Published online Jan 21, 2013. doi: 10.3748/wjg.v19.i3.399
Revised: December 10, 2012
Accepted: December 20, 2012
Published online: January 21, 2013
Processing time: 130 Days and 20.9 Hours
AIM: To study the effects of Helicobacter pylori (H. pylori) tumor necrosis factor-α (TNF) inducing protein (Tip-α) on cytokine expression and its mechanism.
METHODS: We cloned Tip-α from the H. pylori strain 26695, transformed Escherichia coli with an expression plasmid, and then confirmed the expression product by Western blotting. Using different concentrations of Tip-α that affected SGC7901 and GES-1 cells at different times, we assessed cytokine levels using enzyme-linked immunosorbent assay. We blocked SGC7901 cells with pyrrolidine dithiocarbamate (PDTC), a specific inhibitor of nuclear factor κB (NF-κB). We then detected interleukin (IL)-1β and TNF-α levels in SGC7901 cells.
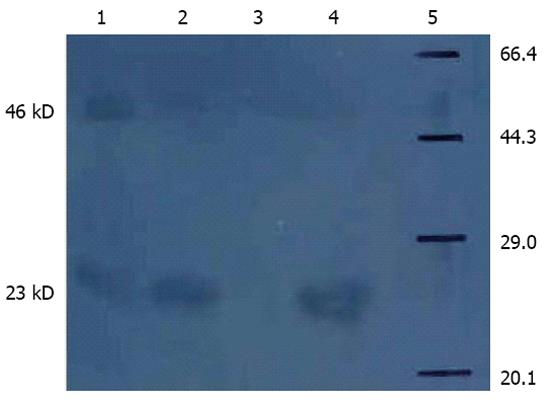
RESULTS: Western blot analysis using an anti-Tip-α antibody revealed a 23-kDa protein, which indicated that recombinant Tip-α protein was recombined successfully. The levels of IL-1β, IL-8 and TNF-α were significantly higher following Tip-α interference, whether GES-1 cells or SGC-7901 cells were used (P < 0.05). However, the levels of cytokines (including IL-1β, IL-8 and TNF-α) secreted by SGC-7901 cells were greater than those secreted by GES-1 cells following treatment with Tip-α at the same concentration and for the same duration (P < 0.05). After blocking NF-κB with PDTC, the cells (GES-1 cells and SGC-7901 cells) underwent interference with Tip-α. We found that IL-1β and TNF-α levels were significantly decreased compared to cells that only underwent Tip-α interference (P < 0.05).
CONCLUSION: Tip-α plays an important role in cytokine expression through NF-κB.
-
Citation: Tang CL, Hao B, Zhang GX, Shi RH, Cheng WF.
Helicobacter pylori tumor necrosis factor-α inducing protein promotes cytokine expressionvia nuclear factor-κB. World J Gastroenterol 2013; 19(3): 399-403 - URL: https://www.wjgnet.com/1007-9327/full/v19/i3/399.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i3.399
Infection with Helicobacter pylori (H. pylori) leads to chronic gastritis, peptic ulcer, and gastric lymphoma[1-3]. H. pylori has also been associated with gastric cancer[4], is classified as a class I carcinogen by the International Agency for Research on Cancer[5], and H. pylori exerts its pathogenesis by secreting toxins, including hemolysin, lipopolysaccharides, CagA and VacA[6-9]. CagA and VacA are the major virulence factors. Persistent infection by H. pylori enables these toxins to stimulate gastric epithelial cells to produce a large number of cytokines such as tumor necrosis factor (TNF-α) and interleukin 1, 6 and 8 (IL-1, IL-6 and IL-8), thus generating an inflammatory reaction[10-14]. Tumor necrosis factor-α inducing protein (Tip-α) is a new toxin discovered recently, and likely accelerates the inflammation and cancers caused by H. pylori[15]. However, its function and the mechanism underlying these effects remain unclear. The present work was conducted to determine the effects of recombinant Tip-α (rTip-α) on human gastric epithelial cells and gastric cancer cytokine expression, as well as explore the mechanisms involved.
H. pylori strain 26695 was obtained from the Shanghai Institute of Digestive Disease. The following reagents were used in this study: Dual Promoter TA Cloning® Kit pCR® II and pET28a vectors (Invitrogen); monoclonal rabbit anti-Tip-α antibody (Beijing Aviva Systems Biology); BamHI, Xho I and Prestained Protein Molecular Weight Markers (Fermentas); DNA and gel extraction kit from Tiangen Biotech (Beijing) Co. Ltd.; DNA marker (TaKaRa); His TrapTMH. pylori affinity chromatography column (GE Healthcare); and enhanced chemiluminescence kit (Pierce Protein Biology Products). The polymerase chain reaction primer sequences were 5’-TTGGATCCATGCTGCAGGCTTG-3’, which contained an Xho I restriction site, and 5’-GGCTCGAGCTACATGGCTATAG-3’, which contained a BamH I restriction site. The primers were synthesized by Invitrogen. The human gastric epithelial cell line GES-1 and gastric cancer SGC7901 cells were purchased from the Shanghai Cancer Institute. Enzyme-linked immunosorbent assay (ELISA) kits were obtained from MultiSciences Biotech (Shanghai) Co., Ltd., while pyrrolidine dithiocarbamate (PDTC) was purchased from Jingmei Biotech Co., Ltd.
Expression, purification, and identification of Tip-α: We cloned Tip-α from the genome of H. pylori strain 26695. The Tip-α gene and pET28a vector (His tag) were digested with BamH I and Xho I, purified, and then ligated together to generate the ET28a-Tip-α plasmid expressing recombinant Tip-α. This plasmid was transformed into Escherichia coli and the resultant protein was purified by Ni-NTA affinity chromatography and verified by Western blotting.
Cell recovery, culture, and passage: Cryopreserved GES-1 and SGC-7901 cells were centrifuged at 1000 rpm for 5 min. After removal of the supernatant, these cells were cultured in 60 mm × 60 mm dishes containing Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum.
IL-1β, IL-8 and TNF-αlevels at different times following interference by 12.5μg/mL rTip-αin GES-1 and SGC7901 cells: GES-1 and SGC7901 cells during their logarithmic growth phase underwent interference with 12.5 μg/mL rTip-α after starvation in serum-free medium for 24 h. The levels of IL-1β, IL-8 and TNF-α cytokines were then assessed at 0, 1, 2, 4 and 8 h post-interference using ELISA.
Levels of IL-1β, IL-8 and TNF-αin GES-1 and SGC7901 cells following incubation with different concentrations of rTip-α: We incubated GES-1 and SGC7901 cells with the following concentrations of rTip-α: 0, 12.5, 25 and 50 μg/mL. After 2 h, we examined the levels of IL-1β, IL-8 and TNF-α using ELISA.
Effects of rTip-αon IL-1βand TNF-αexpression after PDTC-mediated inhibition of NF-κB: Four groups consisting of the same number of GES-1 and SGC7901 cells were starved in serum-free medium for 24 h before undergoing different treatments. Group A was treated with 12.5 μg/mL rTip-α for 2 h. Group B was treated similarly after PDTC blocking of NF-κB for 4 h. Groups C and D were incubated with serum-free medium and dimethyl sulfoxide (the vehicle with which PDTC was diluted), respectively. ELISA was performed to detect the levels of IL-1β and TNF-α in each group.
Data are presented as the mean ± SD and analyzed using SPSS 17.0. The Student’s t test was used to compare two groups, while one-way analysis of variance was used to compare among several groups. A P value < 0.05 was considered statistically significant.
Western blotting analysis demonstrated that the Tip-α recombinant protein and anti-human Tip-α monoclonal antibody could be specifically bound; specific bands were found (Figure 1). Western blotting analysis by non-denaturing gel electrophoresis showed active dimer bands (46 kDa).
The levels of IL-1β, IL-8 and TNF-α were significantly higher after GES-1 and SGC7901 cells underwent interference by 12.5 μg/mL rTip-α for 1, 2, 4 and 8 h than those at 0 h. Cytokine secretion by GES-1 and SGC7901 cells peaked after rTip-α interference for 2 h, indicating no obvious dependence on time (Tables 1 and 2). However, after interference by rTip-α for 2 h, the levels of IL-8 (2.53 ± 0.50) and TNF-α (1.41 ± 0.10) in SGC7901 cells were significantly higher than those in GES-1 cells (0.84 ± 0.11 for IL-8 and 0.72 ± 0.08 for TNF-α). As shown in Table 1, the levels of IL-1β in GES-1 and SGC7901 cells (2.07 ± 0.10 and 2.07 ± 0.30, respectively) were not statistically different after rTip-α interference for 2 h.
| Groups | 0 h | 1 h | 2 h | 4 h | 8 h |
| GES-1 (IL-1β) | 0.34 + 0.04 | 0.88 + 0.09a | 2.07 + 0.30a | 1.35 + 0.20a | 1.41 + 0.15a |
| SGC-7901 (IL-1β) | 0.22 + 0.04 | 0.35 + 0.05 | 2.07 + 0.10a | 1.11 + 0.04a | 1.14 + 0.04a |
| GES-1 (IL-8) | 0.35 + 0.05 | 0.60 + 0.12a | 0.84 + 0.11a | 0.64 + 0.06a | 0.50 + 0.07a |
| SGC-7901 (IL-8) | 0.70 + 0.02 | 0.78 + 0.19 | 2.53 + 0.50a | 2.26 + 0.24a | 2.14 + 0.68a |
| GES-1 (TNF-α) | 0.39 + 0.06 | 0.39 + 0.07 | 0.72 + 0.08a | 0.53 + 0.03a | 0.51 + 0.14a |
| SGC-7901 (TNF-α) | 0.33 + 0.09 | 1.02 + 0.09a | 1.41 + 0.10a | 0.86 + 0.07a | 0.47 + 0.03a |
| Groups | 0 | 12.5μg/mL | 25μg/mL | 50μg/mL |
| GES-1 (IL-1β) | 0.59 + 0.11 | 2.07 + 0.30a | 2.20 + 0.09a | 1.23 + 0.13a |
| SGC-7901 (IL-1β) | 0.36 + 0.01 | 2.07 + 0.10a | 1.22 + 0.03a | 1.02 + 0.04a |
| GES-1 (IL-8) | 0.39 + 0.08 | 0.84 + 0.11a | 0.75 + 0.09a | 0.61 + 0.15a |
| SGC-7901 (IL-8) | 0.78 + 0.09 | 2.53 + 0.50a | 1.50 + 0.16a | 1.41 + 0.14a |
| GES-1 (TNF-α) | 0.30 + 0.06 | 0.72 + 0.08a | 0.54 + 0.13a | 0.63 + 0.10a |
| SGC-7901 (TNF-α) | 0.26 + 0.18 | 1.41 + 0.10a | 0.62 + 0.07a | 0.62 + 0.02a |
The levels of IL-1β, IL-8 and TNF-α were significantly higher than those in the blank control in GES-1 and SGC7901 cells after rTip-α interference for 2 h. Cytokine secretion of GES-1 and SGC7901 cells peaked at 12.5 μg/mL, suggesting that this effect was not concentration-dependent (Table 2).
The levels of IL-1β and TNF-α in SGC7901 cells in Group B (treated with PDTC + rTip-α) were higher than those in Groups C and D (no rTip-α and PDTC), but markedly lower than those in Group A (only treated with rTip-α). As shown in Table 3, these differences were statistically significant (P < 0.05, F = 40.15).
Tip-α is a novel gene that was discovered recently in H. pylori. Located in the H. pylori 0596 open reading frame of the H. pylori 26695 strain, Tip-α is also called H. pylori 0596 protein. Its open reading frame is 519 bp in length and constitutes 173 amino acids. Tip-α has a molecular weight of 19 kDa and can form active homodimers with a molecular weight of 38 kDa through disulfide bonding[15]. Recent studies have found that Tip-α is associated with the adsorption and colonization of H. pylori in gastric mucosa[16]. Some studies have shown that the structure of Tip-α is different from penicillin binding proteins. Tip-α is composed of three closely linked domains that interact with other proteins and nucleic acids. In particular, the homodimer formed by cysteine C25 and C27 is essential for Tip-α to serve its role in the gastric mucosal acidic environment[17]. Detected by gene chip technology, expression of the chemokines Cc 12 Cc17, Cc120, Cx11 and Cx15 was enhanced after Tip-α treatment in gastric cancer cells and gastric epithelial cells[18]. These chemokines can induce chemotaxis of immune cells to local sites of infection, resulting in immune regulation and pathology, and ultimately the inflammatory response[19].
Because our pET28a-Tip-α vector also expresses a 3.74 kDa His tag, the recombinant Tip-α protein we produced possesses a molecular weight of about 23 kDa and can form active homodimers with a molecular weight of 46 kDa through disulfide bonding. Our work indicates that after affecting gastric epithelial and cancer cells, rTip-α can promote the expression of IL-1β, IL-8 and TNF-α. These cytokines are important in promoting the inflammatory response, thus linking Tip-α to inflammation and the occurrence of H. pylori-related gastrointestinal diseases. After incubating the cells for 2 h with rTip-α, the levels of cytokine expression peaked at 12.5 μg/mL, which was the best concentration for interference. The levels of cytokine expression induced by rTip-α were not time- or concentration-dependent. These results suggest that Tip-α affects the host by inducing toxin secretion into the exterior environment of the bacteria through the type II secretion system[20]. The toxins then enter host cells via receptor-mediated endocytosis instead of through injection via the IV secretion system[21-23]. Studies have shown that Tip-α possesses DNA binding activity. In particular, DNA affecting gastric mucosal cells can combine with some transcription factors to promote IL-1β, IL-8 and TNF-α expression, leading to the occurrence and development of H. pylori-related gastrointestinal diseases[24].
PDTC is a specific NF-κB inhibitor that works by blocking degradation of the NF-κB p65 subunit or IκB, thereby reducing NF-κB nuclear translocation[25]. Our data demonstrated no significant increase in IL-1β and TNF-α levels after pretreatment of SGC7901 cells with PDTC followed by rTip-α interference. This finding suggests that the promotion of cytokine expression by Tip-α may be regulated by NF-κB. However, further study is required to determine the underlying mechanism.
In addition, we found that when gastric epithelial and cancer cells were treated with the same concentration of Tip-α for the same duration, the level of cytokine expression in gastric cancer SGC7901 cells was significantly higher than that in gastric epithelial GES-1 cells. This difference may be due to differential effects of rTip-α on NF-κB expression in the cell types or may be related to variations in the DNA binding activity of Tip-α in the cells. Some studies suggest that the increased cytokine expression promoted by Tip-α may hasten the invasion and metastasis of gastric cancer[26]. Overall, Tip-α can activate cytokine expression in an NF-κB-dependent manner. Tip-α plays a major role in the pathogenesis of H. pylori, however, its mechanism requires further investigation.
Helicobacter pylori (H. pylori) exerts its pathogenesis by secreting toxins. Tumor necrosis factor-α (TNF-α) inducing protein (Tip-α) is a new toxin discovered recently, however, its function and mechanism of pathogenesis remain unclear.
The pathogenesis of H. pylori is partially clear, as H. pylori may secrete many types of toxins. With the exception of CagA and VacA, new H. pylori toxins have been discovered, such as Tip-α, mammalian, prokaryotic high-temperature requirement A, and the duodenal ulcer-promoting gene. Only the function of the toxins and their mechanism of pathogenesis are clear, as the mechanism of H. pylori pathogenesis is known.
Other studies discovered that Tip-α promoted the expression of cytokines, however, this article first showed the difference in the promotion of the expression of cytokines between gastric epithelial cells and cancer cells, and that Tip-α activates cytokine expression in a nuclear factor κB (NF-κB)-dependent manner.
Tip-α may become a marker of H. pylori virulence. The virulence of H. pylori may be determined by detecting Tip-α.
Tip-α is the abbreviation for tumor necrosis factor-α inducing protein. It was first discovered that this new H. pylori toxin can promote the expression of TNF-α, therefore, it was called Tip-α.
The authors certified that Tip-α-a new toxin of H. pylori, promoted the expression of cytokine, discovered the difference of this function between gastric epithelial cells and cancer cells, and in an NF-κB-dependent manner, further interpreted the mechanism of pathogenesis of H. pylori.
P- Reviewer Fukuhara K S- Editor Song XX L- Editor Webster JR E- Editor Li JY
| 1. | Nomura A, Stemmermann GN, Chyou PH, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120:977-981. [PubMed] |
| 2. | Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1997;11 Suppl 1:71-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 3. | Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1229] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 4. | Johansson H. Qualification conditions: invest more firmly in continuing education. Vardfacket. 1978;2:28-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Møller H, Heseltine E, Vainio H. Working group report on schistosomes, liver flukes and Helicobacter pylori. Int J Cancer. 1995;60:587-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Segal ED, Tompkins LS. Identification and characterization of a Helicobacter pylori hemolysin. Infect Agents Dis. 1993;2:178-182. [PubMed] |
| 7. | Slomiany BL, Piotrowski J, Sengupta S, Slomiany A. Inhibition of gastric mucosal laminin receptor by Helicobacter pylori lipopolysaccharide. Biochem Biophys Res Commun. 1991;175:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Leunk RD, Johnson PT, David BC, Kraft WG, Morgan DR. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 437] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648-14653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1375] [Cited by in RCA: 1393] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 10. | Crabtree JE, Court M, Aboshkiwa MA, Jeremy AH, Dixon MF, Robinson PA. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J Pathol. 2004;202:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Fan XJ, Chua A, O’Connell MA, Kelleher D, Keeling PW. Interferon-gamma and tumour necrosis factor production in patients with Helicobacter pylori infection. Ir J Med Sci. 1993;162:408-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Huang J, O’Toole PW, Doig P, Trust TJ. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732-1738. [PubMed] |
| 13. | Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 350] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Gionchetti P, Vaira D, Campieri M, Holton J, Menegatti M, Belluzzi A, Bertinelli E, Ferretti M, Brignola C, Miglioli M. Enhanced mucosal interleukin-6 and -8 in Helicobacter pylori-positive dyspeptic patients. Am J Gastroenterol. 1994;89:883-887. [PubMed] |
| 15. | Suganuma M, Kurusu M, Suzuki K, Nishizono A, Murakami K, Fujioka T, Fujiki H. New tumor necrosis factor-alpha-inducing protein released from Helicobacter pylori for gastric cancer progression. J Cancer Res Clin Oncol. 2005;131:305-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Godlewska R, Pawlowski M, Dzwonek A, Mikula M, Ostrowski J, Drela N, Jagusztyn-Krynicka EK. Tip-alpha (hp0596 gene product) is a highly immunogenic Helicobacter pylori protein involved in colonization of mouse gastric mucosa. Curr Microbiol. 2008;56:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Tosi T, Cioci G, Jouravleva K, Dian C, Terradot L. Structures of the tumor necrosis factor a inducing protein Tipa: A novel virulence factor from Helicobacter pylori. FEBS Letters. 2009;583:1581-1585. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Kuzuhara T, Suganuma M, Kurusu M, Fujiki H. Helicobacter pylori-secreting protein Tipalpha is a potent inducer of chemokine gene expressions in stomach cancer cells. J Cancer Res Clin Oncol. 2007;133:287-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1803] [Cited by in RCA: 1792] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 20. | Massari P, Manetti R, Burroni D, Nuti S, Norais N, Rappuoli R, Telford JL. Binding of the Helicobacter pylori vacuolating cytotoxin to target cells. Infect Immun. 1998;66:3981-3984. [PubMed] |
| 21. | Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 967] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 22. | Suganuma M, Yamaguchi K, Ono Y, Matsumoto H, Hayashi T, Ogawa T, Imai K, Kuzuhara T, Nishizono A, Fujiki H. TNF-alpha-inducing protein, a carcinogenic factor secreted from H. pylori, enters gastric cancer cells. Int J Cancer. 2008;123:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Watanabe T, Tsuge H, Imagawa T, Kise D, Hirano K, Beppu M, Takahashi A, Yamaguchi K, Fujiki H, Suganuma M. Nucleolin as cell surface receptor for tumor necrosis factor-alpha inducing protein: a carcinogenic factor of Helicobacter pylori. J Cancer Res Clin Oncol. 2010;136:911-921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Kuzuhara T, Suganuma M, Oka K, Fujiki H. DNA-binding activity of TNF-alpha inducing protein from Helicobacter pylori. Biochem Biophys Res Commun. 2007;362:805-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1186] [Cited by in RCA: 1228] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 25. | Schreck R, Meier B, Männel DN, Dröge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181-1194. [PubMed] |
| 26. | Suganuma M, Kuzuhara T, Yamaguchi K, Fujiki H. Carcinogenic role of tumor necrosis factor-alpha inducing protein of Helicobacter pylori in human stomach. J Biochem Mol Biol. 2006;39:1-8. [PubMed] |