Published online Aug 7, 2013. doi: 10.3748/wjg.v19.i29.4752
Revised: May 30, 2013
Accepted: June 8, 2013
Published online: August 7, 2013
Processing time: 124 Days and 13.8 Hours
AIM: To evaluate the diagnostic yield and safety of a modified technique for the histological diagnosis of subepithelial tumors (SETs).
METHODS: A retrospective review of patients who underwent a modified technique for the histological diagnosis of gastric SETs, consisting of a mucosal incision with a fixed flexible snare (MIF) and deep-tissue biopsy under conventional endoscopic view, from January 2012 to January 2013 was performed. Eleven patients with gastric SETs 10-30 mm in diameter and originating from the third or fourth layer on endoscopic ultrasonography were included.
RESULTS: The mean age was 59.8 (range, 45-76) years, and 5 patients were male. The mean size of the SETs was 21.8 (range, 11-30) mm. The number of biopsy specimens was 6.3 (range 5-8). The mean procedure time was 9.0 min (range, 4-17 min). The diagnostic yield of MIF biopsies was 90.9% (10/11). The histological diagnoses were leiomyoma (4/11, 36.4%), aberrant pancreas (3/11, 27.3%), gastrointestinal stromal tumors (2/11, 18.2%), an inflammatory fibrinoid tumor (1/11, 9.1%); one result was non-diagnostic (1/11, 9.1%). There were six mesenchymal tumors; the specimens obtained in each case were sufficient for an immunohistochemical diagnosis. There was no major bleeding, but one perforation occurred that was successfully controlled by endoscopic clipping.
CONCLUSION: The MIF biopsy was simple to perform, safe, and required a shorter procedure time, with a high diagnostic yield for small SETs.
Core tip: Tissue acquisition from subepithelial tumors (SETs) is essential for a differential diagnosis. Several techniques have been introduced to obtain SET tissue samples. However, the diagnostic efficacy was limited or the procedure was complex and difficult. We investigated a modified technique for the histological diagnosis of SETs, consisting of a mucosal incision with a fixed flexible snare (MIF) and deep-tissue biopsy at the incision site under a conventional endoscopic view. The results of this study suggest that the MIF biopsy is simple to perform, safe, fast, and provides a high diagnostic yield for small SETs.
- Citation: Kim JH, Chung JW, Ha M, Rim MY, Lee JJ, An J, Kim YJ, Kim KO, Kwon KA, Park DK, Kim YS, Choi DJ. A feasible modified biopsy method for tissue diagnosis of gastric subepithelial tumors. World J Gastroenterol 2013; 19(29): 4752-4757
- URL: https://www.wjgnet.com/1007-9327/full/v19/i29/4752.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i29.4752
Gastric subepithelial tumors (SETs) are typically found incidentally during screening endoscopies. The exact incidence of SETs on routine endoscopy is unknown, although one retrospective study reported a prevalence of 0.36%[1]. A wide range of diseases may present as SETs in the upper gastrointestinal tract, including lipoma, leiomyoma, aberrant pancreas, varices, carcinoid, gastrointestinal stromal tumors (GISTs), and lymphomas. Thus, tissue diagnosis for SET differentiation is particularly important because these lesions may have different prognoses and have different therapeutic protocols, such as resection or observation.
Gastric SETs are difficult to definitively diagnose by conventional imaging studies, such as ultrasonography, computed tomography, and magnetic resonance imaging. Endoscopic ultrasonography (EUS) is currently the most effective diagnostic tool for the differential diagnosis of SETs because it can help determine the depth and originating layer of the gastrointestinal wall of the lesion[2]. However, EUS morphological characteristics alone do not provide an accurate diagnosis. EUS has limited utility in distinguishing between benign and malignant lesions[3]. In particular, if the SET is found to be a hypoechoic lesion located in the third or fourth layer on EUS findings, tissue acquisition should be strongly considered for a histological diagnosis[3].
Generally, histological diagnosis may not be necessary in large SETs (more than 3 cm in diameter) or symptomatic lesions because such SETs require resection regardless of the pathological confirmation[4,5]. In contrast, small SETs, such as GISTs less than 3 cm in diameter, do not usually require resection because most are benign. However, the current concept is that every GIST has at least malignant potential, even small GISTs of 1 cm in diameter[6,7].
Presently, there is no consensus regarding the management strategy and surveillance of asymptomatic and small SETs[5,8]. For a definitive diagnosis of SETs, tissue acquisition from a subepithelial lesion is essential for a differential diagnosis and an assessment of the malignant potential.
However, conventional endoscopic biopsies do not typically provide sufficient submucosal tissue specimens for diagnosis because SETs are located deep and are covered with normal mucosa. Thus, several techniques have been introduced to obtain SET tissue samples. However, the diagnostic efficacy seems to be limited for immunohistological diagnosis with these methods, such as EUS-guided fine-needle aspiration (EUS-FNA), EUS-guided trucut biopsy (EUS-TCB), and stacked biopsy[9-15].
We thus investigated a modified technique for the histological diagnosis of SETs, consisting of mucosal incision with a fixed flexible snare (MIF) and deep-tissue biopsy at the incision site under a conventional endoscopic view.
A retrospective review of patients who underwent MIF biopsies from January 2012 to January 2013 was conducted. Among the patients with incidental SETs 10-30 mm in diameter, the inclusion criteria were SETs found in the third and fourth layers, with hypoechoic or mixed echogenic patterns on EUS. We excluded patients with typical findings of a vessel, cyst, or lipoma on EUS. We also excluded patients with EUS characteristics suggestive of malignancy, including those with hyperechogenic foci, anechoic necrotic zones, irregular extraluminal borders, or adjacent malignant-appearing lymphadenopathy[16].
Informed consent, with adequate explanation of the biopsy and possible complications, was obtained from each patient. This study was approved by the Institutional Review Board of Gachon University Gil Medical Center (IRB No. GDIRB2013-05).
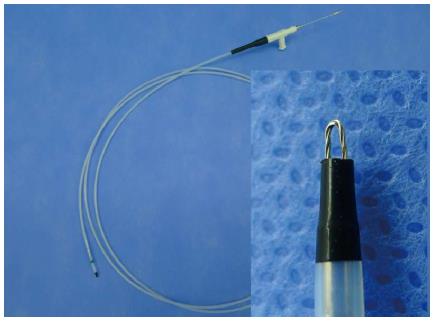
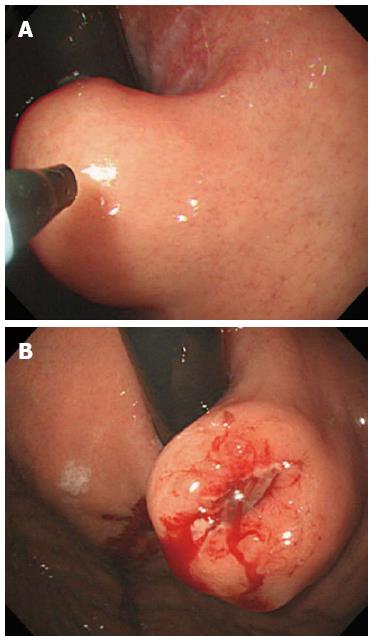
All procedures were performed by one endoscopist (Chung JW) using a conventional single-channel endoscope (GIF Q260 or H260; Olympus Optical Co., Ltd., Tokyo, Japan) with patients under conscious sedation without a transparent hood. An endoscopic-knife (fixed flexible snare; Kachu Technology, Seoul, Korea) connected to an electrosurgical unit (VIO 300D; ERBE, Tübingen, Germany) in “ENDO CUT 1” mode was used for the incision of the mucosa covering the SETs (Figure 1). The length of the tip in this endoscopic knife was 1.5 mm. Under a direct conventional endoscopic view, a mucosal incision was made over the convex zone of the lesion (Figure 2). After the mucosal incision using the fixed flexible snare, we performed a conventional forceps (FB-25K-1; Olympus) biopsy, deep into the incision site of the covering mucosa. Finally, we obtained 5-8 biopsy samples. According to the judgment of the endoscopist, incision site bleeding was controlled using argon plasma coagulation (APC 2; ERBE); the site was closed prophylactically with 2-4 endoclips (HX-610-90L or HX 610-135L; Olympus) in some patients.
Before the MIF biopsy, EUS was performed to characterize the SETs using conventional radial EUS (UM2000; Olympus). All patients were closely monitored for any procedure-related complication in the recovery room and were discharged 2-3 h after the procedure was finished. Oral intake was started 8 h after the procedure. Patients who underwent MIF biopsy empirically received proton pump inhibitors for 1 wk after the procedure. If there was no symptom and/or sign associated with complications, routine follow-up endoscopy was not performed. All patients were instructed to visit our hospital immediately if they had symptoms and/or signs of complications (abdominal pain, hematemesis, melena, dizziness). Patients without symptoms and/or signs of complications visited the outpatient clinic 1-2 wk after the procedure.
Perforation was defined as a split in the muscle layer that occurred during the procedure or the presence of free air detected in post-procedure imaging studies. Major bleeding was defined as bleeding that resulted in a drop in hemoglobin of 2 g/dL or more, that required blood transfusion and/or endoscopic re-intervention, or if surgical intervention caused the hemorrhage. Minor bleeding was defined as bleeding that was controlled by endoscopic hemostasis (argon plasma coagulation or clip) during the procedure.
The forceps biopsy specimens were fixed in a 10% formalin solution and embedded in paraffin wax. The pathologic examinations included identification of cell type, overall cellularity, cytoplasmic features, nuclear atypia, mitotic index, and immunohistochemical findings. The mitotic index was determined on 50 consecutive high-power fields (HPFs). Immunohistochemical analyses of CD117 (c-kit), CD34, desmin, smooth muscle actin, S-100, and Ki-67 markers were performed with commercially available antibodies to classify the tumor subtype. Positive reactions for CD117 and CD34 were considered diagnostic of a GIST. Mesenchymal lesions that were positive for desmin and smooth muscle actin and negative for CD117 and CD34 were diagnosed as smooth muscle tumors such as leiomyoma. Positivity for S-100 protein and negativity for desmin, smooth muscle actin, and CD117 were diagnostic of neural tumors.
Statistical analyses were performed using SPSS software (Ver. 12.0 for Windows; SPSS, Chicago, IL, United States). Continuous data are presented as the means (range), and categorical data are presented as absolute numbers and percentages.
The patient characteristics, location and size of the SETs, histological results, and procedure details are summarized in Table 1. In total, 11 patients were enrolled during the study period. The mean age was 59.8 years (range, 45-76 years); there were five males and six females. The mean size (longest diameter) of the tumors was 21.8 mm (range, 11-30 mm). The number of biopsy specimens was 6.3 (range, 5-8). The mean procedure time was 9.0 min (range 4-17 min).
| Case | Gender | Age (yr) | Location | EUS | MIF biopsy1 | |||||
| Layer | Echogenicity | Size (mm) | Procedure time (min) | Biopsy number (pieces) | Additional procedure | Pathology | ||||
| 1 | Male | 71 | Angle | Fourth | Hypoechoic | 21 | 12 | 7 | Clip | IFT |
| 2 | Female | 46 | LB | Third | Mixed | 15 | 10 | 6 | APC | Aberrant pancreas |
| 3 | Female | 69 | Fundus | Fourth | Hypoechoic | 20 | 5 | 5 | Clip | GISTs |
| 4 | Male | 76 | Cardia | Fourth | Hypoechoic | 21 | 10 | 6 | Clip | Leiomyoma |
| 5 | Female | 65 | HB | Fourth | Mixed | 27 | 7 | 6 | No | GISTs |
| 6 | Female | 47 | LB | Third | Hypoechoic | 30 | 11 | 8 | No | CAG |
| 7 | Female | 45 | Angle | Third | Mixed | 28 | 4 | 7 | No | Aberrant pancreas |
| 8 | Male | 71 | Angle | Fourth | Mixed | 26 | 9 | 6 | Clip | Aberrant pancreas |
| 9 | Female | 46 | Cardia | Fourth | Hypoechoic | 11 | 7 | 7 | No | Leiomyoma |
| 10 | Male | 62 | HB | Fourth | Mixed | 22 | 17 | 6 | Clip | Leiomyoma |
| 11 | Male | 60 | Cardia | Fourth | Hypoechoic | 19 | 7 | 5 | Clip | Leiomyoma |
The MIF biopsy provided specimens that were sufficient for a definitive histological diagnosis in 90.9% (10/11) of cases. The histological diagnoses were leiomyoma (36.4%, 4/11), aberrant pancreas (27.3%, 3/11), GIST (18.2%, 2/11), and inflammatory fibrinoid tumor (9.1%, 1/11), and one result was non-diagnostic (9.1%, 1/11; Table 1). There were six mesenchymal tumors (4 leiomyomas, 2 GISTs), and the specimens obtained were large enough for immunohistochemical diagnoses. Both cases (case No. 3, 5) with GISTs had a spindle cell-type tumor with intermediate mitotic activity (mitotic index 5-10/50 HPFs). These patients had undergone surgical resection (wedge resection), and the results of the biopsy and the surgical resection were consistent.
The patient with a non-diagnostic result (case No. 6) refused a re-biopsy and did not want further evaluation or surgical resection. Thus, this patient was followed annually, and a final histological diagnosis was not reached. One perforation (case No. 10) was observed, and it was successfully controlled by endoscopic clipping. No major bleeding was recorded, but 63.6% (7/11) of patients showed minor bleeding.
We present a modified biopsy technique for the histological diagnosis of SETs. The diagnostic accuracy of MIF biopsies was 90.9% in our study. Adequate samples for diagnosis were obtained from 10 of 11 patients. The success rate was higher than other previously reported conventional methods.
Despite an endoscopist’s intention to obtain tissue from submucosal lesions, conventional methods such as large-capacity “jumbo” forceps biopsies acquire submucosa for diagnosis with an approximately 17% yield[17]. Recent studies have investigated EUS-based methods, which have several limitations, despite a higher success rate than previously reported methods. EUS-FNA can obtain only a limited number of cells and cannot determine the structure of the organization, although the method typically has a 60%-80% success rate[10,14,18,19]. EUS-TCB generally has a similar yield to EUS-FNA, with no additional benefit[10]. Additionally, with EUS-TCB, it is not easy to obtain sufficient tissue with intact tissue architectural details for determining the mitotic index. However, it can provide a higher success rate than EUS-FNA in some patients requiring immunostaining. Combined EUS-FNA and EUS-TCB has been reported to have a diagnostic yield as high as 77%, although with a longer procedure time and higher cost[14].
The MIF biopsy is a simple technique, first making an incision in the mucosa covering the SETs, followed by acquiring SET tissues at the incision site with conventional biopsy forceps. Another advantage of this method is that it is not difficult regardless of the location of the lesion. In contrast, EUS-FNA and EUS-TCB are limited by technical problems in approaching the antrum and at angles because of the stiffness of the device and the rubbery consistency of the subepithelial mass[9,20].
Recently, there have been efforts to resect gastric SETs using ESD techniques, which provided successful resection of SETs in 74.3%-81.1% of cases, with a mean procedure time of 60.9 (range, 20-170) min[21-23]. There has been no report of life-threatening complications, although the incidence of complications was relatively high, at 12%-17%. In our study, the mean procedure time of the MIF biopsy was short (9 min), and the success rate was high (90.9%). Large GISTs with high potential for malignancy should be removed using surgical or endoscopic approaches. However, resection of all small SETs may be an unnecessarily invasive and money-wasting treatment, considering the risk of complications and cost effectiveness. Thus, a pre-resection histological evaluation is essential for SETs, and the MIF biopsy may provide a useful alternative technique in this regard.
One reported method for the adequate tissue acquisition of SETs is to remove the mucosa covering the SETs using an endoscopic knife for an endoscopic submucosal dissection (ESD) and to then to perform a partial resection of the SET[24]. This method provides a 93.7% diagnostic yield, but the procedure is more complex and difficult than the MIF biopsy we describe. Another ESD technique is mucosal incision-assisted biopsy (MIAB), which allows a mucosal incision at the circumferential margin of the lesion using an ESD-associated technique, followed by submucosal dissection to expose the SETs and then biopsy. This method differs from MIF biopsy, which involves an incision in the mucosa covering the top of the convex zone. MIAB appears to be much more complex and difficult than the MIF biopsy[25].
Another method, similar to the MIF biopsy, was reported recently and involves performing a mucosal incision using a needle-knife sphincterotome (Microknife XL; Boston Scientific Inc., Natick, MA, United States), followed by sampling of the tissues inside and then prophylactic clipping (a SINK biopsy)[26]. The difference between the two methods is that we used a fixed flexible snare and did not routinely perform prophylactic endoscopic clipping. We performed APC or clipping for minor bleeding in 63.6% (6/11) of cases, which did not require additional endoscopy or re-admission for post-procedural bleeding. The mucosal incision with multiple deep biopsies appeared to be relatively safe in terms of bleeding, even without prophylactic APC or clipping.
The MIF biopsy needs to be performed carefully, depending on the shape of the SETs. Mucosal incision with deep biopsies should not be technically difficult if the SET is an exophytic “ball shape” growing toward the gastric lumen. However, a slightly elevated lesion, not a ball-shaped protruding lesion, necessitates a careful procedure. One (case No. 6) of our patients could not be diagnosed after the MIF biopsy, and another patient (case No. 10) experienced perforation; the SETs were slightly elevated, i.e., mounded, in both cases. We suggest that these lesions were difficult to target because they were movable, and it was therefore difficult to identify the correct location when making the mucosal incision. Thus, SETs with such shapes require special attention.
Our data suggest that the MIF biopsy is a safe and effective method for the tissue diagnosis of small SETs. However, we recognize the limitations of this study. This was a retrospective study at a single tertiary academic center, and the sample size was small.
In conclusion, MIF biopsy was simple to perform, safe, required a shorter procedure time, and provided a high diagnostic yield for small SETs. Further comparative, prospective studies with larger sample sizes are required.
Gastric subepithelial tumors (SETs) are difficult to definitively diagnose by conventional imaging studies. Therefore, tissue acquisition from SETs is essential for a differential diagnosis. Conventional endoscopic biopsies do not typically provide sufficient submucosal tissue specimens for diagnosis. Several techniques have been introduced to obtain SET tissue samples. However, their diagnostic efficacies were limited, or the procedures were complex and difficult. Authors investigated a modified technique for the histological diagnosis of SETs, consisting of a mucosal incision with a fixed flexible snare (MIF) and deep-tissue biopsy at the incision site under a conventional endoscopic view.
Presently, there is no consensus regarding the management strategy and surveillance of asymptomatic and small SETs. For a definitive diagnosis of SETs, tissue acquisition from a subepithelial lesion is essential for a differential diagnosis and an assessment of the malignant potential. Authors present a modified biopsy technique for the histological diagnosis of small SETs. The diagnostic accuracy of MIF biopsies was 90.9% in our study. The success rate was higher than other previously reported conventional methods.
The results of this study suggest that the MIF biopsy is simple to perform, safe, requires a shorter procedure time, and provides a high diagnostic yield for small SETs. Another advantage of this method is that it is not difficult regardless of the location of the lesion.
Their data suggest that the MIF biopsy is a safe and effective method for the tissue diagnosis of small SETs. A pre-resection histological evaluation is essential for SETs, and the MIF biopsy may provide a useful alternative technique.
The modified technique that uses the MIF biopsy consists of two steps. Under direct conventional endoscopic view, the incision of the mucosa covering the SETs is made over the convex zone of the lesion using an endoscopic knife. After the mucosal incision is created using the fixed flexible snare, a conventional forceps biopsy is performed, deep in the incision site of the covering mucosa.
This paper reports the usefulness of a mucosal incision with a fixed flexible snare and a deep-tissue biopsy for the histological diagnosis of gastric subepithelial tumors. A modified biopsy method is feasible for the tissue diagnosis of gastric subepithelial tumors. The article is well written, and the findings are of practical importance.
P- Reviewers Devanarayana NM, Sioulas AD S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 207] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Humphris JL, Jones DB. Subepithelial mass lesions in the upper gastrointestinal tract. J Gastroenterol Hepatol. 2008;23:556-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology. 2004;126:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2217-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 7. | Huang HY, Li CF, Huang WW, Hu TH, Lin CN, Uen YH, Hsiung CY, Lu D. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Ha CY, Shah R, Chen J, Azar RR, Edmundowicz SA, Early DS. Diagnosis and management of GI stromal tumors by EUS-FNA: a survey of opinions and practices of endosonographers. Gastrointest Endosc. 2009;69:1039-44.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Ginès A, Wiersema MJ, Clain JE, Pochron NL, Rajan E, Levy MJ. Prospective study of a Trucut needle for performing EUS-guided biopsy with EUS-guided FNA rescue. Gastrointest Endosc. 2005;62:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Polkowski M, Bergman JJ. Endoscopic ultrasonography-guided biopsy for submucosal tumors: needless needling? Endoscopy. 2010;42:324-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Polkowski M, Gerke W, Jarosz D, Nasierowska-Guttmejer A, Rutkowski P, Nowecki ZI, Ruka W, Regula J, Butruk E. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: a prospective study. Endoscopy. 2009;41:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Philipper M, Hollerbach S, Gabbert HE, Heikaus S, Böcking A, Pomjanski N, Neuhaus H, Frieling T, Schumacher B. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 436] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 14. | Fernández-Esparrach G, Sendino O, Solé M, Pellisé M, Colomo L, Pardo A, Martínez-Pallí G, Argüello L, Bordas JM, Llach J. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: a randomized crossover study. Endoscopy. 2010;42:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Motomura Y, Honda K, Watanabe M, Nagaie T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077-2082. [PubMed] |
| 16. | Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 220] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc. 2003;57:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Mekky MA, Yamao K, Sawaki A, Mizuno N, Hara K, Nafeh MA, Osman AM, Koshikawa T, Yatabe Y, Bhatia V. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Moon JS. Endoscopic ultrasound-guided fine needle aspiration in submucosal lesion. Clin Endosc. 2012;45:117-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Lee JH, Choi KD, Kim MY, Choi KS, Kim do H, Park YS, Kim KC, Song HJ, Lee GH, Jung HY. Clinical impact of EUS-guided Trucut biopsy results on decision making for patients with gastric subepithelial tumors ≥ 2 cm in diameter. Gastrointest Endosc. 2011;74:1010-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Lee IL, Lin PY, Tung SY, Shen CH, Wei KL, Wu CS. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy. 2006;38:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Bialek A, Wiechowska-Kozłowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpińska K, Ławniczak M, Starzyńska T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012;75:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 23. | Chun SY, Kim KO, Park DS, Lee IJ, Park JW, Moon SH, Baek IH, Kim JH, Park CK, Kwon MJ. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: a preliminary analysis of appropriate indications. Surg Endosc. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Lee CK, Chung IK, Lee SH, Lee SH, Lee TH, Park SH, Kim HS, Kim SJ, Cho HD. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010;71:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Ihara E, Matsuzaka H, Honda K, Hata Y, Sumida Y, Akiho H, Misawa T, Toyoshima S, Chijiiwa Y, Nakamura K. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J Gastrointest Endosc. 2013;5:191-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | de la Serna-Higuera C, Pérez-Miranda M, Díez-Redondo P, Gil-Simón P, Herranz T, Pérez-Martín E, Ochoa C, Caro-Patón A. EUS-guided single-incision needle-knife biopsy: description and results of a new method for tissue sampling of subepithelial GI tumors (with video). Gastrointest Endosc. 2011;74:672-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |