Published online Aug 7, 2013. doi: 10.3748/wjg.v19.i29.4718
Revised: March 20, 2013
Accepted: April 10, 2013
Published online: August 7, 2013
Processing time: 187 Days and 0.2 Hours
AIM: To determine the efficacy of probiotic supplementation on intestinal transit time (ITT) and to identify factors that influence these outcomes.
METHODS: A systematic review of randomized controlled trials (RCTs) of probiotic supplementation that measured ITT in adults was conducted by searching MEDLINE and EMBASE using relevant key word combinations. Main search limits included RCTs of probiotic supplementation in healthy or constipated adults that measured ITT. Study quality was assessed using the Jadad scale. A random effects meta-analysis was performed with standardized mean difference (SMD) of ITT between probiotic and control groups as the primary outcome. Meta-regression and subgroup analyses were conducted to examine the impact of moderator variables on ITT SMD.
RESULTS: A total of 11 clinical trials with 13 treatment effects representing 464 subjects were included in this analysis. Probiotic supplementation was associated with decreased ITT in relation to controls, with an SMD of 0.40 (95%CI: 0.20-0.59, P < 0.001). Constipation (r2 = 39%, P = 0.01), higher mean age (r2 = 27%, P = 0.03), and higher percentage of female subjects (r2 = 23%, P < 0.05) were predictive of decreased ITT with probiotics in meta-regression. Subgroup analyses demonstrated statistically greater reductions in ITT with probiotics in subjects with vs without constipation and in older vs younger subjects [both SMD: 0.59 (95%CI: 0.39-0.79) vs 0.17 (95%CI: -0.08-0.42), P = 0.01]. Medium to large treatment effects were identified with Bifidobacterium Lactis (B. lactis) HN019 (SMD: 0.72, 95%CI: 0.27-1.18, P < 0.01) and B. lactis DN-173 010 (SMD: 0.54, 95%CI: 0.15-0.94, P < 0.01) while other single strains and combination products yielded small treatment effects.
CONCLUSION: Overall, short-term probiotic supplementation decreases ITT with consistently greater treatment effects identified in constipated or older adults and with certain probiotic strains.
Core tip: Clinical trials of probiotics for gut health often utilize intestinal transit time (ITT) as a measure of clinical success although treatment effects are not consistent across studies. We performed the first systematic review and meta-analysis of randomized controlled trials to investigate the efficacy of probiotic supplementation on ITT in adults and to identify factors that influence these outcomes. Overall, short-term probiotic supplementation decreases ITT with consistently greater treatment effects identified in constipated or older adults and with certain probiotic strains.
- Citation: Miller LE, Ouwehand AC. Probiotic supplementation decreases intestinal transit time: Meta-analysis of randomized controlled trials. World J Gastroenterol 2013; 19(29): 4718-4725
- URL: https://www.wjgnet.com/1007-9327/full/v19/i29/4718.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i29.4718
Functional gastrointestinal (GI) disorders are symptom-based conditions that are not explained by definable structural or biochemical causes[1]. The prevalence of at least one functional GI disorder in the last 3 mo has been reported to be as high as 69% in the general population[2]. Slow intestinal transit is a common symptom of functional GI disorders, particularly those involving the bowel[3]. Therapies intended to ameliorate GI-related symptoms by decreasing intestinal transit time (ITT), such as laxatives, are a mainstay treatment of slow-transit bowel disorders although no known therapy is highly efficacious, safe, and cost effective[4].
Probiotics are live micro-organisms that confer a health benefit on the host when administered in adequate dosages[5], which have been extensively studied for treatment of functional GI disorders[6,7]. Additionally, there is speculation that probiotics may even improve gut health in healthy adults. For example, the European Food Safety Authority (EFSA) guidance on health claims related to gut function states that reduced ITT may be considered a beneficial physiological effect in the non-diseased general population, provided that diarrhea does not develop[8]. Consequently, ITT often serves as a primary study endpoint in probiotic clinical trials of gut health.
Based on the recent emphasis in this study endpoint in clinical trials and because accurate estimates of ITT effect size are mandatory for performing power calculations and estimating sample size in clinical trials, we performed the first systematic review and meta-analysis on the efficacy of probiotic supplementation on ITT in adults.
The main objective of this systematic review and meta-analysis of RCTs was to assess the efficacy of probiotic supplementation on ITT in adults. The PRISMA Statement for reporting systematic reviews and meta-analyses served as a template for this report[9].
Studies that were eligible for consideration in this systematic review were RCTs published in English-language journals and indexed in MEDLINE or EMBASE with no date restrictions on the effects of probiotic supplementation on ITT in adults. The following search terms were used for probiotic supplementation (with “*” characterizing a wildcard and “OR” being used as a Boolean function): probiotic*; lactobacill*; bifidobacteri*; yogurt; yoghurt; fermented milk. The following search terms were used for ITT: gastrointestinal; transit; gut; motility; colonic; constipation; irritable bowel. To identify clinical trials, we applied the filters Clinical Trial or Randomized Controlled Trial. The results of each of the three sections were combined by utilizing the “AND” Boolean. In addition, we attempted to identify additional studies by hand-searching references of included studies and relevant review articles.
One reviewer (Miller LE) initially assessed study eligibility. Titles and abstracts were screened to exclude all manuscripts published in non-English journals. Next, review articles, commentaries, letters, and case reports were excluded. We also excluded obviously irrelevant articles. Lastly, we excluded studies of subjects where ITT reduction was undesirable or uninterpretable (i.e., subjects with diarrhea or cohorts with multiple IBS subtypes). Full-text of the remaining manuscripts was retrieved and reviewed. Publications that failed to report ITT or that described non-randomized, non-controlled, or otherwise irrelevant studies were excluded. The last search was performed in December 2012.
Data were extracted and entered into a pre-designed database by one reviewer (Miller LE) and the entries were checked by the other reviewer (Ouwehand AC). Disagreements were settled by consensus.
The following variables were recorded in a pre-designed database: general manuscript information (author, institution name and location, journal, year, volume, page numbers), study design characteristics (study quality, study design, sample size, method of ITT assessment, probiotic strain, daily dosage, product delivery method, and treatment duration), subject characteristics (age, gender, body mass index, and condition), and ITT before and after probiotic supplementation.
We used the Jadad scale to assess study quality of RCTs[10]. Studies were scored according to the presence of three key methodological features: randomization, blinding and subject accountability. Randomization was scored from 0 to 2 with 2 implying appropriate methods of randomization were described, 1 if the study was merely described as “randomized”, and 0 when no details were provided to evaluate randomization. A score of 0 was given if the study was described as randomized, but the method of randomization was clearly inappropriate. Similarly, blinding was scored from 0 to 2 with 2 points awarded if subjects and investigators were blinded using appropriate methods, 1 point if the study was described merely as blinded, and 0 points if the study was described as blinded, but the method of blinding was clearly inappropriate. Subject accountability was scored 0 or 1 with 1 point awarded if all subjects were accounted for in the analysis and reasons for withdrawals were provided. A score of 0 was given when information regarding withdrawals was incomplete. A priori, studies with a Jadad score of 3 to 5 were deemed higher quality and those with a score of 0 to 2 were classified as lower quality.
A random effects meta-analysis model was selected a priori based on the assumption that the true effect may vary among studies based on known differences in probiotic strain, study design characteristics, and subject characteristics. The standardized mean difference (SMD) and 95%CI was selected to report treatment effects because different measures of ITT (e.g., whole gut, colonic, oro-cecal, etc.) were utilized in the included studies. The SMD is a measure of effect size for continuous outcomes defined as the mean difference between groups divided by the pooled standard deviation. SMD values of 0.2, 0.5 and 0.8 are defined as small, medium, and large, respectively[11]. A forest plot was used to illustrate the individual study findings and the random effects meta-analysis results. We used the I2 statistic to estimate heterogeneity of effects across studies with values of ≤ 25%, 50%, and ≥ 75% representing low, moderate, and high inconsistency, respectively[12]. An alpha error P < 0.05 and/or I2≥ 50% were taken as indicators of substantial heterogeneity of treatment effects. Publication bias was visually assessed with a funnel plot (not shown) and quantitatively assessed using Egger’s test[13]. Meta-regressions and pre-defined subgroup analyses were undertaken to quantify the relationship of individual moderators on ITT SMD. All analyses were performed using Comprehensive Meta-analysis (version 2.2, Biostat, Englewood, NJ, United States).
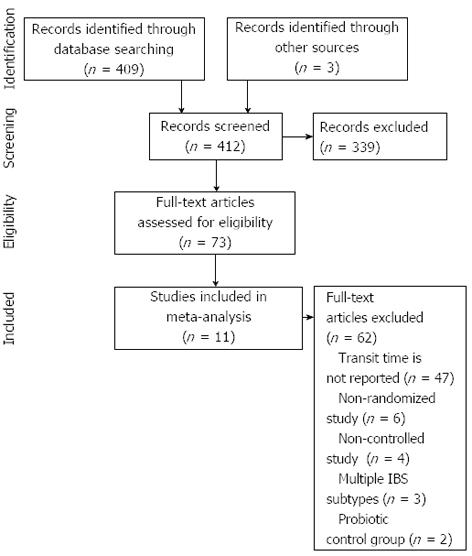
Our initial database search retrieved 409 titles and abstracts and hand searching relevant bibliographies identified 3 additional records. After screening records for inclusion criteria, 73 full text articles were reviewed for eligibility. Ultimately, 11 RCTs with 13 treatment effects representing 464 distinct subjects were included in the final analysis[14-24]. A flow chart of study identification and selection is shown in Figure 1.
Sample sizes were generally small, ranging from 10 to 36 per treatment group for parallel groups designs and from 12 to 83 for cross-over designs. The average detectable effect size, based on sample size and study design by assuming P = 0.05 and statistical power = 80%, was 0.8 (range: 0.3 to 1.3). Eleven RCTs contributed one treatment effect each. The study of Rosenfeldt et al[21] contributed two treatment effects (two different probiotic formulations) and the study of Waller et al[23] contributed two treatment effects (same probiotic strain, two different dosages). Eight of the 11 studies were parallel groups designs while 3 were cross-over studies. The most commonly studied probiotic strains were Bifidobacterium lactis (B. lactis) DN-173 010 (3 treatment effects), B. lactis HN019 (2 treatment effects), and Lactobacillus rhamnosus (L. rhamnosus) GG (2 treatment effects). Daily probiotic dosages varied substantially across studies, ranging from 5 × 108 to 9.75 × 1010 cfu per day (median 1.72 × 1010 cfu per day). Supplementation periods ranged from 10 to 28 d (median 18 d). Intestinal transit time was quantified using radiopaque markers in 10 studies and with carmine red dye in 1 study[19]. The most commonly tested product format was yogurt or other forms of fermented milk. Two studies were confounded by inclusion of other components in the active product that may influence ITT such as lactulose[15] and the combination of inulin and oligofructose[19] (Table 1). Seven treatment effects were calculated based on subjects with constipation or irritable bowel syndrome-C while 6 were based on healthy subjects. Subjects were predominantly female with a mean age ranging from 23 to 50 years and mean body mass index ranging from 21 to 32 kg/m2 (Table 2).
| Ref. | Study design | Active: control (n:n) | Transit timeoutcome, method | Probiotic strain | Daily dosage(109 cfu) | Delivery method | Treatmentduration (d) |
| Agrawal et al[14] | Parallel groups | 17:17 | CTT, radiopaque markers | B. lactis DN-173 010 | 25.0 | Active: Yogurt + probioticControl: Nonfermented milk-based product | 28 |
| Bartram et al[15] | Cross-over | 12 | OATT, radiopaque markers | B. longum | > 0.5 | Active: Yogurt with 2.5 g lactulose + probioticControl: Yogurt | 21 |
| Bouvier et al[16] | Parallel groups | 36:36 | CTT, radiopaque markers | B. lactis DN-173 010 | 97.5 | Active: Probiotic fermented milkControl: Heat-treated probiotic fermented milk | 11 |
| Holma et al[17] | Parallel groups | 12:10 | TITT, radiopaque markers | L. rhamnosus GG | 20 | Active: Buttermilk + probiotic and white wheat breadControl: White wheat bread | 21 |
| Hongisto et al[18] | Parallel groups | 16:14 | TITT, radiopaque markers | L. rhamnosus GG | 15 | Active: Yogurt + probiotic and low fiber toastControl: Low fiber toast | 21 |
| Malpeli et al[19] | Cross-over | 83 | OCTT, carmine red dye | B. lactis BB12L. casei CRL 431 | 2-20 2-12 | Active: Yogurt with 0.625 g inulin and oligofructose + probioticControl: Yogurt | 15 |
| Marteau et al[20] | Cross-over | 32 | CTT, radiopaque markers | B. lactis DN-173 010 | 18.75 | Active: Yogurt + probioticControl: Yogurt | 10 |
| Rosenfeldt et al[21] | Cross-over | 13 | GTT, radiopaque markers | L. rhamnosus 19070-2,L. reuteri DSM 12246 | 20 20 | Active: Freeze-dried powder + probioticControl: Skimmed milk powder w/dextrose | 18 |
| Rosenfeldt et al[21] | Cross-over | 13 | GTT, radiopaque markers | L. casei subsp. alactus CHCC 3137,L. delbrueckii subsp. lactis CHCC 2329,L. rhamnosus GG | 20 20 20 | Active: Freeze-dried powder + probioticControl: Skimmed milk powder w/dextrose | 18 |
| Sairanen et al[22] | Parallel groups | 22:20 | CTT, radiopaque markers | B. longum BB536, B. lactis 420,L. acidophilus 145 | 2.4-181 0.48 | Active: Probiotic fermented milkControl: Fermented milk | 21 |
| Waller et al[23] | Parallel groups | 33:34 | WGTT; radiopaque markers | B. lactis HN019 | 1.8 | Active: Capsule, maltodextrin, probioticControl: Capsule, maltodextrin | 14 |
| Waller et al[23] | Parallel groups | 33:34 | WGTT; radiopaque markers | B. lactis HN019 | 17.2 | Active: Capsule, maltodextrin, probioticControl: Capsule, maltodextrin | 14 |
| Krammer et al[24] | Parallel groups | 12:12 | CTT, radiopaque markers | L. casei Shirota | 6.5 | Active: Probiotic fermented milk drinkControl: Nonfermented milk drink | 28 |
| Ref. | Age (yr) | Female gender | BMI (kg/m2) | Condition |
| Agrawal et al[14] | 40 | 100% | 25 | IBS-C |
| Bartram et al[15] | 23 | 58% | - | None |
| Bouvier et al[16] | 33 | 50% | 22 | None |
| Holma et al[17] | 44 | 92%1 | 24 | Constipation |
| Hongisto et al[18] | 43 | 100% | 24 | Constipation |
| Malpeli et al[19] | 41 | 100% | - | Constipation |
| Marteau et al[20] | 27 | 100% | 21 | None |
| Rosenfeldt et al[21] | 25 | 0% | - | None |
| Rosenfeldt et al[21] | 25 | 0% | - | None |
| Sairanen et al[22] | 39 | 64% | 25 | None |
| Waller et al[23] | 44 | 65% | 31 | Constipation |
| Waller et al[23] | 44 | 65% | 32 | Constipation |
| Krammer et al[24] | 50 | 100% | - | Constipation |
Overall, the quality of RCT reporting was medium with a median Jadad score of 3 (range: 1-5). Eight of 13 treatment effects were based on higher quality (Jadad score 3-5) trials. The method of randomization was unclear in most studies. Descriptions of blinding were adequate overall. Subject accountability in RCTs was mentioned in only 7 of 13 cases (Table 3).
| Ref. | Jadad scale | |||
| Randomization(range: 0-2) | Double blinding(range: 0-2) | Subject account(range: 0-1) | Total score1(range: 0-5) | |
| Agrawal et al[14] | 1 | 2 | 1 | 4 |
| Bartram et al[15] | 1 | 2 | 0 | 3 |
| Bouvier et al[16] | 1 | 2 | 0 | 3 |
| Holma et al[17] | 1 | 0 | 1 | 2 |
| Hongisto et al[18] | 1 | 0 | 0 | 1 |
| Malpeli et al[19] | 0 | 2 | 1 | 3 |
| Marteau et al[20] | 1 | 2 | 1 | 4 |
| Rosenfeldt et al[21] | 1 | 1 | 0 | 2 |
| Rosenfeldt et al[21] | 1 | 1 | 0 | 2 |
| Sairanen et al[22] | 1 | 1 | 0 | 2 |
| Waller et al[23] | 2 | 2 | 1 | 5 |
| Waller et al[23] | 2 | 2 | 1 | 5 |
| Krammer et al[24] | 1 | 1 | 1 | 3 |
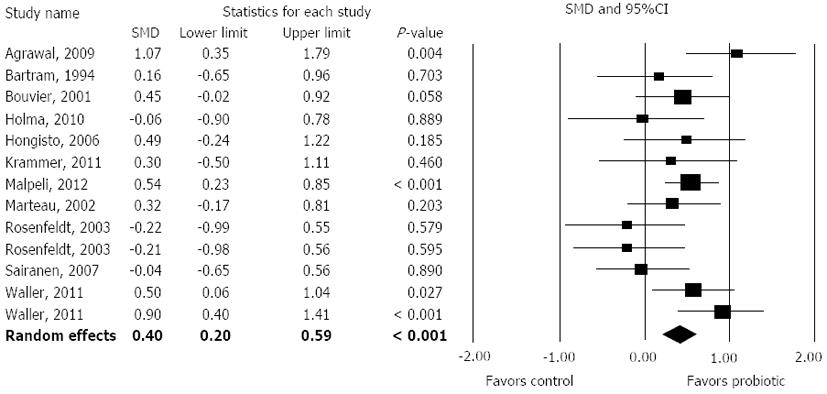
Overall, probiotic supplementation was associated with reduced ITT, with an SMD of 0.40 (95%CI: 0.20-0.59, P < 0.001) (Figure 2). There was low heterogeneity among studies (I2 = 29%, P = 0.15) with no evidence of publication bias (Egger’s regression test: P = 0.13). Only 4 of 13 individual treatment effects statistically favored probiotic supplementation.
We performed meta-regression analysis including predefined covariates to explore the potential predictors of SMD. Constipation (r2 = 39%, P = 0.01), higher mean age (r2 = 27%, P = 0.03), and higher percentage of female subjects (r2 = 23%, P < 0.05) were predictive of decreased ITT with probiotics in meta-regression (Table 4). Additionally, we performed a pre-defined subgroup analysis to observe the influence of study- and subject-related characteristics on SMD (Table 5). Subgroup analyses demonstrated statistically greater reductions in ITT with probiotics in subjects with vs without constipation and in older vs younger subjects (both SMD: 0.59 vs 0.17, P = 0.01). Study design, body mass index, treatment duration, and daily probiotic dosage had no influence on probiotic treatment effects in any analysis. Analysis of outcomes by probiotic strain identified medium to large treatment effects with B. lactis HN019 (SMD: 0.72, P < 0.01) and B. lactis DN-173 010 (SMD: 0.54, P < 0.01) while treatment effects with other single strains and combination products were small (SMD: 0.17-0.25) and not statistically significant (Table 6).
| Variable | Unit of measure | Intercept | Point estimate | Explained variance | P-value |
| Constipation | 0 = no, 1 = yes | 0.171 | 0.415 | 39% | 0.01 |
| Age | Per 10 years | -0.445 | 0.230 | 27% | 0.03 |
| Female gender proportion | Per 10% | 0.024 | 0.053 | 23% | < 0.05 |
| Body mass index1 | Per 5 kg/m2 | -0.544 | 0.200 | 25% | 0.11 |
| Daily probiotic dosage | Per 10 × 109 cfu | 0.454 | -0.013 | 1% | 0.62 |
| Treatment duration | Per 1 wk | 0.535 | -0.048 | 1% | 0.67 |
| Study | SMD | 95%CI | P-value(within groups) | P-value(between groups) |
| Subject condition | ||||
| Constipation/IBS-C (n = 7) | 0.59 | 0.39-0.79 | < 0.001 | 0.01 |
| Healthy (n = 6) | 0.17 | -0.08-0.42 | 0.18 | |
| Age | ||||
| ≥ 40 years (n = 7) | 0.59 | 0.39-0.79 | < 0.001 | 0.01 |
| < 40 years (n = 6) | 0.17 | -0.08-0.42 | 0.18 | |
| Study design | ||||
| Parallel groups (n = 8) | 0.49 | 0.24-0.75 | < 0.001 | 0.23 |
| Cross-over (n = 5) | 0.25 | -0.06-0.56 | 0.11 | |
| Body mass index1 | ||||
| ≥ 25 kg/m2 (n = 4) | 0.61 | 0.27-0.95 | < 0.001 | 0.29 |
| < 25 kg/m2 (n = 4) | 0.34 | -0.02-0.70 | 0.06 | |
| Female gender proportion | ||||
| ≥ 75% (n = 6) | 0.44 | 0.17-0.76 | < 0.01 | 0.47 |
| < 75% (n = 7) | 0.32 | 0.05-0.60 | 0.02 | |
| Treatment duration | ||||
| < 20 d (n = 7) | 0.43 | 0.18-0.67 | < 0.001 | 0.62 |
| ≥ 20 d (n = 6) | 0.32 | -0.02-0.66 | 0.07 | |
| Daily probiotic dosage | ||||
| ≥ 1010 cfu (n = 8) | 0.41 | 0.14-0.68 | < 0.01 | 0.84 |
| < 1010 cfu (n = 5) | 0.36 | 0.05-0.68 | 0.02 |
| Probiotic strain | Treatment effects (n) | SMD | 95%CI | P-value |
| B. lactis HN019 | 2 | 0.72 | 0.27-1.18 | < 0.01 |
| B. lactis DN-173 010 | 3 | 0.54 | 0.15-0.94 | < 0.01 |
| L. rhamnosus GG | 2 | 0.25 | -0.38-0.87 | 0.44 |
| Other single strains | 2 | 0.23 | -0.41-0.87 | 0.48 |
| Strain combinations | 4 | 0.17 | -0.18-0.52 | 0.34 |
Clinical trials of probiotic supplementation often utilize ITT as a primary efficacy outcome. However, inconsistent treatment effects among trials have been observed, likely due to differences among study designs, probiotic strains, dosing regimens, and subject characteristics. We performed the first systematic review and meta-analysis on this topic and demonstrated that, overall, short-term (10-28 d) probiotic supplementation is able to reduce ITT in adults. We also demonstrated that the treatment effect of probiotics is strongly dependent on: (1) the presence or absence of constipation; (2) subject age; and (3) probiotic strain.
Presence of constipation and older age were predictive of greater ITT treatment effects with probiotic supplementation. Constipation was the primary influencer of probiotic treatment effects on ITT, explaining 39% of the variance in SMD. The independent influence of subject age, after accounting for constipation, is unknown and may be confounded since the seven studies that enrolled the oldest subjects were the same studies that enrolled constipated subjects. The number of treatment effects per strain is limited; B. lactis DN-173 010 (3), B. lactis HN019 (2) and L. rhamnosus GG (2). Drawing definite conclusions is therefore perilous, but the finding that the former two strains have notably greater treatment effects on ITT suggests that these strains could be considered when aiming to relieve slow intestinal transit.
The clinical importance of ITT is highly dependent on the underlying pathology. In healthy adults with no evidence of GI disturbances or delayed transit, there is arguably little benefit in lowering ITT[25]. In contrast to this position, EFSA considers that a reduction in ITT within the normal range to be a possibly beneficial physiological effect in healthy adults[8]. Overall, probiotic supplementation for this sole purpose cannot be strongly recommended given the questionable clinical benefit and the small effect size (SMD: 0.17) identified in this meta-analysis. In adults with constipation or IBS, a reduction in ITT is moderately associated with improvements in stool form and frequency[25,26]. Therefore, probiotic supplementation appears to be a reasonably effective option to achieve this therapeutic goal provided diarrhea does not develop. Current evidence suggests that probiotics contribute to lowering intestinal pH, decreasing colonization and invasion by pathogenic organisms, and modifying the host immune response with few known side effects[27]. However, there is no strong evidence from RCTs that probiotics improve symptoms such as abdominal pain or bloating in these patients[28]. The clinical importance of decreased ITT in the absence of symptom amelioration is controversial and requires further exploration.
Interestingly, this meta-analysis identified a positive benefit of probiotic supplementation on ITT although only 4 of 13 treatment effects demonstrated such a benefit. This is likely because the majority of clinical trials were underpowered due to small sample size. In fact, only 1 treatment effect was identified from a study with a minimum detectable effect size ≤ 0.5 (moderate effect) and only 5 had a minimum detectable effect size ≤ 0.8 (large effect). Considering the overall ITT SMD with probiotic supplementation is only 0.4, it is clear that small sample size and, consequently, inadequate statistical power was the main driver of the high failure rate of individual studies.
The use of estimated SMD is an integral component of study design development and sample size estimation for RCTs. Sample sizes for RCTs based on estimated SMD are shown in Table 7. Based on the SMDs calculated in this meta-analysis, enrollment of approximately 90 subjects would be required in a study of probiotics for constipation or irritable bowel syndrome-C with a parallel groups design or 19 subjects if utilizing a cross-over design. In comparison, for a trial of healthy volunteers, required sample sizes would be 786 and 156 for parallel groups and cross-over designs, respectively, in order to achieve adequate statistical power. Although cross-over trials always require a smaller sample size for a given SMD since subjects serve as their own controls, the main disadvantages of this design include a longer time on study, higher attrition rates due to the extended trial duration, and difficulties in estimating an appropriate washout duration. As such, cross-over designs are inappropriate for clinical trials with extended treatment durations or long or unknown washout periods.
The strengths of this systematic review and meta-analysis include selection of only RCTs to minimize bias and the comprehensive assessment of the impact of moderator variables on the primary outcome. Nevertheless, our analysis was associated with several limitations. First, treatment duration in the reviewed studies ranged from 10 to 28 d and, therefore, the treatment effect of longer term probiotic supplementation on ITT is unknown. Second, the therapeutic benefit of probiotics is considered to be strain-specific; however, the small number of studies performed with each strain prevented robust strain-specific comparisons. Third, there was a significant over-representation of subjects who were young to middle-aged, female, and with a normal body mass index. Abundant caution must be exercised when extrapolating the treatment effects observed in this review to a broader population. Finally, we noted significant heterogeneity among ITT measurement methods as well as product delivery methods and additional included ingredients (e.g., prebiotics) among studies. There is potential for these differences to confound the results of our analysis.
In conclusion, short-term probiotic supplementation decreases ITT with consistently greater treatment effects identified in constipated or older adults and with certain probiotic strains.
Functional gastrointestinal (GI) disorders are common in the general population, with slow intestinal transit a common symptom. No known therapy is highly efficacious, safe, and cost effective for treatment of slow-transit bowel disorders. Probiotics are live micro-organisms that confer a health benefit on the host when administered in adequate dosages and have been extensively studied for treatment of functional GI disorders.
Clinical trials of probiotic supplementation on intestinal transit time (ITT) yield widely variable outcomes. The reasons for these discrepant outcomes have not been explored to date. Authors performed the first systematic review and meta-analysis on the efficacy of probiotic supplementation on ITT in adults with a secondary focus on identifying the factors that influence these outcomes.
Authors demonstrated that, overall, short-term (10-28 d) probiotic supplementation reduces ITT in adults. However, the treatment effect of probiotics is strongly dependent on: (1) the presence or absence of constipation; (2) subject age; and (3) probiotic strain.
The effect of probiotics on ITT is highly dependent on probiotic strain and patient characteristics. The reason for these differences requires exploration in future clinical trials. Thus far, no evidence supports the use of probiotics to decrease ITT in younger subjects or in those without constipation.
Probiotics are live micro-organisms that confer a health benefit on the host when administered in adequate dosages. Intestinal transit time is a general term that refers to the time taken for a food bolus to travel through the gastrointestinal system. The standardized mean difference is a statistical measure of effect size for continuous outcomes, defined as the mean difference between groups divided by the pooled standard deviation.
Considering the high prevalence of functional GI disorders nowadays and the numerous studies on the role of probiotics in treating such conditions, it is important to know where we stand. This meta-analysis demonstrates the efficacy of probiotic supplementation in improving intestinal transit time. It is very well written, with methods clearly presented. Conclusions are drawn regarding the clinical importance of these findings and their relevance to clinical trials design, representing valuable information.
P- Reviewer Gheonea DI S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1479] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 2. | Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569-1580. [PubMed] |
| 3. | Ansari R, Sohrabi S, Ghanaie O, Amjadi H, Merat S, Vahedi H, Khatibian M. Comparison of colonic transit time between patients with constipation-predominant irritable bowel syndrome and functional constipation. Indian J Gastroenterol. 2010;29:66-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Tack J, Müller-Lissner S. Treatment of chronic constipation: current pharmacologic approaches and future directions. Clin Gastroenterol Hepatol. 2009;7:502-508; quiz 496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Oelschlaeger TA. Mechanisms of probiotic actions - A review. Int J Med Microbiol. 2010;300:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 409] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 6. | Malaguarnera G, Leggio F, Vacante M, Motta M, Giordano M, Bondi A, Basile F, Mastrojeni S, Mistretta A, Malaguarnera M. Probiotics in the gastrointestinal diseases of the elderly. J Nutr Health Aging. 2012;16:402-410. [PubMed] |
| 7. | Girardin M, Seidman EG. Indications for the use of probiotics in gastrointestinal diseases. Dig Dis. 2011;29:574-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 8. | European Food Safety Authority. Guidance on the scientific requirements for health claims related to gut and immune function. EFSA J. 2011;9:1984. [RCA] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-W94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3566] [Cited by in RCA: 4315] [Article Influence: 269.7] [Reference Citation Analysis (0)] |
| 10. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12886] [Article Influence: 444.3] [Reference Citation Analysis (1)] |
| 11. | Cohen J. Statistical power analysis for the behavioral sciences. Hillside, NJ: Lawrence Erlbaum Associates 1987; . |
| 12. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46520] [Article Influence: 2114.5] [Reference Citation Analysis (3)] |
| 13. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] |
| 14. | Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, Schlumberger A, Jakob S, Whorwell PJ. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Bartram HP, Scheppach W, Gerlach S, Ruckdeschel G, Kelber E, Kasper H. Does yogurt enriched with Bifidobacterium longum affect colonic microbiology and fecal metabolites in health subjects? Am J Clin Nutr. 1994;59:428-432. [PubMed] |
| 16. | Bouvier M, Meance S, Bouley C, Berta J, Grimaud J. Effects of consumption of a milk fermented by the probiotic strain Bifidobacterium animalis DN-173 010 on colonic transit time in healthy humans. Bioscience Microflora. 2001;20:43-48. |
| 17. | Holma R, Hongisto SM, Saxelin M, Korpela R. Constipation is relieved more by rye bread than wheat bread or laxatives without increased adverse gastrointestinal effects. J Nutr. 2010;140:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Hongisto SM, Paajanen L, Saxelin M, Korpela R. A combination of fibre-rich rye bread and yoghurt containing Lactobacillus GG improves bowel function in women with self-reported constipation. Eur J Clin Nutr. 2006;60:319-324. [PubMed] |
| 19. | Malpeli A, González S, Vicentin D, Apás A, González HF. Randomised, double-blind and placebo-controlled study of the effect of a synbiotic dairy product on orocecal transit time in healthy adult women. Nutr Hosp. 2012;27:1314-1319. [PubMed] |
| 20. | Marteau P, Cuillerier E, Meance S, Gerhardt MF, Myara A, Bouvier M, Bouley C, Tondu F, Bommelaer G, Grimaud JC. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study. Aliment Pharmacol Ther. 2002;16:587-593. [PubMed] |
| 21. | Rosenfeldt V, Paerregaard A, Nexmann Larsen C, Moller PL, Tvede M, Sandstrom B, Jakobsen M, Michaelsen KF. Faecal recovery, mucosal adhesion, gastrointestinal effects and tolerance of mixed cultures of potential prebiotic lactobacilli. Microbial Ecology in Health and Disease. 2003;15:2-9. |
| 22. | Sairanen U, Piirainen L, Gråsten S, Tompuri T, Mättö J, Saarela M, Korpela R. The effect of probiotic fermented milk and inulin on the functions and microecology of the intestine. J Dairy Res. 2007;74:367-373. [PubMed] |
| 23. | Waller PA, Gopal PK, Leyer GJ, Ouwehand AC, Reifer C, Stewart ME, Miller LE. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J Gastroenterol. 2011;46:1057-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Krammer HJ, Seggem HV, Schaumburg J, Neumer F. Effect of Lactobacillus casei Shirota on colonic transit time in patients with chronic constipation. Coloproctology. 2011;33:109-113. |
| 25. | Saad RJ, Rao SS, Koch KL, Kuo B, Parkman HP, McCallum RW, Sitrin MD, Wilding GE, Semler JR, Chey WD. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010;105:403-411. [PubMed] [DOI] [Full Text] |
| 26. | Törnblom H, Van Oudenhove L, Sadik R, Abrahamsson H, Tack J, Simrén M. Colonic transit time and IBS symptoms: what’s the link? Am J Gastroenterol. 2012;107:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Williams NT. Probiotics. Am J Health Syst Pharm. 2010;67:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 321] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 28. | Chmielewska A, Szajewska H. Systematic review of randomised controlled trials: probiotics for functional constipation. World J Gastroenterol. 2010;16:69-75. [PubMed] |