Published online Aug 7, 2013. doi: 10.3748/wjg.v19.i29.4808
Revised: May 2, 2013
Accepted: May 16, 2013
Published online: August 7, 2013
Processing time: 152 Days and 13.4 Hours
AIM: To investigate the potential role of positron emission tomography (PET) in the diagnosis, staging and prognosis predicting of pancreatic carcinoma (PC).
METHODS: A systematic review of relevant literatures in PubMed, Embase and Cochrane Library was performed. The sensitivity and specificity of diagnostic and staging studies, and HRs for prognosis predicting studies were pooled. The bivariate model was used for diagnostic studies and the random-effect model for prognostic studies. Heterogeneity between included studies was tested using χ2 test, and subgroup analysis was performed to explain the heterogeneities. All of the calculations were performed using Stata version 11.0.
RESULTS: A total of 39 studies were included. The pooled sensitivity of PET in diagnosing PC (30 studies, 1582 patients), evaluating N stating (4 studies, 101 patients) and liver metastasis (7 studies, 316 patients) were 0.91 (95%CI: 0.88-0.93), 0.64 (95%CI: 0.50-0.76), and 0.67 (95%CI: 0.52-0.79), respectively; and the corresponding specificity was 0.81 (95%CI: 0.75-0.85), 0.81 (95%CI: 0.25-0.85), and 0.96 (95%CI: 0.89-0.98), respectively. In prognosis analysis (6 studies, 198 patients), significant difference of overall survival was observed between high and low standardized uptake value groups (HR = 2.39, 95%CI: 1.57-3.63). Subgroup analysis showed that PET/CT was more sensitive than PET alone in evaluating liver metastasis of PC, 0.82 (95%CI: 0.48-0.98) and 0.67 (95%CI: 0.52-0.79), respectively.
CONCLUSION: PET can be used as a valuable diagnostic and predictive tool for PC, but its effect in the staging of PC remains indeterminate.
Core tip: Positron emission tomography (PET) is an important tool for the diagnosis, staging and prognosis predicting of tumors. However, no consensus has been reached with regard to the role of PET in pancreatic carcinoma (PC) diagnosis. We performed meta-analysis of 39 included studies. The pooled results showed that PET can be used as a valuable diagnostic and predictive tool for PC, but its effect in the staging remains indeterminate. New tracers and PET scanning technology, as well as other parameters besides of standardized uptake value should be noticed in order to improve the diagnostic and predictive accuracy of PET in PC.
- Citation: Wang Z, Chen JQ, Liu JL, Qin XG, Huang Y. FDG-PET in diagnosis, staging and prognosis of pancreatic carcinoma: A meta-analysis. World J Gastroenterol 2013; 19(29): 4808-4817
- URL: https://www.wjgnet.com/1007-9327/full/v19/i29/4808.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i29.4808
Pancreatic carcinoma (PC) is one of the leading causes of cancer death worldwide and is steadily increasing in incidence in most countries[1]. In industrialized countries, the incidence of PC ranks second after colorectal cancer among all gastrointestinal malignancies[1]. Despite recent significant advances in cancer diagnosis and treatment, the prognosis of PC remains extremely unfavorable with a reported 5-year survival rate of only 1%-10%[2,3]. For PC, surgery remains the only curative treatment, and the success depends on the stage of disease at diagnosis, but not the histological type[4]. Unfortunately, only 10%-15% of cancers are found to be resectable at the time of diagnosis for the late onset of the symptoms[5]. Therefore, to choose the most appropriate treatment and to avoid unnecessary surgical risk, timely diagnosis and staging is essential in the evaluation of patients with PC.
Although significant advances have been achieved in diagnostic technologies such as computed tomography (CT), endoscopic ultrasonography (EUS) and magnetic resonance imaging (MRI), the preoperative diagnosis and staging of PC remains suboptimal, which restricts the treatment planning of this disease[5]. The discrimination between inflammatory processes and PC, and the assessment of local resectability and distant metastases of the PC are still challenging with different imaging modalities[6]. Over the years, positron emission tomography (PET) has played an important role in oncology, especially for diagnosis, staging, and for evaluating the response to treatment and the prognosis of tumors[7]. However, there has been no consensus with regard to the role of PET in PC now. Some researchers held that PET could be used as a valuable measure in the diagnosis, staging and prognosis predicting of PC[8]; but others did not find enough evidences to justify the use of PET in PC[9]. Therefore, a systemic review aimed to evaluate the effect of PET in the diagnosis, staging and prognosis predicting of PC is urgently needed.
In this study, we assessed the pertinent literatures and conducted a meta-analysis to further investigate the potential role of PET in PC.
A systematic literature search was performed to identify studies assessing the effect of PET in the diagnosis, staging and prognosis predicting of PC. The PubMed, Embase and Cochrane Library databases were searched with the MeSH headings (“pancreatic neoplasms” and “tomography emission computed”) and keywords (“pancreas or pancreatic neoplasms” or “pancreatic tumor/tumour” or “pancreatic cancer” or “PC” or “cancer of the pancreas”) and (PET or “diffusion” or “weighted imaging”). The upper limit of search date was not limited, and the lower limit was December 2012. The language was not limited. In addition, reference lists from the included studies were hand searched.
Inclusion criteria for this meta-analysis were: (1) Studies assessing the effect of PET in the diagnosis, staging and prognosis predicting of PC. The participants were clinically suspected of PC, and diagnosed with PC by histology or follow-up exceeding 6 mo; (2) For diagnosis and staging, the results were judged with histopathology or clinical follow-up exceeding 6 mo; (3) For diagnosis and staging, true-positive (TP), false-positive (FP), true-negative (TN), and false-negative (FN) results of imaging methods could be calculated for per-patient; for prognosis, HRs and their 95%CIs for overall survival (OS) data were available or able to be calculated from original articles; (4) For eligible studies with data published more than once, we only included the articles with the largest sample size of patients; and (5) PET was performed with intravenous administration of 18F-FDG.
Exclusion criteria for this meta-analysis were: (1) studies included patients with non-primary PC in staging or prognosis analysis (e.g., metastatic cancer); (2) primary data were confounding and not able to be analyzed; (3) for staging, studies included patients who received radiotherapy or chemotherapy preoperatively, which may cause downstaging because neo-adjuvant protocols can lead to tumor downstaging and affect the diagnostic accuracy of imaging; (4) vitro studies and animal experiments; (5) Studies with a sample size less than 10; and (6) papers were not original research in type (e.g., review articles).
Two authors extracted data using pre-defined tables, which included the following items: authors and publication time, country, study design, participants, sample size, quality score, and outcomes (TP, FP, TN and FN for diagnosis and staging analysis; HRs and their 95%CIs of OS for prognosis analysis). Follow-up period was recorded for prognosis analysis.
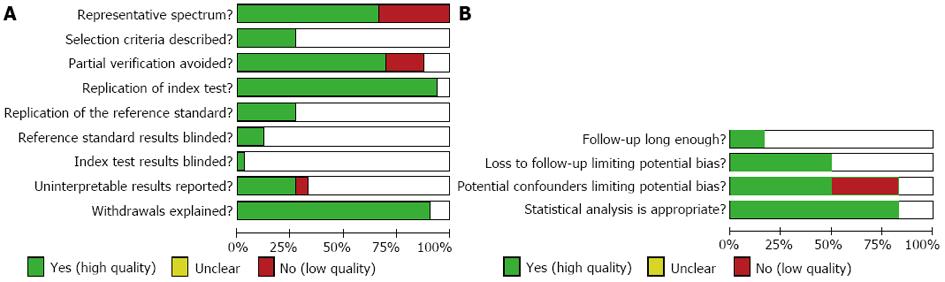
For diagnosis and staging, nine items of QUADAS closely related to this study were used to assess the methodological quality of eligible studies (the other five items of QUADAS were not related to this test)[10]. For prognosis, four items (closely related to this study) from previous literatures were selected as the quality standard[11]. Each item was described as Yes (high quality), Unclear, or No (low quality).
For diagnosis and staging analysis, the calculation was based on max standardized uptake value (SUV), and pooled estimates of sensitivity and specificity of PET (with corresponding 95%CIs) were analyzed using the bivariate model[12], which was considered as a more valid statistical model for diagnostic meta-analysis[13,14]. The bivariate model uses a random effect approach for both sensitivity and specificity, which allows for heterogeneity beyond chance as a result of clinical and methodological differences between studies. To graphically present the results, we plotted the hierarchical summary receiver operating characteristic (HSROC) curves[13]. As a concern for meta-analysis of diagnostic trials, publication bias was tested using the funnel plot and Deeks test[15], which was conducted by a regression of diagnostic log OR against 1/sqrt (effective sample size), weighting by effective sample size, with P < 0.1 for the slope coefficient indicating significant asymmetry.
For prognosis analysis, HRs and their CIs for OS were retrieved from each primary study. In case they were not directly reported in primary literatures, we derived them from the survival curves using published method[16,17]. Kaplan-Meier curves of included studies were read by Engauge Digitizer version 2.11 (free software downloaded from http://sourceforge.net). HR calculation spreadsheet was freely downloaded from http://www.trialsjournal.com/content/supplementary/1745-6215-8-16-s1.xls. HRs for OS were pooled using a random-effect model.
Heterogeneity between included studies was tested using χ2 test (P < 0.1 was considered significant). If heterogeneities were present, subgroup analysis was attempted to explain them.
All of the calculations were performed using Stata version 11.0. All P values were two-sided and all CIs had a two-sided probability coverage of 95%.
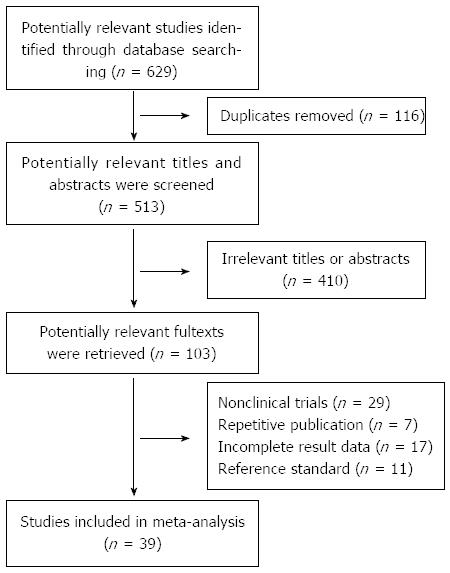
According to the search strategy, a total of 629 papers were selected: 362 in PubMed, 216 in EMBASE, 37 in Cochrane Library and 14 by hand search. After browsing the titles and abstracts, we found that many studies were irrelevant and some were identified in more than one database; and 103 articles remained for potential inclusion and full texts were obtained. After screening the full text, 64 articles were excluded. The main reasons for exclusion were: nonclinical trials (such as review articles), repetitive publication, incomplete data, and inappropriate reference standard. At last, 39 studies were eligible for inclusion[18-56]. The number of studies evaluating primary tumor diagnosis, N staging, liver metastasis and prognosis was 30[18-21,25,32-56], 4[18-21], 7[19-25] and 6[26-31], respectively. The study selection process is summarized in Figure 1. The characteristics of included studies are listed in Tables 1, 2, 3 and 4. And the quality of included studies is shown in Figure 2.
| Ref. | Study design | Imaging | Population | n (M/F) | Results | |||
| TP | FP | FN | TN | |||||
| Stollfuss et al[32] | NR | PET | Suspected PC or CP | 73 (54/19) | 41 | 3 | 2 | 27 |
| Wang et al[33] | NR | PET | Pancreatic mass | 40 (27/13) | 26 | 3 | 1 | 10 |
| Rose et al[34] | R | PET | Suspected PC | 65 (NR) | 48 | 2 | 4 | 11 |
| Kauhanen et al[18] | P | PET | Suspected PC | 38 (19/19) | 17 | 3 | 1 | 17 |
| Herrmann et al[35] | P | PET | Suspected PC or CP | 41 (27/14) | 30 | 4 | 3 | 4 |
| PET/CT | 31 (NR) | 24 | 5 | 1 | 1 | |||
| Nakamoto et al[36] | P | PET | Suspected PC | 47 (31/16) | 22 | 3 | 5 | 17 |
| Friess et al[37] | P | PET | Suspected PC or CP | 74 (57/17) | 41 | 4 | 1 | 28 |
| Keogan et al[38] | P | PET | Suspected PC | 37 (22/15) | 22 | 2 | 3 | 10 |
| Koyama et al[39] | NR | PET | Suspected PC | 86 (50/36) | 53 | 4 | 12 | 17 |
| Nishiyama et al[19] | NR | PET | Suspected PC | 86 (64/22) | 49 | 11 | 6 | 20 |
| Inokuma et al[40] | P | PET | Suspected PC | 46 (25/21) | 33 | 2 | 2 | 9 |
| Bares et al[20] | P | PET | Suspected PC | 40 (25/15) | 25 | 2 | 2 | 11 |
| Van et al[41] | NR | PET | Suspected PC or CP | 109 (65/44) | 29 | 10 | 3 | 67 |
| Zimny et al[42] | P | PET | Suspected PC | 106 (NR) | 63 | 5 | 11 | 27 |
| Kato et al[43] | NR | PET | Patients with PC or CP | 24 (20/4) | 14 | 2 | 1 | 7 |
| Ruf et al[21] | R | PET | Suspected PC | 32 (20/12) | 14 | 10 | 1 | 7 |
| Rasmussen et al[44] | P | PET | Suspected PC | 20 (12/8) | 9 | 1 | 3 | 7 |
| Delbeke et al[45] | R | PET | Suspected PC | 65 (33/32) | 52 | 3 | 0 | 10 |
| Farma et al[46] | R | PET/CT | Suspected PC | 82 (43/39) | 58 | 2 | 7 | 15 |
| Borbath[25] | R | PET | Suspected PC | 59 (29/30) | 42 | 5 | 6 | 6 |
| Sendler et al[47] | P | PET | Suspected PC | 42 (21/21) | 22 | 4 | 9 | 7 |
| Bang et all[48] | NR | PET | Suspected PC | 102 (76/26) | 90 | 2 | 3 | 7 |
| Papós et al[49] | NR | PET | Suspected PC | 22 (13/9) | 6 | 2 | 0 | 14 |
| Rajput et al[50] | R | PET | Suspected PC | 11 (NR) | 8 | 0 | 1 | 2 |
| Ho et al[51] | NR | PET | Suspected PC | 14 (7/7) | 8 | 2 | 0 | 4 |
| Mertz et al[52] | P | PET | Suspected PC | 35 (NR) | 27 | 2 | 4 | 2 |
| Takanami et al[53] | R | PET/CT | Suspected PC | 16 (13/3) | 7 | 0 | 2 | 7 |
| Sperti et al[54] | P | PET | Suspected PC | 64 (33/31) | 24 | 1 | 2 | 37 |
| Tann et al[55] | R | PET | Suspected PC | 30 (16/14) | 4 | 8 | 3 | 15 |
| PET/CT | 30 (16/14) | 6 | 2 | 1 | 21 | |||
| Bares et al[56] | NR | PET | Suspected PC | 15 (11/4) | 12 | 0 | 1 | 2 |
| Ref. | Study design | Imaging method | Population | n (M/F) | Results | |||
| TP | FP | FN | TN | |||||
| Kauhanen et al[18] | P | PET | Histologically proved PC | 8 (NR) | 2 | 0 | 5 | 1 |
| Nishiyama et al[19] | NR | PET | PC diagnosed by histology or follow-up | 55 (NR) | 14 | 1 | 6 | 34 |
| Bares et al[20] | P | PET | Histologically proved PC | 23 (NR) | 10 | 2 | 3 | 8 |
| Ruf et al[21] | R | PET | PC diagnosed by histology or follow-up | 15 (9/6) | 8 | 2 | 5 | 0 |
| Ref. | Study design | Imaging | Population | n (M/F) | Results | |||
| TP | FP | FN | TN | |||||
| Strobel et al[22] | R | PET | Histologically proved PC | 50 (25/25) | 5 | 0 | 6 | 39 |
| PET/CT | 50 (25/25) | 9 | 1 | 2 | 38 | |||
| Nakamoto et al[23] | NR | PET | Histologically proved PC | 34 (22/12) | 11 | 2 | 1 | 20 |
| Nishiyama et al[24] | NR | PET | Histologically proved PC | 42 (26/16) | 10 | 3 | 3 | 26 |
| Nishiyama et al[19] | NR | PET | PC diagnosed by histology or follow-up | 55 (NR) | 11 | 0 | 7 | 37 |
| Bares et al[20] | P | PET | Histologically proved PC | 23 (NR) | 4 | 1 | 3 | 15 |
| Ruf et al[21] | R | PET | PC diagnosed by histology or follow-up | 15 (9/6) | 3 | 2 | 5 | 5 |
| Borbath et al[25] | R | PET | PC diagnosed by histology or follow-up | 47 (NR) | 10 | 1 | 2 | 34 |
| Ref. | Study design | Imaging method | Population | n (M/F) | Follow-up period | HR (95%CI) |
| Sperti et al[26] | R | PET | Histologically proved PC | 60 (34/26) | NR | 3.96 (1.92-8.17) |
| Maisey et al[27] | P | PET | Histologically proved PC | 11 (7/4) | NR | 3.4 (2.01-5.73) |
| Zimny et al[28] | NR | PET | Histologically proved PC | 52 (33/19) | NR | 2.27 (1.69-3.05) |
| Nakata et al[29] | NR | PET | Histologically proved PC | 37 (21/16) | NR | 0.93 (0.70, 1.25)1 |
| 4.9 (1.19-20.2)2 | ||||||
| Maemura et al[30] | NR | PET | PC diagnosed by histology or follow-up | 24 (NR) | NR | 2.1 (1.5-2.92) |
| Nakata et al[31] | NR | PET | Histologically proved PC | 14 (NR) | 6-17 mo | 2.99 (2.25-3.97) |
In the diagnosis of primary tumors, 30 studies (1582 patients) were eligible for meta-analysis[18-21,25,32-56]. The pooled sensitivity and specificity of PET in the diagnosis of PC were 0.91 (95%CI: 0.88-0.93) and 0.81 (95%CI: 0.75-0.85), respectively.
For lymph node metastasis, 4 studies (101 patients) were eligible for meta-analysis[18-21]. The pooled sensitivity, specificity and negative predictive value of PET in the diagnosis of N staging were 0.64 (95%CI: 0.50-0.76), 0.81 (95%CI: 0.25-0.85), and 0.65 (95%CI: 0.28-0.90), respectively.
For liver metastasis, 7 studies (316 patients) were eligible for meta-analysis[19-25]. The pooled sensitivity and specificity of PET in the diagnosis of liver metastasis were 0.67 (95%CI: 0.52-0.79) and 0.96 (95%CI: 0.89-0.98), respectively.
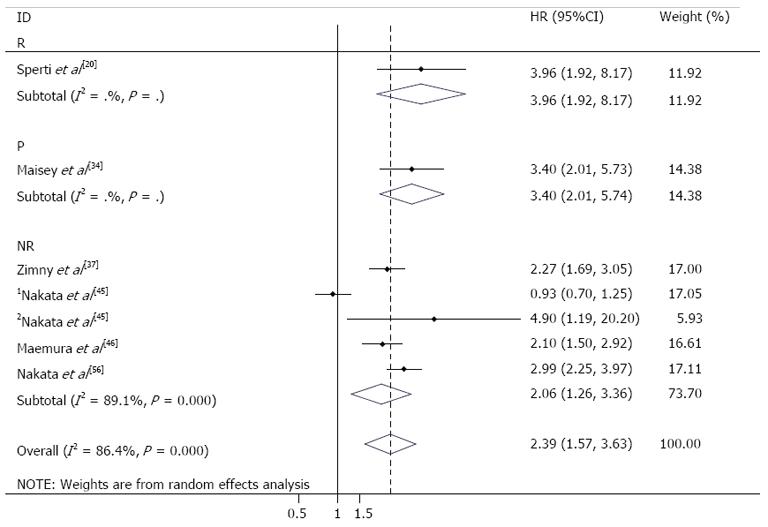
For predicting the prognosis, 6 studies (198 patients) were eligible for meta-analysis[26-31]. In the study by Nakata et al[29], the data about resectable and unresectable tumors were reported separately. The pooled HR for OS was 2.39 (95%CI: 1.57-3.63), which suggested that patients in low SUV group had a significant longer OS than patients in high SUV group (Figure 3).
The P values of heterogeneity test for the meta-analysis were all less than 0.1. Considering that the results might be influenced by the study design and imaging method, we performed subgroup analysis according to the design and imaging method of included studies. The results of subgroup analysis are listed in Table 5.
| Groups | Diagnosis | N staging | Liver metastasis | Prognosis | ||||
| Sen (95%CI) | Spe (95%CI) | Sen (95%CI) | Spe (95%CI) | Pv- (95%CI) | Sen (95%CI) | Spe (95%CI) | HR (95%CI) | |
| Overall | 0.91 (0.88-0.93) | 0.81 (0.75-0.85) | 0.64 (0.50-0.76) | 0.81 (0.25-0.85) | 0.65 (0.28-0.90) | 0.67 (0.52-0.79) | 0.96 (0.89-0.98) | |
| P subgroup | 0.89 (0.84-0.92) | 0.84 (0.76-0.89) | 0.56 (0.15-0.90) | 0.79 (0.48-0.94) | - | 0.57 (0.21-0.88) | 0.94 (0.68-0.99) | 2.39 (1.57-3.63) |
| R subgroup | 0.90 (0.83-0.95) | 0.75 (0.58-0.87) | 0.61 (0.32-0.85) | 0.17 (0.04-0.81) | - | 0.56 (0.28-0.81) | 0.94 (0.65-0.99) | 3.40 (2.01-5.74) |
| NR subgroup | 0.93 (0.88-0.96) | 0.82 (0.74-0.87) | 0.70 (0.46-0.87) | 0.97 (0.84-0.99) | - | 0.74 (0.52-0.88) | 0.92 (0.83-0.96) | 3.96 (1.92-8.17) |
| PET subgroup | 0.91 (0.88-0.93) | 0.80 (0.74-0.85) | - | - | - | 0.67 (0.52-0.79) | 0.96 (0.89-0.98) | 2.06 (1.26-3.36) |
| PET/CT subgroup | 0.90 (0.79-0.95) | 0.85 (0.38-0.98) | - | - | - | 0.82 (0.48-0.98) | 0.97 (0.87-1.00) | - |
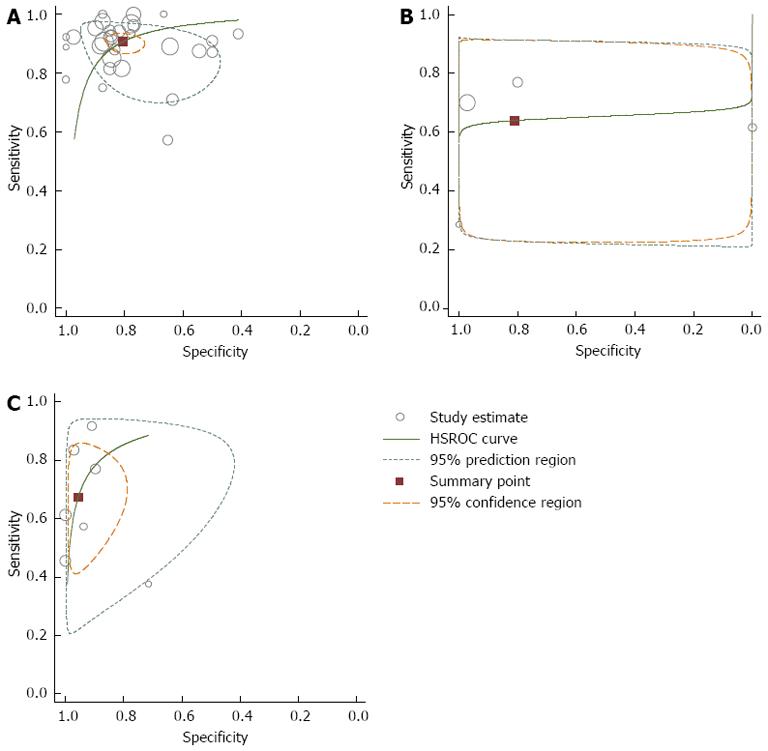
We plotted HSROC curves to graphically present the results of diagnosis and staging (Figure 4). In HSROC curves, the index test’s sensitivity (TP rate) was plotted on the y axis against 1-specificity (FN rate) on the x axis. Additionally, the 95%CI and a 95% prediction region around the pooled estimates were plotted to illustrate the precision with which the pooled values were estimated (confidence ellipse of a mean) and to show the between-study variation (prediction ellipse; the likely range of values for a new study)[13].
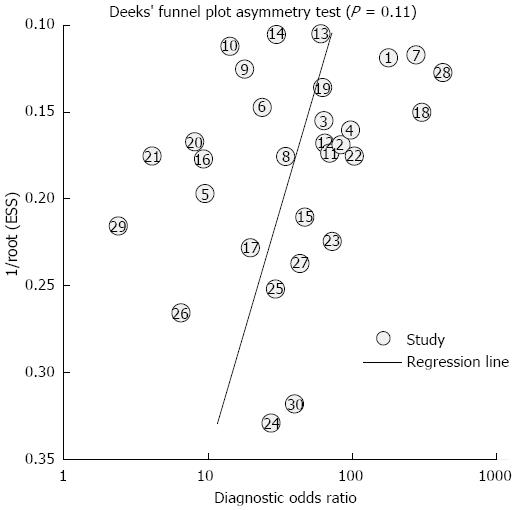
Because the included studies for staging and prognosis were too few (less than 10), we explored publication bias using the data of PET/CT in the diagnosis of primary tumors, which included 30 studies. As a result, the funnel plot seemed symmetrical with a P value of 0.11, which suggested a low risk of publication bias (Figure 5).
In recent years, PET imaging has been increasingly used to identify and stage PC, and also utilized as a prognostic indicator. However, the value of PET in the management of PC remains indeterminate. In our study, we collected existing data to assess the value of PET in the diagnosis, staging and prognosis predicting of PC. We found that PET could be used as a valuable diagnostic and predictive tool for PC; but for staging, PET has a moderate sensitivity and a relatively high specificity (Table 5).
Clinically, the diagnostic pathway for detection and staging of PC usually starts with abdominal ultrasound (US) followed by CT or MRI of the upper abdomen. However, even combined diagnostic approaches are limited by a low sensitivity for the detection of small lesions (a diameter of less than 2 cm) and for differentiation between malignant and benign lesions[57]. Recently, promising results in the diagnostic value of PET as a diagnostic and staging tool in PC have been reported[19-21]. In our review, we found PET had an acceptable sensitivity and specificity [sensitivity: 0.91 (95%CI: 0.88-0.93); specificity: 0.81 (95%CI: 0.75-0.85)] in the diagnosis of PC, which demonstrated that PET was valuable in the diagnosis of PC. This result was consistent with previous reports[18,22,32,33]. Considering that diagnostic accuracy might be influenced by study design and the usage of CT, we conducted subgroup analysis. However, the other confounding factors (such as tumor diameter, serum glucose and C-reactive protein levels) were not considered because of incomplete data in included studies. This reduced the reliability of our results to some extent, although the impact of these factors on diagnostic accuracy is indeterminate [19,34].
Because the only curative treatment for PC is surgery, accurate staging is necessary to properly select patients (surgical resection benefits only those patients with localized disease). Previous studies reported that both PET and CT were poor for N staging, although the diagnostic accuracy of PET was a little higher than CT[58]. This is consistent with our study (Table 5). As for liver metastasis, the value of PET is still controversial[7]. In our study, we found a sensitivity of 0.67 (95%CI: 0.52-0.79) for PET in detecting liver metastasis of PC. This suggests that the value of PET in assessing liver metastasis of PC remains indeterminate, although it has a relatively high specificity [0.96 (95%CI: 0.89-0.98)]. Recently, studies found that combined PET/CT could improve detection rates in the staging of PC[3]. In our study, we found that combined PET/CT was more sensitive than PET alone in assessing liver metastasis (82% vs 67%, Table 5), this confirmed the previous findings. Efforts have been made to to improve the diagnostic accuracy of PET in PC. It has been found that delayed PET scanning helped differentiate malignant lesions from benign ones, and new tracers such as 18F fluorothymidine (FLT) could improve the diagnostic accuracy[19,59]. However, these findings need to be further validated.
Patients with PC usually have extremely poor prognosis among gastrointestinal malignancies. With conventional imaging modalities, it is often difficult to predict the prognosis of patients with PC preoperatively. Recently, studies found that the metabolic activity of the pancreas tumor, measured by PET usually through SUV, seemed to be useful in evaluating the prognosis of PC[29]. This result was consistent with ours, which suggested that patients with a higher SUV were associated with worse prognosis (HR = 2.39, 95%CI: 1.57-3.63). Additionally, the result did not change in the subgroup analysis (Table 5). This demonstrated that what we found was reliable. However, some researchers considered that the usage of SUV for prognostic assessment had some serious limitations (besides tumor characteristics, absolute value of SUV can also be influenced by several institution-dependent factors)[60]. And they found that SUVmax difference (between pre- and post-treatment scans) or the usage of relative values (such as the retention index) allowed more accurate prognostic evaluation[60,61]. Of course, more studies are needed to confirm these findings in the future.
In this study, we designed a systematic search strategy, selected studies according to the strict inclusion criteria, assessed the methodological quality using uniform criteria, and performed subgroup analysis in the presence of heterogeneity. Thirty-nine studies were included. These increased the reliability of the results to some extent. However, several concerns must also be addressed when interpreting the pooled results. First, clinical follow-up was used as the reference standard in most of the included studies. Although the follow-up period was long enough, it might not correctly classify the target condition in some cases, which would affect the accuracy of the results. Second, some parameters (such as tumor diameter, glucose and C-reactive protein levels) which would affect the accuracy of the results were not considered in our study because of incomplete data, we failed to perform subgroup analysis or meta-regression, which might find out other possible causes of heterogeneity. Finally, publication bias was not tested because the few number of included studies in evaluating the staging and prognosis of PC may induce potential bias.
In conclusion, PET can be used as a valuable diagnostic and predictive tool for PC, but its effect in the staging of PC remains unclear. New tracers and PET scanning technology, as well as other parameters of PET besides SUV, should be noticed in order to improve the diagnostic and predictive accuracy of PET in PC.
Pancreatic carcinoma (PC) is one of the leading causes of cancer death worldwide and is steadily increasing in incidence in most countries. In industrialized countries, the incidence of PC ranks second after colorectal cancer among all gastrointestinal malignancies. Although significant advances have been achieved in diagnostic technologies, the preoperative diagnosis and staging of PC remains suboptimal.
Over the years, positron emission tomography (PET) has played an important role in oncology, especially in the diagnosis, staging and prognosis prediction of tumors. However, there is no consensus with regard to the role of PET in PC now.
PET had an acceptable sensitivity and specificity [sensitivity: 0.91 (95%CI: 0.88-0.93); specificity: 0.81 (95%CI: 0.75-0.85)] in the diagnosis of PC. And higher standard uptake value measured by PET was associated with worse prognosis of PC patients (HR = 2.39, 95%CI: 1.57-3.63). However, the accuracy of PET in evaluating N staging and liver metastasis of PC was unsatisfied. This article gives them a comprehensive update based on previous studies.
PET can be used as a valuable diagnostic and predictive tool for PC, but its effect in the staging of PC remains indeterminate.
Based on previous studies, this study evaluated the comprehensive role of PET in PC, including the diagnosis, staging and prognosis prediction. The authors found that PET can be used as a valuable diagnostic and predictive tool for PC, but its effect in the staging of PC remains indeterminate. The study is well designed, methodologically correct, elaborately prepared and full of significance in the field.
P- Reviewers Citak N, Roca B S- Editor Wen LL L- Editor Ma JY E- Editor Ma S
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7953] [Cited by in RCA: 8101] [Article Influence: 506.3] [Reference Citation Analysis (2)] |
| 2. | Sharma C, Eltawil KM, Renfrew PD, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010. World J Gastroenterol. 2011;17:867-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 146] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (5)] |
| 3. | Heinrich S, Goerres GW, Schäfer M, Sagmeister M, Bauerfeind P, Pestalozzi BC, Hany TF, von Schulthess GK, Clavien PA. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg. 2005;242:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | American Cancer Society. Pancreatic cancer. Available from: http://www.cancer.org. |
| 5. | Katz MH, Savides TJ, Moossa AR, Bouvet M. An evidence-based approach to the diagnosis and staging of pancreatic cancer. Pancreatology. 2005;5:576-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Wu LM, Hu JN, Hua J, Liu MJ, Chen J, Xu JR. Diagnostic value of diffusion-weighted magnetic resonance imaging compared with fluorodeoxyglucose positron emission tomography/computed tomography for pancreatic malignancy: a meta-analysis using a hierarchical regression model. J Gastroenterol Hepatol. 2012;27:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Grassetto G, Rubello D. Role of FDG-PET/CT in diagnosis, staging, response to treatment, and prognosis of pancreatic cancer. Am J Clin Oncol. 2011;34:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | van Kouwen MC, Oyen WJ, Nagengast FM, Jansen JB, Drenth JP. FDG-PET scanning in the diagnosis of gastrointestinal cancers. Scand J Gastroenterol Suppl. 2004;85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Mansour JC, Schwartz L, Pandit-Taskar N, D’Angelica M, Fong Y, Larson SM, Brennan MF, Allen PJ. The utility of F-18 fluorodeoxyglucose whole body PET imaging for determining malignancy in cystic lesions of the pancreas. J Gastrointest Surg. 2006;10:1354-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2570] [Cited by in RCA: 2732] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 11. | Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1144] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 12. | Arends LR, Hamza TH, van Houwelingen JC, Heijenbrok-Kal MH, Hunink MG, Stijnen T. Bivariate random effects meta-analysis of ROC curves. Med Decis Making. 2008;28:621-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Geersing GJ, Janssen KJ, Oudega R, Bax L, Hoes AW, Reitsma JB, Moons KG. Excluding venous thromboembolism using point of care D-dimer tests in outpatients: a diagnostic meta-analysis. BMJ. 2009;339:b2990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2049] [Cited by in RCA: 2473] [Article Influence: 123.7] [Reference Citation Analysis (0)] |
| 15. | Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1792] [Cited by in RCA: 2225] [Article Influence: 111.3] [Reference Citation Analysis (1)] |
| 16. | Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834. [PubMed] |
| 17. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4951] [Article Influence: 275.1] [Reference Citation Analysis (0)] |
| 18. | Kauhanen SP, Komar G, Seppänen MP, Dean KI, Minn HR, Kajander SA, Rinta-Kiikka I, Alanen K, Borra RJ, Puolakkainen PA. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Nishiyama Y, Yamamoto Y, Monden T, Sasakawa Y, Tsutsui K, Wakabayashi H, Ohkawa M. Evaluation of delayed additional FDG PET imaging in patients with pancreatic tumour. Nucl Med Commun. 2005;26:895-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Bares R, Klever P, Hauptmann S, Hellwig D, Fass J, Cremerius U, Schumpelick V, Mittermayer C, Büll U. F-18 fluorodeoxyglucose PET in vivo evaluation of pancreatic glucose metabolism for detection of pancreatic cancer. Radiology. 1994;192:79-86. [PubMed] |
| 21. | Ruf J, Lopez Hänninen E, Böhmig M, Koch I, Denecke T, Plotkin M, Langrehr J, Wiedenmann B, Felix R, Amthauer H. Impact of FDG-PET/MRI image fusion on the detection of pancreatic cancer. Pancreatology. 2006;6:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Strobel K, Heinrich S, Bhure U, Soyka J, Veit-Haibach P, Pestalozzi BC, Clavien PA, Hany TF. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J Nucl Med. 2008;49:1408-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Nakamoto Y, Higashi T, Sakahara H, Tamaki N, Kogire M, Imamura M, Konishi J. Contribution of PET in the detection of liver metastases from pancreatic tumours. Clin Radiol. 1999;54:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Nishiyama Y, Yamamoto Y, Yokoe K, Monden T, Sasakawa Y, Tsutsui K, Satoh K, Ohkawa M. Contribution of whole body FDG-PET to the detection of distant metastasis in pancreatic cancer. Ann Nucl Med. 2005;19:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Borbath I, Van Beers BE, Lonneux M, Schoonbroodt D, Geubel A, Gigot JF, Deprez PH. Preoperative assessment of pancreatic tumors using magnetic resonance imaging, endoscopic ultrasonography, positron emission tomography and laparoscopy. Pancreatology. 2005;5:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Sperti C, Pasquali C, Chierichetti F, Ferronato A, Decet G, Pedrazzoli S. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J Gastrointest Surg. 2003;7:953-959; discussion 959-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Maisey NR, Webb A, Flux GD, Padhani A, Cunningham DC, Ott RJ, Norman A. FDG-PET in the prediction of survival of patients with cancer of the pancreas: a pilot study. Br J Cancer. 2000;83:287-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Zimny M, Fass J, Bares R, Cremerius U, Sabri O, Buechin P, Schumpelick V, Buell U. Fluorodeoxyglucose positron emission tomography and the prognosis of pancreatic carcinoma. Scand J Gastroenterol. 2000;35:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Nakata B, Nishimura S, Ishikawa T, Ohira M, Nishino H, Kawabe J, Ochi H, Hirakawa K. Prognostic predictive value of 18F-fluorodeoxyglucose positron emission tomography for patients with pancreatic cancer. Int J Oncol. 2001;19:53-58. [PubMed] |
| 30. | Maemura K, Takao S, Shinchi H, Noma H, Mataki Y, Kurahara H, Jinnouchi S, Aikou T. Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer. J Hepatobiliary Pancreat Surg. 2006;13:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Nakata B, Chung YS, Nishimura S, Nishihara T, Sakurai Y, Sawada T, Okamura T, Kawabe J, Ochi H, Sowa M. 18F-fluorodeoxyglucose positron emission tomography and the prognosis of patients with pancreatic adenocarcinoma. Cancer. 1997;79:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Stollfuss JC, Glatting G, Friess H, Kocher F, Berger HG, Reske SN. 2-(fluorine-18)-fluoro-2-deoxy-D-glucose PET in detection of pancreatic cancer: value of quantitative image interpretation. Radiology. 1995;195:339-344. [PubMed] |
| 33. | Wang X, Yu LJ. 18F-FDG PET/CT in detection of pancreatic cancer: Value of synthetic analysis interpretation. Zhongguo Yixue Yingxiang Jishu. 2007;23:1709-1712. |
| 34. | Rose DM, Delbeke D, Beauchamp RD, Chapman WC, Sandler MP, Sharp KW, Richards WO, Wright JK, Frexes ME, Pinson CW. 18Fluorodeoxyglucose-positron emission tomography in the management of patients with suspected pancreatic cancer. Ann Surg. 1999;229:729-737; discussion 737-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Herrmann K, Erkan M, Dobritz M, Schuster T, Siveke JT, Beer AJ, Wester HJ, Schmid RM, Friess H, Schwaiger M. Comparison of 3’-deoxy-3’-[18F]fluorothymidine positron emission tomography (FLT PET) and FDG PET/CT for the detection and characterization of pancreatic tumours. Eur J Nucl Med Mol Imaging. 2012;39:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Nakamoto Y, Higashi T, Sakahara H, Tamaki N, Kogire M, Doi R, Hosotani R, Imamura M, Konishi J. Delayed (18)F-fluoro-2-deoxy-D-glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer. 2000;89:2547-2554. [PubMed] |
| 37. | Friess H, Langhans J, Ebert M, Beger HG, Stollfuss J, Reske SN, Büchler MW. Diagnosis of pancreatic cancer by 2[18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Gut. 1995;36:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 106] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Keogan MT, Tyler D, Clark L, Branch MS, McDermott VG, DeLong DM, Coleman RE. Diagnosis of pancreatic carcinoma: role of FDG PET. AJR Am J Roentgenol. 1998;171:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Koyama K, Okamura T, Kawabe J, Nakata B, Chung KH, Ochi H, Yamada R. Diagnostic usefulness of FDG PET for pancreatic mass lesions. Ann Nucl Med. 2001;15:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Inokuma T, Tamaki N, Torizuka T, Magata Y, Fujii M, Yonekura Y, Kajiyama T, Ohshio G, Imamura M, Konishi J. Evaluation of pancreatic tumors with positron emission tomography and F-18 fluorodeoxyglucose: comparison with CT and US. Radiology. 1995;195:345-352. [PubMed] |
| 41. | van Kouwen MC, Jansen JB, van Goor H, de Castro S, Oyen WJ, Drenth JP. FDG-PET is able to detect pancreatic carcinoma in chronic pancreatitis. Eur J Nucl Med Mol Imaging. 2005;32:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Zimny M, Bares R, Fass J, Adam G, Cremerius U, Dohmen B, Klever P, Sabri O, Schumpelick V, Buell U. Fluorine-18 fluorodeoxyglucose positron emission tomography in the differential diagnosis of pancreatic carcinoma: a report of 106 cases. Eur J Nucl Med. 1997;24:678-682. [PubMed] |
| 43. | Kato T, Fukatsu H, Ito K, Tadokoro M, Ota T, Ikeda M, Isomura T, Ito S, Nishino M, Ishigaki T. Fluorodeoxyglucose positron emission tomography in pancreatic cancer: an unsolved problem. Eur J Nucl Med. 1995;22:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Rasmussen I, Sörensen J, Långström B, Haglund U. Is positron emission tomography using 18F-fluorodeoxyglucose and 11C-acetate valuable in diagnosing indeterminate pancreatic masses? Scand J Surg. 2004;93:191-197. [PubMed] |
| 45. | Delbeke D, Rose DM, Chapman WC, Pinson CW, Wright JK, Beauchamp RD, Shyr Y, Leach SD. Optimal interpretation of FDG PET in the diagnosis, staging and management of pancreatic carcinoma. J Nucl Med. 1999;40:1784-1791. [PubMed] |
| 46. | Farma JM, Santillan AA, Melis M, Walters J, Belinc D, Chen DT, Eikman EA, Malafa M. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15:2465-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 47. | Sendler A, Avril N, Helmberger H, Stollfuss J, Weber W, Bengel F, Schwaiger M, Roder JD, Siewert JR. Preoperative evaluation of pancreatic masses with positron emission tomography using 18F-fluorodeoxyglucose: diagnostic limitations. World J Surg. 2000;24:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Bang S, Chung HW, Park SW, Chung JB, Yun M, Lee JD, Song SY. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J Clin Gastroenterol. 2006;40:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Papós M, Takács T, Trón L, Farkas G, Ambrus E, Szakáll S, Lonovics J, Csernay L, Pávics L. The possible role of F-18 FDG positron emission tomography in the differential diagnosis of focal pancreatic lesions. Clin Nucl Med. 2002;27:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Rajput A, Stellato TA, Faulhaber PF, Vesselle HJ, Miraldi F. The role of fluorodeoxyglucose and positron emission tomography in the evaluation of pancreatic disease. Surgery. 1998;124:793-797; discussion 793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Ho CL, Dehdashti F, Griffeth LK, Buse PE, Balfe DM, Siegel BA. FDG-PET evaluation of indeterminate pancreatic masses. J Comput Assist Tomogr. 1996;20:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Mertz HR, Sechopoulos P, Delbeke D, Leach SD. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc. 2000;52:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 53. | Takanami K, Hiraide T, Tsuda M, Nakamura Y, Kaneta T, Takase K, Fukuda H, Takahashi S. Additional value of FDG PET/CT to contrast-enhanced CT in the differentiation between benign and malignant intraductal papillary mucinous neoplasms of the pancreas with mural nodules. Ann Nucl Med. 2011;25:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Sperti C, Bissoli S, Pasquali C, Frison L, Liessi G, Chierichetti F, Pedrazzoli S. 18-fluorodeoxyglucose positron emission tomography enhances computed tomography diagnosis of malignant intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2007;246:932-937; discussion 937-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Tann M, Sandrasegaran K, Jennings SG, Skandarajah A, McHenry L, Schmidt CM. Positron-emission tomography and computed tomography of cystic pancreatic masses. Clin Radiol. 2007;62:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Bares R, Klever P, Hellwig D, Hauptmann S, Fass J, Hambuechen U, Zopp L, Mueller B, Buell U, Schumpelick V. Pancreatic cancer detected by positron emission tomography with 18F-labelled deoxyglucose: method and first results. Nucl Med Commun. 1993;14:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, Truninger K, Ammann R, Cavallini G, Charnley RM. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 374] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 58. | Asagi A, Ohta K, Nasu J, Tanada M, Nadano S, Nishimura R, Teramoto N, Yamamoto K, Inoue T, Iguchi H. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas. 2013;42:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 59. | Quon A, Chang ST, Chin F, Kamaya A, Dick DW, Loo BW, Gambhir SS, Koong AC. Initial evaluation of 18F-fluorothymidine (FLT) PET/CT scanning for primary pancreatic cancer. Eur J Nucl Med Mol Imaging. 2008;35:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Lyshchik A, Higashi T, Nakamoto Y, Fujimoto K, Doi R, Imamura M, Saga T. Dual-phase 18F-fluoro-2-deoxy-D-glucose positron emission tomography as a prognostic parameter in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging. 2005;32:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Topkan E, Parlak C, Kotek A, Yapar AF, Pehlivan B. Predictive value of metabolic 18FDG-PET response on outcomes in patients with locally advanced pancreatic carcinoma treated with definitive concurrent chemoradiotherapy. BMC Gastroenterol. 2011;11:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |