CA, United States). Tissue extracts of equal protein concentrations were mixed with equal volumes of 2 × Laemmli Sample Buffer (Bio-Rad; Hercules, CA, United States) and boiled for 5 min. Protein lysates were loaded on a 12% SDS polyacrylamide gel, and electrophoresis was completed as recommended for Bio-Rad CriterionTM gels. Proteins were transferred to polyvinylidene diflouride membrane (Immobilon-S; Millipore, Billaerica, MA, United States) by CriterionTM Blotter (Bio-Rad; Hercules, CA, United States). Membranes were blocked and incubated with primary and secondary antibodies as previously reported by Moeser et al[21].
Data were analyzed using the general linear models procedure of SAS (Cary; NC 27513, United States) appropriate for a 2 ×2 factorial design, with feeding level (FSF vs HSF) and infection (± rotavirus) as the factors.
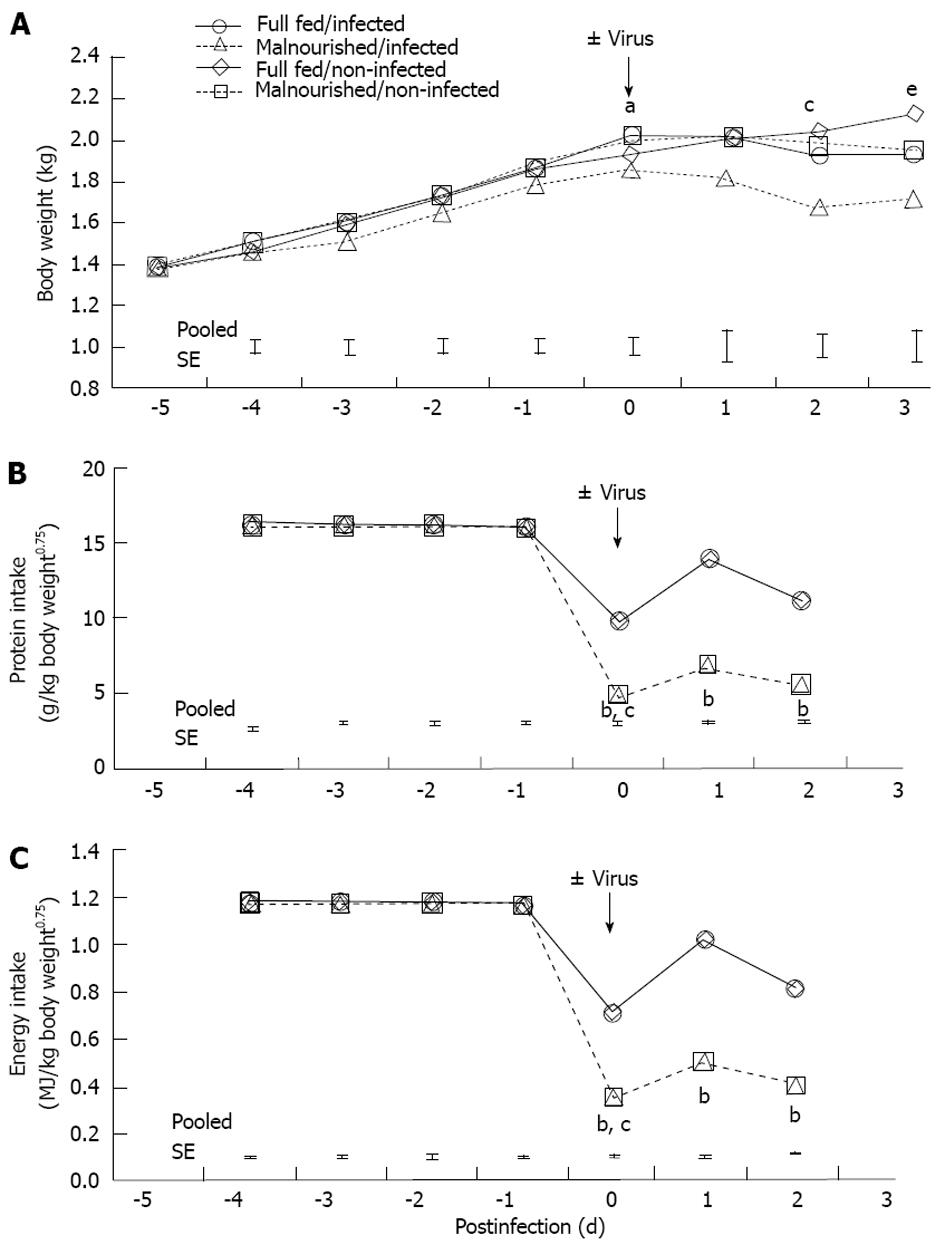
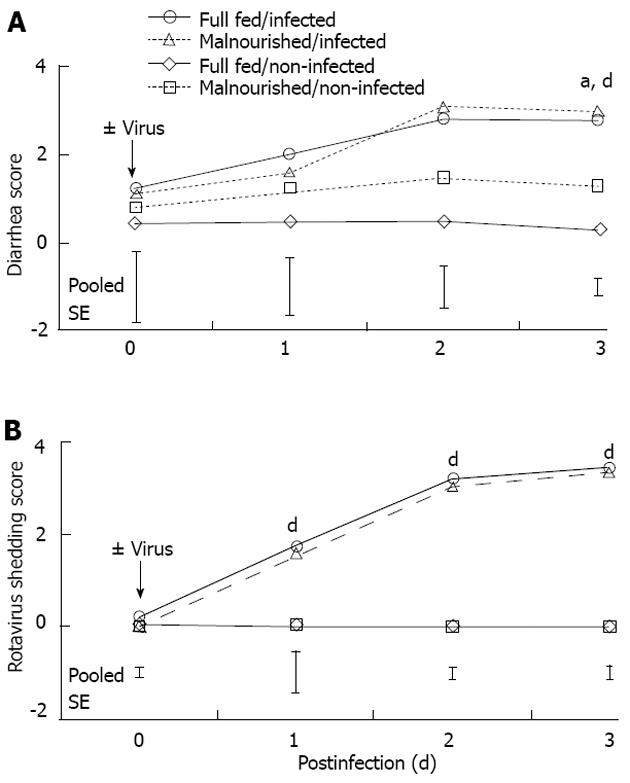
Body weight for 1-d-old pigs was 1.4 ± 0.2 kg. The pigs were adapted to the feeding system until day 5 when they were switched to either FSF (well-nourished) or HSF (malnourished) diets. Twenty-four hours after pigs were assigned to dietary treatments they were inoculated with rotavirus. There was a feeding level by infection interaction on day 0 (Figure 1A; P < 0.05). The interaction was due to the drop in body weight of the HSF/infected piglets compared with pigs in other dietary treatment groups. On days 1-3 post-infection there was no interaction of feeding level and infection. However, on days 2 and 3 post-infection there were main effects of both feeding level and infection. Full-strength formula/non-infected pigs maintained a higher body weight compared HSF/infected pigs (P < 0.05). Feed intake of non-infected pigs was pair-fed to the level of their infected counterpart, so there were no major differences in protein and energy intake between the non-infected and infected pigs from the same dietary treatment (Figure 1B and C). Malnourished pigs received a 50% reduction in nutrient intake, but daily intakes of water, sodium, potassium and chloride were similar to full-fed pigs, because electrolyte solution was used for formula dilution. The significant effect of viral infection on body weight on day 3 could be related to multiple factors, however, we have controlled nutrition by pair feeding, and measured growth, so the most likely cause of weight loss was diarrheic water loss. Pigs had no diarrhea prior to rotavirus inoculation (data not shown). However, viral infection resulted in diarrhea scores of 3 (severe, watery diarrhea) for both rotavirus infected groups. On day 3 post-infection there was a main effect of virus and feeding level on diarrhea score (P < 0.01 and P < 0.05, respectively; Figure 2A); however, there was no additive effect of malnutrition. Additionally, rotavirus-inoculated pigs had a significant increase in viral shedding from days 1 to 3 post-infection (Figure 2B).
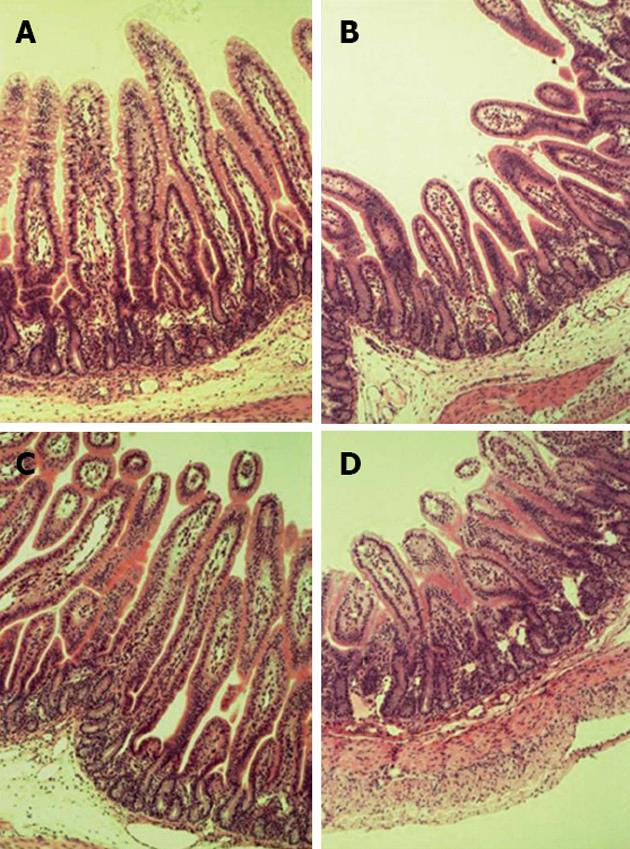
Rotavirus infected pigs had significantly reduced lactase activity (Table 1; P < 0.05) on day 3 post-infection. In addition, there was a main effect of virus on villus height, crypt depth and villus height:crypt depth ratio (Figure 3 and Table 1; P < 0.05). There also was a main effect of feeding level on crypt depth with malnourished pigs having a greater depth than FSF pigs (Figure 3 and Table 1; P < 0.05). However, there was not a significant interaction between rotavirus and feeding level on intestinal lactase or morphology.
| Fully fed | Malnourished | |||||
| Non-infected | Infected | Non-infected | Infected | SE | Effect1 | |
| Lactase activity [mmol/(min∙g protein)] | 169.8 | 9.6 | 160.3 | 19.7 | 21.0 | V |
| Intestinal morphology | ||||||
| Villus height (mm) | 0.87 | 0.37 | 0.85 | 0.34 | 0.13 | V |
| Crypt depth (mm) | 0.10 | 0.13 | 0.13 | 0.16 | 0.01 | V, F |
| Height:depth ratio | 8.96 | 3.12 | 6.86 | 2.12 | 1.24 | V |
Transepithelial electrical resistance (TER) data were recorded on mid-jejunum tissues from pigs on each dietary and viral treatment (Figure 4A). There was no significant interaction of feeding-level by virus and no main effects on TER data. However, there was a trend for FSF/rotavirus infected pigs to have decreased TER compared with FSF/non-infected pigs (Figure 4A; P = 0.1). Malnourished animals had similar TER readings regardless of rotavirus infection.
To assess the effect of nutritional status and rotavirus infection on intestinal permeability, 3H-manitol flux was measured on mid-jejunum tissues from pigs. The feeding-level by rotavirus infection interaction was not significant (Figure 4B). However, there was a main effect of rotavirus infection on intestinal permeability (P < 0.05). Pigs infected with rotavirus had 50% greater intestinal permeability than non-infected pigs. Conversely, there was no main effect of feeding level on intestinal permeability.
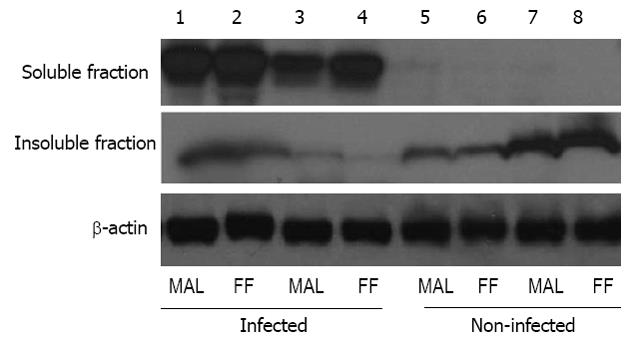
Western blotting for the tight-junction protein, occludin, demonstrated that rotavirus infected pigs had greater quantity of occludin protein in the soluble fraction of protein than in the insoluble fraction (Figure 5). Additionally, non-infected pigs had no occludin protein in the soluble fraction and higher levels of occludin in the insoluble fraction. As was observed in TER and flux measures of barrier function, feeding level did not alter the cellular localization of the tight-junction protein occludin (Figure 5).
Rotavirus gastroenteritis accounts for 30%-40% of pediatric diarrheal deaths worldwide[22], and PEM is also a primary cause of childhood morbidity and mortality especially in developing nations[1,23]. While rotavirus vaccine safety and efficacy has improved over the last 10 years for children in developed countries, the efficacy of rotavirus vaccine for children in poor settings is compromised due to environmental factors associated with reduction in vaccine effectiveness[24]. Our laboratory and others working in neonatal piglet models have sought to determine possible nutritional therapies which could reduce the severity of the infection or enhance the effectiveness of vaccines[13-16]. Nutritional therapies will be affected by previous nutritional status of individuals, and nutritional deprivation is a key component to overcome for treatment of enteric diseases afflicting impoverished children. In mouse models of rotavirus infection and malnutrition there is a significant increase in gut permeability to environmental macromolecules, as well as a significant decrease in minimal infectious dose needed to produce diarrhea[25,26]. Therefore, understanding the mechanisms associated with gut barrier function under normal rotavirus infection as well as rotavirus infection compounded by malnutrition is an important component for developing efficacious treatment and prevention strategies in young children and animals.
The purpose of this study was to examine the effects of PEM and rotavirus infection on intestinal barrier function in neonatal piglets. Diarrhea and malnutrition are two major problems in pediatric patients and a better understanding of the interaction and underlying mechanisms may lead to improved treatment. The study design was a 2 × 2 factorial with two levels of nutrition and either non-infected or infected with rotavirus.
Rotavirus infection caused decreased body weight, reduced protein intake, reduced energy intake, diarrhea, decreased lactase activity, trends for decreased jejunum TER, increased jejunum permeability, and decreased cell membrane localization of occludin. However, malnutrition did not have a significant additive effect to rotavirus infection on intestinal barrier function measured 3 d post-infection. The lack of additive effects on malnutrition is most likely related to the time line of the study. The 3 d post-infection time point may have been too short to evaluate repair of the intestine in this model. It is also possible that if we had applied the nutritional treatment prior to rotavirus infection there might have been a significant effect of PEM in the piglets.
Previously, we have shown that rotavirus infection causes intestinal damage and diarrhea within 2 d post-infection in the neonatal piglet model[4]. Additionally, the effects of the viral infection began to subside nearly 1 wk earlier in full-fed pigs than in the malnourished, infected pigs[4]. Herein, we report similar results of rotavirus infection causing significant weight loss and increased diarrhea by day 2 post-infection. Although there was a significant interaction of feeding level and infection on weight loss on day 0 of inoculation the interaction was not sustained for the next 3 d post-infection. However, the main effects of rotavirus and feeding level on weight loss were significant on days 2 and 3 post-infection. The reduced weight gain, increased diarrhea scores and increased viral shedding were expected following inoculation. However, we did anticipate there would be additive effects of malnutrition on all three outcomes, which we did not observe. Others have shown that PEM alters physiological and immunological properties of the intestine leaving individuals more susceptible to diarrhea associated illnesses[25-29]. Nevertheless, this bidirectional relationship between PEM and susceptibility to enteric pathogens in pediatric patients is related to the type of pathogen and many environmental factors playing a significant role in susceptibility to infection[30]. We may have underestimated the time needed to detect a plain of nutrition response with rotavirus infection. In fact, in mouse models of malnutrition and intestinal permeability the dietary treatments were applied to the mice a minimum of 5 d before rotavirus inoculation, and the research showed increased ovalbumin absorption in malnourished, infected mice compared to well nourished, infected mice[25].
Dietary nutrients are essential for gastrointestinal growth and health[12]. Malnutrition reduces the integrity of intestinal epithelium, facilitating bacterial translocation with subsequent enteritis and diarrhea[8], and it can also impair epithelial cell proliferation in crypts of the small intestine, resulting in delayed cellular migration along the crypt-villus axis[9]. This impairment is inhibitory to intestinal repair processes associated with gastrointestinal enteritis. We found that PEM did not further reduce lactase activity or villus blunting in the small intestine beyond the reduction seen with rotavirus infection alone. Additionally, crypt depth was increased by PEM and viral infection, but there was no additive effect. Previously, Zijlstra et al[4] reported a reduction in crypt depth with infected, malnourished pigs compared to infected, full-fed pigs. However, our data suggest that HSF/infected pigs had increased crypt depth compared to all other treatment groups. This may be related to the exact location of sampling between our study and Zijlstra et al[4]. Zijlstra et al[4] found the reductions in crypt depth were more distal in the small intestine on day 2 post-infection, and the reduction in crypt depth in the proximal to mid small intestine was not significant until day 9 post-infection. Additionally, in piglet models of transmissible gastroenteritis, increased jejunal crypt depth following infection have been reported[31-33].
Maintenance of migrating crypt cells is essential in maintaining gut barrier function following intestinal insult to seal the basement membrane and close leaky epithelial intercellular spaces and tight junctions. Rotavirus is known to disrupt tight junctions and decrease TER in Caco-2 cells between 8 and 24 h post-infection. We found in vivo treatments of rotavirus showed a trend for reduced TER in mid-jejunal tissue from neonatal pigs. TER data showed similar resistance between infected pigs and HSF/uninfected pigs; however there was not an additive effect of malnutrition and rotavirus infection in the neonatal pigs. Serosal to mucosal flux of 3H-mannitol was significantly greater for infected pigs with no effect of PEM on mucosal paracellular permeability. The numerical reduction in TER for all treatment groups except FSF/non-infected piglets does not completely align with the differences in mannitol flux we observed, and this could be explained by recent understandings of tight junction pore and leak pathways[34]. Piglets in the full-fed/infected groups had diarrhea accompanied by alterations in tight junctions, TER, and mannitol flux and we reason that virus infection in the well-nourished state caused tight junction pores to open (allowing electrolytes and water passage) together with a pore/shunt pathway allowing macromolecule passage. In contrast, malnourished piglets, regardless of infection, had numerically reduced TER, but mannitol flux was only increased in malnourished/infected piglets suggesting that malnutrition alone was sufficient to alter passage of small ions across the barrier associated with reductions in TER and diarrhea, but rotavirus infection altered the tight junctions to allow increased macromolecule flux through pore/shunt pathways.
Expression of the tight junction protein, occludin, revealed that although virus infection impacted the proportion of occludin in the membrane (insoluble fraction) versus the cytosolic (soluble) fraction there was no effect of feeding level on occludin localization. Rotavirus infection significantly increased occludin in the soluble fraction of the protein extraction compared to non-infected pigs which had greater occludin in the membrane fraction of the protein extraction. This finding corroborates the effects seen in the TER and flux data showing no additive effects of PEM to the rotavirus infected pigs intestinal barrier function. Other models of starvation and injury in rats suggest an additive effect of malnourishment and intestinal insult on gut barrier permeability[35]. However, our results are consistent with previously reported data showing that PEM did not affect jejunal tissue protein synthesis rates and phosphorylation of p70S6K, a key enzyme activated by mammalian target of rapamycin in regulating protein synthesis[15]. Zijlstra et al[4] showed that the growth factors, insulin-like growth factor (IGF)-I and IGF-II, were not significantly reduced in the malnourished, infected pigs until 9 d post-infection. These growth factors are known trophic factors in the intestine and have been shown to increase jejunal uptake of glucose in the intestine[36]. Because IGF-I and IGF-II concentrations were probably not decreased in our pigs by 3 d post-infection the effects of PEM on gut barrier function may not have been detectable at this early time point post-infection.
In conclusion, the present study provides clear evidence that rotavirus infection significantly affects small intestinal TER, and is the first report of increased paracellular permeability in neonatal pigs resulting from altered tight junction protein localization in enterocytes. However, additive effects of PEM on intestinal TER, paracellular permeability, and tight junction protein localization are not seen by 3 d post-infection in neonatal pigs. Though it is likely that during a more extended timeline wherein metabolic hormone responses decrease following viral infection and PEM there is potential for reduction in crypt cell proliferation[37]. This reduction in proliferation may potentially exacerbate the effect of viral infection in PEM neonates. Further identification of paracellular permeability mechanisms associated with rotavirus and PEM would be useful in developing treatments for pediatric patients facing environments where malnutrition and diarrhea are intertwined.
The authors thank Dr. Lori Gatlin and Oulayvanh Phillips for their assistance with virus inoculant preparation and animal care.
Rotavirus enteritis and malnutrition are common problems for children in the developing countries. Rotavirus infections are common for all children under the age of five. However, children in less than desirable circumstances must deal with rotavirus infection compound by malnourishment. There is limited information on the interactions between the mechanisms of rotavirus and malnutrition on gut barrier function.
In the present study, investigation of the interactions between rotavirus enteritis and malnutrition on gut barrier function were studied in the neonatal piglet model to determine the mechanisms involved in barrier function failure.
Rotavirus infection significantly blunted villus height in neonatal piglets, increased gut barrier permeability and reduced localization of tight junction proteins in the cell membrane of intestinal enterocytes during acute infection. Determination of the mechanisms of rotavirus and malnutrition interaction could lead to development of new nutritional or pharmacological treatments in high risk children in developing countries.
The effects of rotavirus and malnutrition in the neonatal piglet experimental model demonstrated the virus significantly effects gut barrier function and could potentially be used to study effective nutritional or pharmacological treatments for rotavirus in children. Further investigation into signaling mechanisms controlling intestinal tight junctions may provide insights into protein targets for development of effective therapies.
Protein energy malnutrition is a common problem that compounds gastroenteritis in developing countries and neonatal health.
This study is well constructed and it is based on previous work on nutritional impacts on viral infections especially in developing countries. This is an interesting and relevant study which confirms that rotavirus acutely impacts gut barrier function in the neonatal pig making animals more susceptible to flux across intestinal epithelial layer. Moreover, this study suggests that longer term studies should be completed to investigate the interaction of malnutrition with rotavirus infection in the developing neonate.
P- Reviewer He ST S- Editor Zhai HH L- Editor A E- Editor Li JY
| 1. | Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1912] [Cited by in RCA: 1890] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 2. | Martella V, Bányai K, Matthijnssens J, Buonavoglia C, Ciarlet M. Zoonotic aspects of rotaviruses. Vet Microbiol. 2010;140:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 3. | Caulfield LE, de Onis M, Blössner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193-198. [PubMed] |
| 4. | Zijlstra RT, Donovan SM, Odle J, Gelberg HB, Petschow BW, Gaskins HR. Protein-energy malnutrition delays small-intestinal recovery in neonatal pigs infected with rotavirus. J Nutr. 1997;127:1118-1127. [PubMed] |
| 5. | Ciarlet M, Estes MK. Interactions between rotavirus and gastrointestinal cells. Curr Opin Microbiol. 2001;4:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Estes MK, Kang G, Zeng CQ, Crawford SE, Ciarlet M. Pathogenesis of rotavirus gastroenteritis. Novartis Found Symp. 2001;238:82-96; discussion 96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Lorrot M, Vasseur M. Physiopathology of Rotavirus diarrhea. Arch Pediatr. 2007;14 Suppl 3:S145-S151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Deitch EA, Ma WJ, Ma L, Berg RD, Specian RD. Protein malnutrition predisposes to inflammatory-induced gut-origin septic states. Ann Surg. 1990;211:560-567; discussion 567-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Butzner JD, Gall DG. Impact of refeeding on intestinal development and function in infant rabbits subjected to protein-energy malnutrition. Pediatr Res. 1990;27:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Dickman KG, Hempson SJ, Anderson J, Lippe S, Zhao L, Burakoff R, Shaw RD. Rotavirus alters paracellular permeability and energy metabolism in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G757-G766. [PubMed] |
| 11. | Obert G, Peiffer I, Servin AL. Rotavirus-induced structural and functional alterations in tight junctions of polarized intestinal Caco-2 cell monolayers. J Virol. 2000;74:4645-4651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Jacobi SK, Odle J. Nutritional factors influencing intestinal health of the neonate. Adv Nutr. 2012;3:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Corl BA, Odle J, Niu X, Moeser AJ, Gatlin LA, Phillips OT, Blikslager AT, Rhoads JM. Arginine activates intestinal p70(S6k) and protein synthesis in piglet rotavirus enteritis. J Nutr. 2008;138:24-29. [PubMed] |
| 14. | Corl BA, Harrell RJ, Moon HK, Phillips O, Weaver EM, Campbell JM, Arthington JD, Odle J. Effect of animal plasma proteins on intestinal damage and recovery of neonatal pigs infected with rotavirus. J Nutr Biochem. 2007;18:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Rhoads JM, Corl BA, Harrell R, Niu X, Gatlin L, Phillips O, Blikslager A, Moeser A, Wu G, Odle J. Intestinal ribosomal p70(S6K) signaling is increased in piglet rotavirus enteritis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G913-G922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Andres A, Donovan SM, Kuhlenschmidt TB, Kuhlenschmidt MS. Isoflavones at concentrations present in soy infant formula inhibit rotavirus infection in vitro. J Nutr. 2007;137:2068-2073. [PubMed] |
| 17. | Oliver WT, Mathews SA, Phillips O, Jones EE, Odle J, Harrell RJ. Efficacy of partially hydrolyzed corn syrup solids as a replacement for lactose in manufactured liquid diets for neonatal pigs. J Anim Sci. 2002;80:143-153. [PubMed] |
| 18. | Lecce JG, Balsbaugh RK, Clare DA, King MW. Rotavirus and hemolytic enteropathogenic Escherichia coli in weanling diarrhea of pigs. J Clin Microbiol. 1982;16:715-723. [PubMed] |
| 19. | Argenzio RA, Liacos JA. Endogenous prostanoids control ion transport across neonatal porcine ileum in vitro. Am J Vet Res. 1990;51:747-751. [PubMed] |
| 20. | Blikslager AT, Roberts MC, Argenzio RA. Prostaglandin-induced recovery of barrier function in porcine ileum is triggered by chloride secretion. Am J Physiol. 1999;276:G28-G36. [PubMed] |
| 21. | Moeser AJ, Nighot PK, Ryan KA, Simpson JE, Clarke LL, Blikslager AT. Mice lacking the Na+/H+ exchanger 2 have impaired recovery of intestinal barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G791-G797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200 Suppl 1:S9-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 23. | Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4217] [Cited by in RCA: 3457] [Article Influence: 203.4] [Reference Citation Analysis (0)] |
| 24. | Holmgren J, Svennerholm AM. Vaccines against mucosal infections. Curr Opin Immunol. 2012;24:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Uhnoo IS, Freihorst J, Riepenhoff-Talty M, Fisher JE, Ogra PL. Effect of rotavirus infection and malnutrition on uptake of a dietary antigen in the intestine. Pediatr Res. 1990;27:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Riepenhoff-Talty M, Uhnoo I, Chegas P, Ogra PL. Effect of nutritional deprivation on mucosal viral infections. Immunol Invest. 1989;18:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Roy VV, Mathur R, Reddy V. Etiology of acute gastroenteritis in malnutrition. Indian J Med Res. 1986;84:173-177. [PubMed] |
| 28. | Reddy V, Srikantia SG. Interaction of nutrition and the immune response. Indian J Med Res. 1978;68 Suppl:48-57. [PubMed] |
| 29. | Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P, Liu L, Haque R, Petri WA. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2012;54:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 30. | Mondal D, Haque R, Sack RB, Kirkpatrick BD, Petri WA. Attribution of malnutrition to cause-specific diarrheal illness: evidence from a prospective study of preschool children in Mirpur, Dhaka, Bangladesh. Am J Trop Med Hyg. 2009;80:824-826. [PubMed] |
| 31. | Rhoads JM, MacLeod RJ, Hamilton JR. Alanine enhances jejunal sodium absorption in the presence of glucose: studies in piglet viral diarrhea. Pediatr Res. 1986;20:879-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Keljo DJ, Bloch KJ, Bloch M, Arighi M, Hamilton JR. In vivo intestinal uptake of immunoreactive bovine albumin in piglet enteritis. J Pediatr Gastroenterol Nutr. 1987;6:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Keljo DJ, Butler DG, Hamilton JR. Altered jejunal permeability to macromolecules during viral enteritis in the piglet. Gastroenterology. 1985;88:998-1004. [PubMed] |
| 34. | Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 659] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 35. | Wirén M, Söderholm JD, Lindgren J, Olaison G, Permert J, Yang H, Larsson J. Effects of starvation and bowel resection on paracellular permeability in rat small-bowel mucosa in vitro. Scand J Gastroenterol. 1999;34:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Drozdowski L, Thomson AB. Intestinal hormones and growth factors: effects on the small intestine. World J Gastroenterol. 2009;15:385-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |