Published online Jan 21, 2013. doi: 10.3748/wjg.v19.i3.375
Revised: September 24, 2012
Accepted: September 29, 2012
Published online: January 21, 2013
AIM: To compare the outcomes of concomitant cholangiocarcinoma (C-CCA) and subsequent cholangiocarcinoma (S-CCA) associated with hepatolithiasis.
METHODS: From December 1987 to December 2007, 276 patients underwent hepatic resection for hepatolithiasis in Changhua Christian Hospital. Sixty-five patients were excluded due to incomplete medical records and the remaining 211 patients constituted our study population base. Ten patients were diagnosed with C-CCA based on the preoperative biopsy or postoperative pathology. During the follow-up period, 12 patients developed S-CCA. The diagnosis of S-CCA was made by image-guided biopsy or by pathology if surgical intervention was carried out. Patient charts were reviewed to collect clinical information. Parameters such as CCA incidence, interval from operation to CCA diagnosis, interval from CCA diagnosis to disease-related death, follow-up time, and mortality rate were calculated for both the C-CCA and S-CCA groups. The outcomes of the C-CCA and S-CCA groups were mathematically compared and analysed.
RESULTS: Our study demonstrates the clinical implications and the survival outcomes of C-CCA and S-CCA. Among the patients with unilateral hepatolithiasis, the incidence rates of C-CCA and S-CCA were fairly similar (4.8% vs 4.5%, respectively, P = 0.906). However, for the patients with bilateral hepatolithiasis, the incidence rate of S-CCA (12.2%) was higher than that of C-CCA (4.7%), although the sample size was limited and the difference between two groups was not statistically significant (P = 0.211). The average follow-up time was 56 mo for the C-CCA group and 71 mo for the S-CCA group. Regard to the average time intervals from operation to CCA diagnosis, S-CCA was diagnosed after 67 mo from the initial hepatectomy. The average time intervals from the diagnoses of CCA to disease-related death was 41 mo for the C-CCA group and 4 mo for the S-CCA group, this difference approached statistical significance (P = 0.075). Regarding the rates of overall and disease-related mortality, the C-CCA group had significantly lower overall mortality (70% vs 100%, P = 0.041) and disease-related mortality (60% vs 100%, P = 0.015) than the S-CCA group. For the survival outcomes of two groups, the Kaplan-Meier curves corresponding to each group also demonstrated better survival outcomes for the C-CCA group (log rank P = 0.005). In the C-CCA group, three patients were still alive at the time of data analysis, all of them had free surgical margins and did not have pathologically proven lymph node metastasis at the time of the initial hepatectomy. In the S-CCA group, only one patient had chance to undergo a second hepatectomy, and all 12 S-CCA patients had died at the time of data analysis.
CONCLUSION: C-CCA has better outcomes than S-CCA. The first hepatectomy is crucial because most patients with recurrent CCA or S-CCA are not eligible for repeated surgical intervention.
- Citation: Lin CC, Lin PY, Chen YL. Comparison of concomitant and subsequent cholangiocarcinomas associated with hepatolithiasis: Clinical implications. World J Gastroenterol 2013; 19(3): 375-380
- URL: https://www.wjgnet.com/1007-9327/full/v19/i3/375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i3.375
Hepatolithiasis is the presence of stones in the intrahepatic duct (IHD) proximal to the confluence of the right and left main hepatic ducts. This disease is endemic in Southeast Asia and is also referred to as “IHD stones”, “recurrent pyogenic cholangitis”, “oriental cholangiohepatitis” and “Hong Kong disease” in the literature[1-3]. Since Sanes and MacCallum pointed out the possible association between hepatolithiasis and cholangiocarcinoma (CCA) in 1942, numerous studies have reported similar observations and formulated theories about CCA pathogenesis[4-7].
Currently, hepatic resection is frequently used for the definitive treatment of hepatolithiasis. Among patients who undergo hepatectomy for hepatolithiasis, approximately 5%-10% are incidentally found to have concomitant cholangiocarcinoma (C-CCA)[8-10]. On the other hand, subsequent cholangiocarcinoma (S-CCA) may appear mo to years after the initial hepatectomy. To the best of our knowledge, no previous study has reported on the clinical course and outcomes of S-CCA. During our daily practice, we observed that many patients with S-CCA progressed to death rather quickly. We wondered if patients with S-CCA really have worse outcomes than those with C-CCA. If this premise is true, it would be reasonable to treat hepatolithiasis more aggressively to minimize the deleterious consequences of S-CCA.
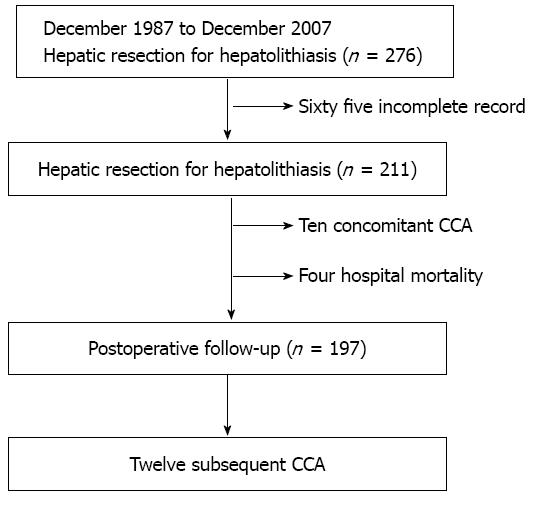
From December 1987 to December 2007, a total of 276 patients underwent hepatic resection for hepatolithiasis in Changhua Christian Hospital. After excluding 65 patients with incomplete medical records, the remaining 211 patients constituted the base of our study. For each case, the diagnosis of hepatolithiasis was made by the means of image studies preoperatively (liver ultra-sonography, computed tomography, endoscopic retrograde cholangiopancreatography, or magnetic resonance cholangiopancreatography), which was then confirmed by postoperative pathologic examination. The C-CCA diagnosis was based on the preoperative biopsy or the postoperative pathologic examination. The S-CCA diagnosis was made by image-guided aspiration cytology, or by pathology if surgical intervention was carried out. By this assessment, 10 patients were diagnosed with C-CCA. During the follow-up period, 12 patients developed S-CCA (Figure 1).
Patient charts were reviewed to collect clinical information. Parameters such as CCA incidence, interval from operation to CCA diagnosis, interval from CCA diagnosis to disease-related death, follow-up time, and mortality were calculated for both the C-CCA group and the S-CCA group. Finally, a mathematical comparison between the two groups was performed.
All data were recorded in a computerized database. Continuous variables are expressed as mean and range. A non-parametric test was used to examine the differences between the two groups. The Mann-Whitney U test was used for comparing continuous variables. Categorical variables were tested by the Person χ2 test. Kaplan-Meier analysis with log rank test was used to compare survival between the two groups. P values less than 0.05 were considered statistically significant. The statistical analysis was performed with SPSS version 16.0 (Statistical Package for the Social Sciences; SPSS, Inc., Chicago, IL).
Patient clinical characteristics details are shown in Table 1. Among the patients with unilateral hepatolithiasis, the incidences of C-CCA and S-CCA were similar (4.8% vs 4.5%, respectively; P = 0.906). However, when we looked at bilateral hepatolithiasis, the C-CCA incidence (4.7%) was comparable with that for unilateral hepatolithiasis, but 12.2% of patients with bilateral hepatolithiasis developed S-CCA. However, the case number was too small to allow determination of a statistically significant difference (P = 0.211).
| C-CCA group | S-CCA group | P value | |
| Incidence (%) | 10/211 (4.7) | 12/197 (6.1) | 0.546 |
| Unilateral stones | 8/168 (4.8) | 7/156 (4.5) | 0.906 |
| Bilateral stones | 2/43 (4.7) | 5/41 (12.2) | 0.211 |
| Age, yr (range) | 61 (39-82) | 59 (46-69) | 0.742 |
| Gender (%) | |||
| Male | 1 (10) | 1 (8.3) | 0.892 |
| Female | 9 (90) | 11 (91.7) | |
| Mean interval from operation to CCA, mo (range) | - | 67 (7-138) | - |
| Mean interval from CCA to disease-related death, mo (range) | 41 (3-107) | 4 (0-13) | 0.075 |
| Follow-up time, mo (range) | 56 (2-140) | 71 (7-144) | 0.291 |
| Mortality, n (%) | |||
| Overall | 7 (70) | 12 (100) | 0.041 |
| Disease-related | 6 (60) | 12 (100) | 0.015 |
The average ages for the C-CCA group and the S-CCA group were 61 and 59 years old, respectively (P = 0.742). In both groups, the gender distribution were similar, and only approximately 10% of the patients were male (P = 0.892). After the patients were diagnosed with C-CCA, the average life span until disease-related death was 41 mo. On average, S-CCA is diagnosed after 67 mo from the initial hepatectomy. However, after the diagnosis is established, the average life span in these patients was only 4 mo. This difference between intervals from CCA to disease-related death approached statistical significance (P = 0.075).
The clinical outcomes of the C-CCA patients are summarized in Table 2. At the time of data analysis, 70% (7/10) of the C-CCA patients had died. Two of them (cases 89 and 142) had regional lymph node metastasis at the time of hepatectomy. Three C-CCA patients (cases 119, 212 and 217) had positive surgical margins reported by pathology. One patient (case 227) had a free surgical margin and negative regional lymph nodes; however, she suffered from CCA recurrence at the common bile duct 4 years following the initial hepatectomy. After excision of the common bile duct (free surgical margin), she lived for 5 more years. One patient (case 128) had a free surgical margin and negative regional lymph nodes. However, a hypopharyngeal tumor was diagnosed 3 mo after the initial hepatectomy, and the patient finally expired due to acute respiratory failure. After excluding this last patient, the disease-related mortality was only 60% (6/10). The three patients (cases 90, 253 and 143) who were still alive share some common characteristics. They all had free surgical margins and did not have pathologically-proven lymph node metastasis at the time of the initial hepatectomy. Patients 90 and 253 had been completely disease-free for 140 and 49 mo, respectively. Patient 143 was a victim of bilateral hepatolithiasis, and recurrent IHD stones were detected during the follow-up period. However, she refused treatment until recurrent CCA at the common bile duct was found 5 years following the initial hepatectomy. After excising the common bile duct (free surgical margin), she was disease-free for more than 43 mo. The C-CCA diagnosis was made pre-operatively in one patient (case 142), intra-operatively in four patients (cases 89, 128, 217 and 253), and post-operatively in five patients (cases 90, 119, 143, 212 and 227).
| Case | Age/sex | Stone location | Procedure | Residual/recurrent stones | Margin | pTNM | Interval from CCA to death (mo) |
| 89 | 50/F | R | S5/6/7 | -/- | Free | T1 N1 M0 | 3 |
| 90 | 68/F | L | LH | -/- | Free | T1 Nx M0 | Alive (140) |
| 119 | 82/F | L | LL | -/- | Atypical mucinous epithelium | T1 Nx M0 | 73 |
| 128 | 60/M | L | LH | -/- | Free | T1 N0 M0 | 3 (hypopharyngeal tumor) |
| 142 | 39/F | L | LL | -/- | Free | T4 N1 M0 | 3 |
| 212 | 76/F | L | S2 | -/- | Inadequate | T1 Nx M0 | 56 |
| 227 | 61/F | L | LH + RA | -/+ | Free | T1 N0 M0 | 107 (re-operated 4 years later due to CCA recurrence at CBD) |
| 253 | 72/F | L | LH | -/- | Free | T1 N0 M0 | Alive (49) |
| 143 | 50/F | BI | LH + S5 | -/+ | Free | T1 Nx M0 | Alive (119) (re-operated 5 years later due to CCA recurrence at CBD) |
| 217 | 56/F | BI | RH | +/- | Inadequate | T2a Nx M0 | 5 |
The clinical outcomes of S-CCA patients are summarized in Table 3. All 12 of the patients with S-CCA had died at the time of data analysis, resulting in a 100% overall, and disease-related, mortality. Among them, only one patient (case 11) had undergone a second hepatectomy. However, the surgical margin was positive for cancer and the patient expired 4 mo later.
| Case | Age/sex | Stone location | Procedure | Residual/recurrent stones | Interval from hepatectomy to S-CCA (mo) | Second hepatectomy for S-CCA | Interval S-CCA to death (mo) |
| 1 | 56/F | L | LH | - / - | 87 | No | < 1 |
| 11 | 59/F | R | S5/6 | - / - | 91 | Yes | 4 |
| (with involved surgical margin) | |||||||
| 66 | 65/F | R | RH | - / + | 114 | No | 6 |
| 69 | 61/F | R | RP | - / + | 67 | No | 3 |
| 137 | 69/F | L | LL | - / - | 95 | No | 2 |
| 203 | 60/F | L | LH | - / - | 47 | No | 13 |
| 236 | 63/F | L | LL | - / - | 7 | No | < 1 |
| 37 | 46/F | BI | LL | - / - | 138 | No | 6 |
| 96 | 65/F | BI | LL + S6 | - / - | 115 | No | 2 |
| 157 | 53/F | BI | LL + S6 | - / - | 19 | No | 4 |
| 159 | 63/F | BI | LL | + / - | 19 | No | 6 |
| 163 | 50/M | BI | LH | + / - | 10 | No | 2 |
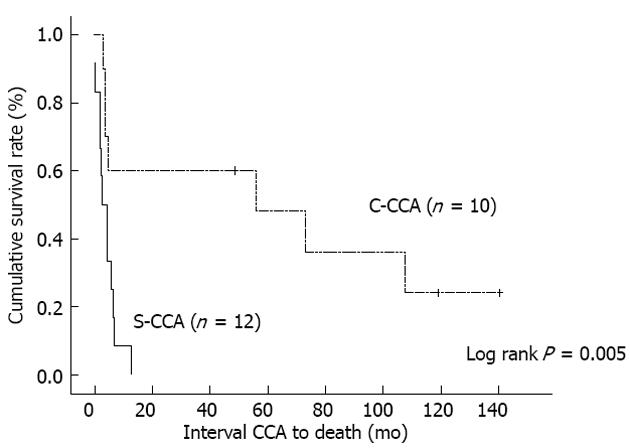
Figure 2 shows the Kaplan-Meier curves corresponding to each group. The C-CCA group demonstrated clearly better survival outcomes than the S-CCA group (P = 0.005, log rank).
The S-CCA incidence in unilateral hepatolithiasis is 4.5%, which is comparable to that of C-CCA (4.8%). However, the S-CCA incidence in bilateral hepatolithiasis (12.2%) is more than double that of C-CCA incidence (4.7%). Our case number was too small for this difference to be reflected in statistical significance (P = 0.211). We believe, however, that this is quite possibly a true biological difference. This difference can be explained by recent studies that showed that hepatectomy for unilateral hepatolithiasis is associated with high stone clearance rates and low stone recurrence rates[8,11-13]. However, with bilateral hepatolithiasis, there are still very few reports in the literature discussing the best management and long-term outcomes[14]. According to a recent report and our unpublished data, treating bilateral hepatolithiasis with hepatic resection did not show encouraging results as did resection in unilateral disease[15]. We speculate that hepatic resection seems to be less effective in stopping the disease progression toward S-CCA in patients with bilateral hepatolithiasis.
This study showed clearly that the C-CCA group had lower overall mortality (P = 0.041) and disease-related mortality (P = 0.015) than the S-CCA group. This better survival outcome is demonstrated by the Kaplan-Meier curves (Figure 2, P = 0.005, log rank). Considering that the complete removal of the malignancy with a free surgical margin is essential for achieving good survival outcomes[16-18], the role of the first hepatectomy is definitely crucial. Paradoxically, preoperative C-CCA diagnosis is sometimes difficult due to the presence of IHD stones[19-21]. Among our 10 C-CCA patients, only one patient was diagnosed pre-operatively. In order to solve this problem, making good use of intraoperatively-collected frozen sections is recommended. In the case of a strongly suspected C-CCA in the absence of conclusive tissue proof, carrying out the hepatectomy aggressively can increase the likelihood of creating a safe surgical margin.
For the five patients (cases 90, 119, 143, 212 and 227) whose C-CCA diagnoses were made incidentally by post-operative pathology, the survival outcomes were good if they had safe surgical margins (cases 90, 143 and 227). The lymph node status is also an important prognostic factor for these patients; however, these data were incomplete because regional lymph node dissection was not routinely performed on these patients.
All 12 S-CCA patients died shortly after definitive S-CCA diagnosis. A possible explanation is that most patients at the time of S-CCA diagnosis are not eligible for a second hepatectomy due to distant metastasis, locally advanced disease, peritoneal seeding, or insufficient remaining liver volume. Only one S-CCA patient in our series (case 11) had the opportunity to undergo a second hepatectomy. However, the tumor could not be removed completely, and the patient died 4 mo after the operation.
The interval from the initial hepatectomy to S-CCA development ranged from 7 to 138 mo (average 67 mo). After hepatic resection for hepatolithiasis, we observed that in patients who presented residual or recurrent IHD stones, the S-CCA incidence (11.8%, 4/34) was higher than in S-CCA patients who had achieved complete stone clearance and disease-free status after hepatectomy (4.9%, 8/163). However, 67% of S-CCA patients (8/12) had neither residual nor recurrent IHD stones. We can presume that undiagnosed CCA might be left in the liver remnant, resulting in S-CCA, especially for patients who developed S-CCA relatively soon after the initial hepatectomy (e.g., cases 157, 159, 163 and 236). Interestingly, however, one patient (case 37) had neither residual nor recurrent IHD stones but ultimately developed S-CCA after a follow-up period of 138 mo. Beyond the presence of residual and recurrent IHD stones, we should also consider other predisposing factors for the development of S-CCA such as cancer-related genes, female gender, choledochoenterostomy, liver atrophy, smoking, family history, and viral hepatitis infection[22-26].
This retrospective study is the first published investigation that subdivided and compared hepatolithiasis-related cholangiocarcinoma in concomitant and subsequent groups. The study design included patients who underwent hepatic resection for hepatolithiasis during the past two decades (1987 to 2007) in Changhua Christian Hospital. Unexpectedly, incomplete information in 65 patient medical records was noticed during data collection, forcing us to exclude these patients. The majority of the excluded patients were treated in the first decade (1987 to 1997). However, all CCA patients in our study were operated on after 1997. We believe that these missing records should cause minimal effect on our results, although the data collected in our study were skewed towards the second decade (1997 to 2007). Another limitation of this study could be the relatively small number of enrolled CCA patients. However, considering that the C-CCA incidence is only 5%-10% in our patient population, the difficulty in obtaining very large patient numbers is appreciable.
We want to reemphasize the importance of a successful first hepatectomy, because most patients with recurrent CCA or that develop S-CCA are not candidates for repeated surgical intervention.
Hepatolithiasis is the presence of stones in the intrahepatic duct (IHD) proximal to the confluence of the right and left main hepatic ducts. This disease is endemic in Southeast Asia and is also referred to as “IHD stones”, “recurrent pyogenic cholangitis”, “oriental cholangiohepatitis” and “Hong Kong disease” in the literature.
Concomitant cholangiocarcinoma (C-CCA) has better outcomes than subsequent cholangiocarcinoma (S-CCA). The first hepatectomy is crucial because most patients with recurrent CCA or S-CCA are not eligible for repeated surgical intervention.
Parameters such as cholangiocarcinoma (CCA) incidence, interval from operation to CCA diagnosis, interval from CCA diagnosis to disease-related death, follow-up time, and mortality rate were calculated for both the C-CCA and S-CCA groups. The outcomes of the C-CCA and S-CCA groups were mathematically compared and analysed.
C-CCAs associated to hepatolithiasis is a crucial problem in hepatobiliary medicine. The authors can heal well unilateral hepatolithiasis today, but there are not effective methods in a treatment of bilateral hepatolithiasis. S-CCAs associated to hepatolithiasis is a larger problem than unilateral hepatolithiasis. The long-term outcomes are bed. The authors show the average life span only 4 mo. Literature’s data are similar. The subject is very interesting, rarely discussed in the literature, and the period of observation and follow-up are commendable. Manuscript is well written. Data table are well-documented. Refferences is appropriately selected.
P- Reviewers O'Dwyer P, Smigielski JA S- Editor Gou SX L- Editor A E- Editor Lu YJ
| 1. | Cook J, Hou PC, Ho HC, Mcfadzean AJ. Recurrent pyogenic cholangeitis. Br J Surg. 1954;42:188-203. [PubMed] |
| 3. | Mage S, Morel AS. Surgical experience with cholangio-hepatitis (hong kong disease) in canton chinese. Ann Surg. 1965;162:187-190. [PubMed] |
| 4. | Sanes S, Maccallum JD. Primary Carcinoma of the Liver: Cholangioma in Hepatolithiasis. Am J Pathol. 1942;18:675-687. [PubMed] |
| 5. | Koga A, Ichimiya H, Yamaguchi K, Miyazaki K, Nakayama F. Hepatolithiasis associated with cholangiocarcinoma. Possible etiologic significance. Cancer. 1985;55:2826-2829. [PubMed] |
| 6. | Nakanuma Y, Terada T, Tanaka Y, Ohta G. Are hepatolithiasis and cholangiocarcinoma aetiologically related? A morphological study of 12 cases of hepatolithiasis associated with cholangiocarcinoma. Virchows Arch A Pathol Anat Histopathol. 1985;406:45-58. [PubMed] |
| 7. | Falchuk KR, Lesser PB, Galdabini JJ, Isselbacher KJ. Cholangiocarcinoma as related to chronic intrahepatic cholangitis and hepatolithiasis. Case report and review of the literature. Am J Gastroenterol. 1976;66:57-61. [PubMed] |
| 8. | Chen DW, Tung-Ping Poon R, Liu CL, Fan ST, Wong J. Immediate and long-term outcomes of hepatectomy for hepatolithiasis. Surgery. 2004;135:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Chen MF, Jan YY, Wang CS, Hwang TL, Jeng LB, Chen SC, Chen TJ. A reappraisal of cholangiocarcinoma in patient with hepatolithiasis. Cancer. 1993;71:2461-2465. [PubMed] |
| 10. | Ohta G, Nakanuma Y, Terada T. Pathology of hepatolithiasis: cholangitis and cholangiocarcinoma. Prog Clin Biol Res. 1984;152:91-113. [PubMed] |
| 11. | Jan YY, Chen MF, Wang CS, Jeng LB, Hwang TL, Chen SC. Surgical treatment of hepatolithiasis: long-term results. Surgery. 1996;120:509-514. [PubMed] |
| 12. | Uchiyama K, Kawai M, Ueno M, Ozawa S, Tani M, Yamaue H. Reducing residual and recurrent stones by hepatectomy for hepatolithiasis. J Gastrointest Surg. 2007;11:626-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Lee KF, Chong CN, Ng D, Cheung YS, Ng W, Wong J, Lai P. Outcome of surgical treatment for recurrent pyogenic cholangitis: a single-centre study. HPB (Oxford). 2009;11:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Yang T, Lau WY, Lai EC, Yang LQ, Zhang J, Yang GS, Lu JH, Wu MC. Hepatectomy for bilateral primary hepatolithiasis: a cohort study. Ann Surg. 2010;251:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Li SQ, Liang LJ, Peng BG, Hua YP, Lv MD, Fu SJ, Chen D. Outcomes of liver resection for intrahepatic stones: a comparative study of unilateral versus bilateral disease. Ann Surg. 2012;255:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Lee CC, Wu CY, Chen GH. What is the impact of coexistence of hepatolithiasis on cholangiocarcinoma? J Gastroenterol Hepatol. 2002;17:1015-1020. [PubMed] |
| 17. | Li HY, Zhou SJ, Li M, Xiong D, Singh A, Guo QX, Liu CA, Gong JP. Diagnosis and cure experience of hepatolithiasis-associated intrahepatic cholangiocarcinoma in 66 patients. Asian Pac J Cancer Prev. 2012;13:725-729. [PubMed] |
| 18. | Boland B, Kim A, Nissen N, Colquhoun S. Cholangiocarcinoma: aggressive surgical intervention remains justified. Am Surg. 2012;78:157-160. [PubMed] |
| 19. | Chen MF, Jan YY, Hwang TL, Jeng LB, Yeh TS. Impact of concomitant hepatolithiasis on patients with peripheral cholangiocarcinoma. Dig Dis Sci. 2000;45:312-316. [PubMed] |
| 20. | Menias CO, Surabhi VR, Prasad SR, Wang HL, Narra VR, Chintapalli KN. Mimics of cholangiocarcinoma: spectrum of disease. Radiographics. 2008;28:1115-1129. [PubMed] |
| 21. | Hur H, Park IY, Sung GY, Lee DS, Kim W, Won JM. Intrahepatic cholangiocarcinoma associated with intrahepatic duct stones. Asian J Surg. 2009;32:7-12. [PubMed] |
| 22. | Kuroki T, Tajima Y, Kanematsu T. Hepatolithiasis and intrahepatic cholangiocarcinoma: carcinogenesis based on molecular mechanisms. J Hepatobiliary Pancreat Surg. 2005;12:463-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Isse K, Specht SM, Lunz JG, Kang LI, Mizuguchi Y, Demetris AJ. Estrogen stimulates female biliary epithelial cell interleukin-6 expression in mice and humans. Hepatology. 2010;51:869-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Liu ZY, Zhou YM, Shi LH, Yin ZF. Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study. Hepatobiliary Pancreat Dis Int. 2011;10:626-631. [PubMed] |
| 25. | Suzuki Y, Mori T, Abe N, Sugiyama M, Atomi Y. Predictive factors for cholangiocarcinoma associated with hepatolithiasis determined on the basis of Japanese Multicenter study. Hepatol Res. 2012;42:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Zhou Y, Zhao Y, Li B, Huang J, Wu L, Xu D, Yang J, He J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer. 2012;12:289. [PubMed] |