Published online Jan 21, 2013. doi: 10.3748/wjg.v19.i3.418
Revised: December 17, 2012
Accepted: December 22, 2012
Published online: January 21, 2013
Processing time: 80 Days and 3.8 Hours
We report a case of metachronous multiple primary malignancies involving both rectum and liver with colonic metastasis from hepatocellular carcinoma (HCC) through hematogenous pathway. A 72-year-old woman was admitted to the emergency department with right upper abdominal pain for 4 h. Considering her surgical history of Mile’s procedure plus liver resection for rectal cancer with liver metastasis three years ago and the finding of urgent computed tomography scan on admission, the preoperative diagnosis was spontaneous rupture of rectal liver metastasis located in caudate lobe and colonic metastasis from rectal cancer. The patient underwent an emergency isolated caudate lobectomy at a hemorrhagic shock status. Pathology reported a primary HCC in the caudate lobe and colonic metastasis of HCC with tumor embolus in the surrounding vessels of the intestine. No regional lymph node involvement was found. It is hypothesized that HCC may disseminate hematogenously to the ascending colon, thus making it a rare case.
- Citation: Sun LH, Han HQ, Wang PZ, Tian WJ. Emergency caudate lobectomy for ruptured hepatocellular carcinoma with multiple primary cancers. World J Gastroenterol 2013; 19(3): 418-421
- URL: https://www.wjgnet.com/1007-9327/full/v19/i3/418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i3.418
The caudate lobe of the liver is located deep in the hepatic parenchyma and surrounded by some major vessels. Because of its surgically difficult-to- approach anatomic location and perception of early involvement of the inferior vena cava (IVC) or portal vein by tumors, caudate lobectomy was once used infrequently. With more precise anatomical knowledge, development of surgical technique, and improvement in perioperative care, it has become more common recently. But in order to succeed in the procedure, the optimal operative approach should be planned and determined carefully before operation. Emergency isolated caudate lobectomy was rarely reported worldwide, especially for the ruptured primary hepatocellular carcinoma (HCC). The incidence of colorectal cancers has increased in recent years. As the overall survival rate has improved due to the development of multiple therapies, it is not surprising to see more patients with multiple primary malignancies involving the colorectum and other organs. For colorectal cancer, stomach was the most common extracolonic site of both the synchronous and metachronous multiple primary malignancies, but liver is very rarely involved. Extrahepatic metastasis of HCC occurs occasionally and increases with prolonged survival. However, HCC with gastrointestinal (GI) tract metastasis is seldom reported, and it is only found in less than 6% of the cases in autopsy series. GI metastasis through hematogenous pathway is especially rare.
A 72-year-old woman was admitted with right upper abdominal pain. The pain started in the epigastrium. A few hours before admission, the pain disseminated to the upper right abdominal quadrant and right back region and became severe.
The patient had a previous history of rectal adenocarcinoma with liver metastasis. Three years earlier she underwent the Miles procedure and partial hepatectomy. Following the operation, she received combined chemotherapy with 5-fluorouracil, folinic acid and oxaliplatin. Six months ago, computerized tomography (CT) scan of the abdomen showed a 5 cm × 5 cm × 5 cm, diverticulum like, exophytic mass located in the ascending colon near the ileocecus, without lymph node enlargement. It was considered a metastasis of rectal cancer. The mass showed no obvious response to systemic adjuvant chemotherapy.
On admission, her temperature was 36.9 °C, pulse was 112 beats/min and blood pressure was 90/50 mmHg. The oxygen saturation was 95% while the patient was breathing ambient air. On physical examination, the patient was ill-looking, conscious with skin and conjunctival pallor. The abdomen was distended, and bowel sounds were absent. There was tenderness in the right upper abdominal quadrant, with guarding and rebound tenderness. Lungs and heart sounds were normal.
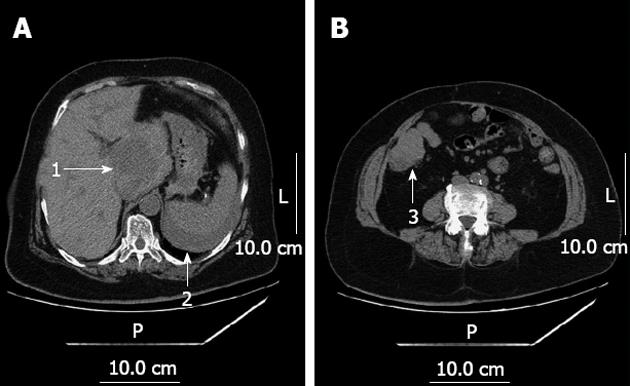
Her blood work-up on admission showed a hemoglobin level of 6.8 g/L, white cell count of 8.9 × 109/L and neutrophils of 72%. Coagulation tests, liver function, renal function and electrolytes were all within the normal range. Urgent CT scan of the abdomen showed multiple masses localized in the liver, a 6 cm × 6 cm × 6 cm mass in the caudate lobe of the liver (Couinaud’s segment 1), and signs of abdominal haemorrhage (Figure 1). The preoperative diagnoses were hemorrhagic shock, spontaneous rupture of rectal liver metastasis, colonic metastasis of rectal cancer or multiple primary cancers.
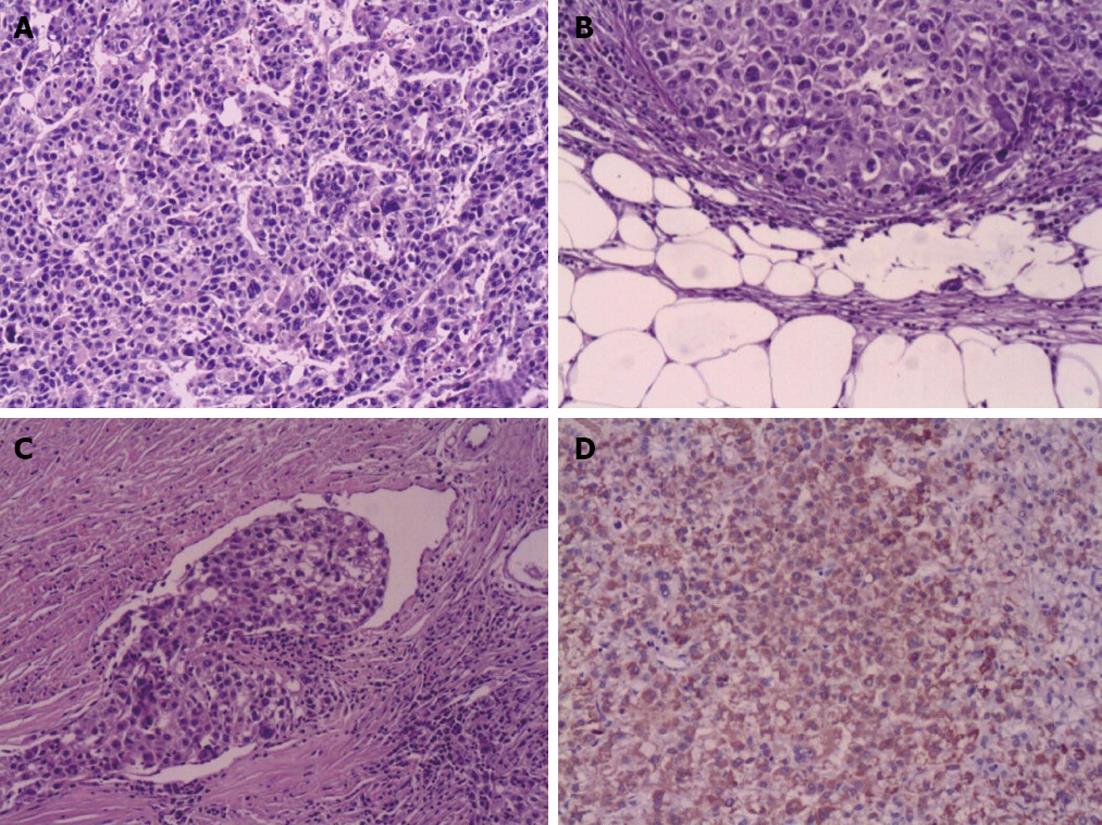
Hemodynamic stability was achieved following rehydration with intravenous fluid and multiple blood transfusions. At the same time, the patient was promptly sent to the operating room. Open exploratory laparotomy was performed via a J-shaped skin incision, with more than 2 liters of blood and clots and intra-abdominal adhesions due to the last operation visualized in the peritoneum. Inspection of the liver revealed a ruptured mass of 6 cm × 6 cm × 6 cm in the hepatic segment I with active bleeding. A 5 cm × 5 cm × 5 cm erosive tumor lesion was localized in the beginning of the ascending colon and nearly occupied the whole lumen. Isolated caudate lobectomy was performed via bilateral approach, in addition to the resection of ileocecum. The operation time was 290 min and blood loss was 2000 mL. Ten units of packed red blood cells and 2000 mL of fresh frozen plasma were transfused during the perioperative period. Histological examination demonstrated a primary HCC located in the caudate lobe and a colonic metastasis of HCC with tumor embolus in the surrounding vessels of the intestine (Figure 2). No regional lymph node metastasis was found. Immunohistochemical findings revealed that polyclonal alpha fetoprotein and hepatocyte antigen were positive and carcinombryonic antigen was negative. The patient made a full recovery from the surgery and was subsequently discharged 10 d after admission. Eight months later, the patient died of cachexia.
The caudate lobe of the liver is located deep in the hepatic parenchyma behind both major lobes and between the confluence of the three main hepatic veins, IVC, and hepatic hilum. It is generally divided into three parts, as described by Kumon: the left Spiegel lobe (Couinaud’s segment 1), the caudate process, and the paracaval portion (Couinaud’s segment 9). Because of its surgically difficult-to-approach anatomic location and perception of early involvement of the IVC or portal vein by tumors, caudate lobectomy was once called a forbidden zone of hepatic surgery and not performed frequently until it was first described by Lerut. In the early days, patients underwent resection of the caudate lobe along with removal of adjacent portions of the liver, i.e., right or left lobectomy, in order to get a better exposure. But for patients who concurrently had chronic liver disease, removal of too much normal hepatic parenchyma would delay recovery or even cause liver failure. With more precise anatomical knowledge, development of surgical technique, and improvement in perioperative care, isolated caudate lobectomy[1] has become common recently. Nevertheless, clinical assessment should be conducted strictly before operative period[2]. Laboratory investigations, including tests of complete blood count, coagulation, liver function, renal function, electrolytes, indocyanine green retention rate at 15 min, and virology, should be carried out to evaluate the patient’s surgical fitness; and radiological studies, including ultrasonography, CT scan, and three dimensional reconstructive CT, should be undertaken to evaluate the hepatic lobe size, extent and location of the tumor, vascular involvement, lymph node affection, and the severity of cirrhosis, and to rule out intra-abdominal metastasis. The most appropriate operative procedure should be planned and determined preoperatively[3]. Consequently, emergency isolated caudate lobectomy was rarely reported worldwide, especially for the ruptured primary HCC. For these patients, routine treatments included compression and packing hemostasis, transcatheter arterial chemoembolization (TACE), and partial hepatectomy. Because the caudate lobe requires the arterial blood supply from the two major lobes of the liver, TACE cannot achieve the goal of hemostasis. The necrotic tumor tissue and important vessels surrounding the caudate lobe also affect the compression and packing hemostasis. As a result, emergency isolated caudate lobectomy is considered to be the best choice for these patients. There are four approaches for the resection of the caudate lobe of the liver, left side approach, right side approach[4], bilateral approach, and anterior transhepatic approach by splitting parenchyma of the liver. The approach to the caudate lobe depends on the location of the tumor and the expertise of the surgeon. Hepatic surgery is a very bloody procedure, especially the caudate lobectomy, because of the unique anatomical relationship between the caudate lobe and IVC and the portal triad. Vascular control combined with positioning and central venous pressure control is recommended to minimize intraoperative blood loss. Full mobilization of liver is indicated before the dissection of the caudate lobe. Dividing the fibrous retrocaval ligament is extremely important in fully mobilizing the caudate lobe to expose the hepatic veins along the anterior surface of the IVC. Once the left lateral edge of the caudate lobe is free, ligation of the short hepatic veins draining directly into IVC is performed. Our patient underwent a partial hepatectomy three years ago for rectal liver metastasis resulting in complicated intra-abdominal adhesions, which further increased the difficulties of the operative procedure[5]. Likewise, the pathophysiological changes of hemorrhagic shock also make it more difficult to keep the stability of the haemodynamics of the patient.
Considering the history of rectal cancer three years ago in this case, the preoperative diagnosis was spontaneous rupture of rectal liver metastasis and colonic metastasis of rectal cancer. But histological examination demonstrated a primary HCC located in the caudate lobe and a colonic metastasis of HCC. Multiple primary cancers are relatively rare[6]. The overall incidence rate is between 0.73% and 11.7%. However, more multiple primary cancers have been encountered due to an increase in the number of elderly patients and advancement in diagnostic techniques. In addition, cancer patients are assumed to be at an increased risk of developing cancers in other organs due to genetic alterations or exposure to the same environmental carcinogens[7]. It is generally defined according to the criteria of Warren and Gates: (1) each tumor must be clearly malignant on histologic examination; (2) each tumor must be distinct; and (3) the possibility that the second tumor represents a metastasis must be excluded. Multiple primary cancers can be synchronous or metachronous depending on the interval between their diagnosis. Synchronous malignancies are secondary cancers occurring simultaneously or within 6 mo after the diagnosis of primary cancers, while metachronous malignancies are secondary cancers that developed more than 6 mo after the diagnosis of primary cancers. The incidence of colorectal cancers has increased in recent years. As the overall survival rate has improved due to the development of multiple therapies, it is not surprising to see more patients with multiple primary malignancies involving the colorectum and other organs[8]. For colorectal cancer, stomach is the most common extracolonic site of the synchronous multiple primary cancers. The thyroid gland is the second organ involved. The stomach is also the most common extracolonic site of the metachronous multiple primary malignancies, followed by the cervix, lung, and skin. The metachronous multiple primary malignancies involving both rectum and liver are very rare. Although the mechanisms underlying the development of multiple primary cancers are not fully understood, some factors such as sustaining environmental carcinogens, susceptibility to carcinogen, cancer cell immunodeficiency, and immunosuppression caused by radiotherapy and chemotherapy have been implicated. Under these circumstances, it is expected that as people live longer, they have more chances to develop multiple primary malignancies. The prognosis of the patients with multiple primary cancers can be predicted independently by the stage of each tumor.
HCC is one of the most common malignancies worldwide[9]. Extrahepatic metastasis of HCC occurs occasionally and increases with prolonged survival. The most frequent sites of metastasis are lungs, bones, regional lymph nodes, and adrenal glands. However, HCC with GI tract metastasis is very rare, being found in only less than 6% of cases in autopsy series[10]. The metastasis sites only reported in some case reports included duodenum, stomach, colon and jejunum. The major pathway of the metastasis is direct invasion to the contiguous GI tract via adhesion to the serosal side by a bulky tumor mass. However, the frequency of metastatic colon cancer arising from HCC is low. In this case, the solitary metastasis from HCC appeared in ileocecus, separated from other liver tissues. The mode of the metastasis to the colon is still unclear. It is hypothesized that HCC may disseminate hematogenously to the distant GI tracts. Tumor embolus in the vessels surrounding the intestine may be the key evidence of hematogenous metastasis. Absence of regional lymph node metastasis may indicate low possibility of lymphatic metastasis. The prognosis of GI metastasis from HCC is known to be poor with a median survival period of seven months.
In conclusion, we report a rare case of metachronous multiple primary malignancies involving both rectum and liver with colonic metastasis through hematogenous pathway. This patient was manifested by spontaneous rupture of HCC located in the caudate lobe, and underwent an emergency isolated caudate lobectomy, with a full recovery after surgery.
P- Reviewer Wong GLH S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Sakoda M, Ueno S, Kubo F, Hiwatashi K, Tateno T, Kurahara H, Mataki Y, Shinchi H, Natsugoe S. Surgery for hepatocellular carcinoma located in the caudate lobe. World J Surg. 2009;33:1922-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg. 2007;94:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, Nagorney DM, Belghiti J, Ng IO, Yamaoka Y. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005;140:450-457; discussion 457-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Chaib E, Ribeiro MA, Silva Fde S, Saad WA, Cecconello I. Caudate lobectomy: tumor location, topographic classification, and technique using right- and left-sided approaches to the liver. Am J Surg. 2008;196:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Ishizawa T, Hasegawa K, Ikeda M, Aoki T, Sano K, Imamura H, Kokudo N, Makuuchi M. Transhepatic approach for a small paracaval tumor in repeat resection. Dig Surg. 2007;24:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Ueno M, Muto T, Oya M, Ota H, Azekura K, Yamaguchi T. Multiple primary cancer: an experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int J Clin Oncol. 2003;8:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Yamamoto S, Yoshimura K, Ri S, Fujita S, Akasu T, Moriya Y. The risk of multiple primary malignancies with colorectal carcinoma. Dis Colon Rectum. 2006;49:S30-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Ahmed F, Goodman MT, Kosary C, Ruiz B, Wu XC, Chen VW, Correa CN. Excess risk of subsequent primary cancers among colorectal carcinoma survivors, 1975-2001. Cancer. 2006;107:1162-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Greten TF, Papendorf F, Bleck JS, Kirchhoff T, Wohlberedt T, Kubicka S, Klempnauer J, Galanski M, Manns MP. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer. 2005;92:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Nozaki Y, Kobayashi N, Shimamura T, Akiyama T, Inamori M, Iida H, Endo H, Fujita K, Yoneda M, Takahashi H. Colonic metastasis from hepatocellular carcinoma: manifested by gastrointestinal bleeding. Dig Dis Sci. 2008;53:3265-3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |