Published online Jul 21, 2013. doi: 10.3748/wjg.v19.i27.4437
Revised: May 6, 2013
Accepted: June 18, 2013
Published online: July 21, 2013
Processing time: 112 Days and 0.6 Hours
Hepatoid adenocarcinoma of the stomach (HAS) is a rare form of gastric cancer that has unique clinicopathological features and an extremely poor prognosis. Here, we report on three patients with suspected gastric cancer who were referred to our hospital. Gastrointestinal fiberscopy on the three patients revealed two lesions in the antrum and a third lesion in the gastroesophageal junction. The alpha fetoprotein (AFP) serum levels were markedly elevated in all cases. At the time of diagnosis, two cases were advanced stages with lymph nodes and/or liver metastases. Two patients underwent exploratory laparotomy. A total gastrectomy was performed on the operable lesion, and an expanded gastrectomy was completed in the case with hepatic metastases. Histopathological analysis revealed that the tumors displayed two pathological changes: hepatoid-like foci and adenocarcinomatous. Furthermore, the tumor cells were immunohistochemically positive for AFP, alpha-1 antitrypsin, and alpha-1 antichymotrypsin. All three patients received chemotherapy. The follow-up duration ranged from 8-36 mo. Our experience and previous published studies have suggested that HAS is an aggressive type of adenocarcinoma. However, radical surgery and chemotherapy may positively impact clinical outcomes.
Core tip: Hepatoid adenocarcinoma of the stomach is a rare but important type of gastric cancer that has unique clinicopathological features and an extremely poor prognosis. We analyzed the relationship between clinicopathological features, treatment courses and prognosis using three case reports combined with previously published studies.
- Citation: Ye MF, Tao F, Liu F, Sun AJ. Hepatoid adenocarcinoma of the stomach: A report of three cases. World J Gastroenterol 2013; 19(27): 4437-4442
- URL: https://www.wjgnet.com/1007-9327/full/v19/i27/4437.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i27.4437
Hepatoid adenocarcinoma (HAC) is a rare type of extrahepatic tumor that has a morphological similarity to hepatocellular carcinoma (HCC)[1]. The first description of HAC was in the stomach, which is the most common location; however, HAC has been reported to develop in a variety of organs, such as gallbladder, lung, urinary bladder, esophagus, pancreas, peritoneum, Jejunum, colon, rectum, renal pelvis, ureter, ovaries, uterus and papilla of Vater[2-5]. Hepatoid adenocarcinoma of the stomach (HAS) is a relatively rare gastric carcinoma and has an extremely poor diagnosis. Only a few cases have been previously reported. In this report, we describe three cases of HAS, and review the literature concerning its clinicopathological aspects.
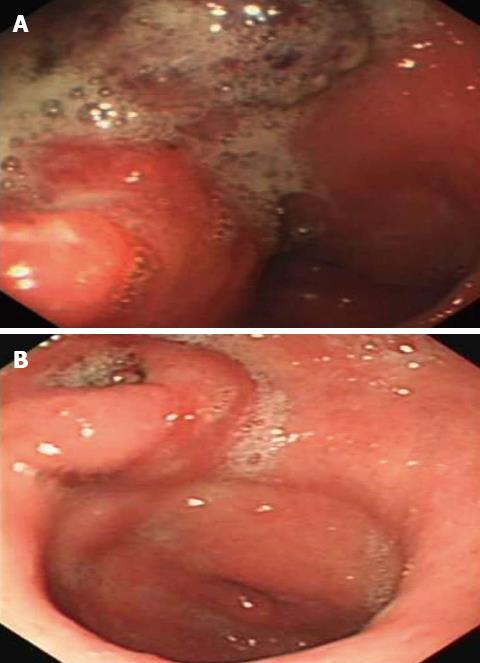
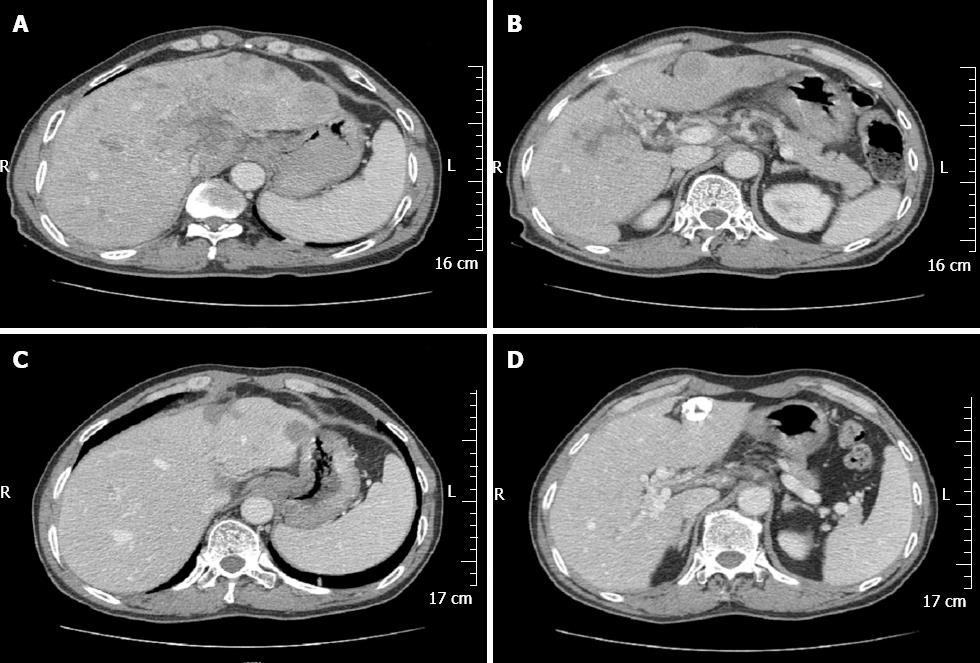
A 58-year-old Chinese male was admitted to our gastroenterology ward on February 12, 2010 with upper abdominal dull pain that had persisted for 1 mo. The patient had no relevant past medical history. The results of the physical examinations were unremarkable. A laboratory investigation showed that the patient’s serum alpha fetoprotein (AFP) level was elevated to 5845 ng/mL. However, additional tumor markers were normal. Additionally, hepatitis B surface antigen and antibody and hepatitis C antibody were all negative. A gastroduodenoscopy revealed a gigantic mass with a central ulceration at the lesser curvature extending from the angle to the antrum of the stomach (Figure 1A). A gastric biopsy revealed poorly differentiated adenocarcinoma. Computed tomography (CT) scans of the abdomen and pelvis was performed and revealed thickening of the wall of the antrum, enlarged lymph nodes at the lesser curvature, multiple hepatic tumors in the bilateral lobes of the liver and tumor thrombus in the portal vein and its branches (Figure 2A and B). However, no cirrhotic change was observed in the liver. A diagnosis of AFP-producing gastric cancer with multiple liver metastases was made. The patient received systemic chemotherapies, including a regimen of four cycles of epirubicin-oxaliplatin-fluorouracil and a regimen of six cycles of oxaliplatin plus capecitabine. During the chemotherapy intermission, the liver metastases were treated with transcatheter arterial chemoembolization and CT-guided radiofrequency ablation. All treatments were provided by our gastric multidisciplinary team. These treatments resulted in partial remission (Figure 1B), and the patient’s serum AFP levels decreased to 518.8 ng/mL. The CT scan showed that the patient’s enlarged lymph nodes had decreased in size and that the liver metastatic foci were stable (Figure 2C and D). A distal gastrectomy with a lymph node dissection and a left lateral liver lobectomy was performed on June 11, 2011.
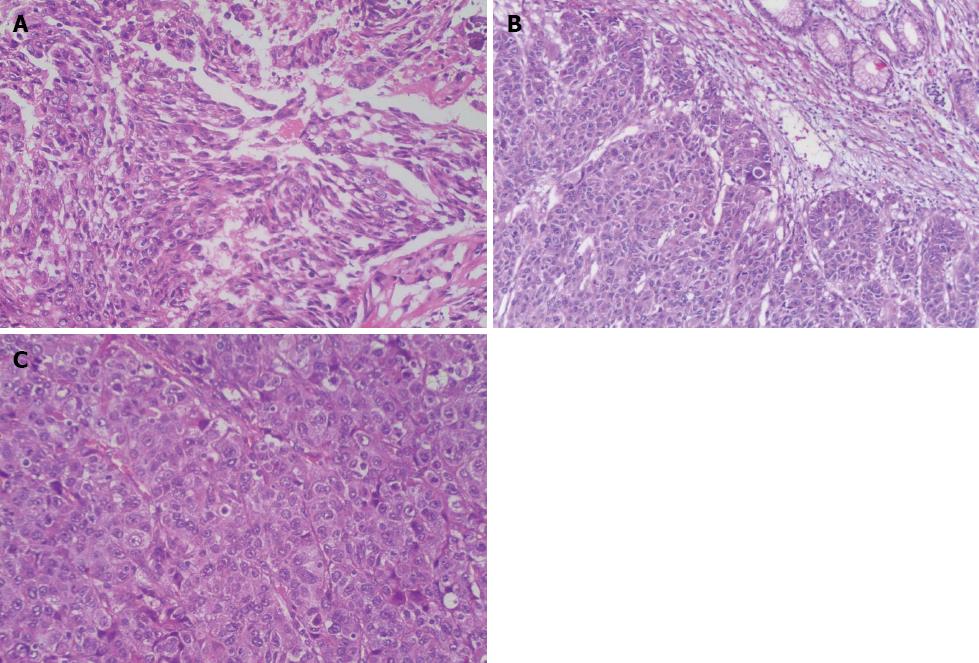
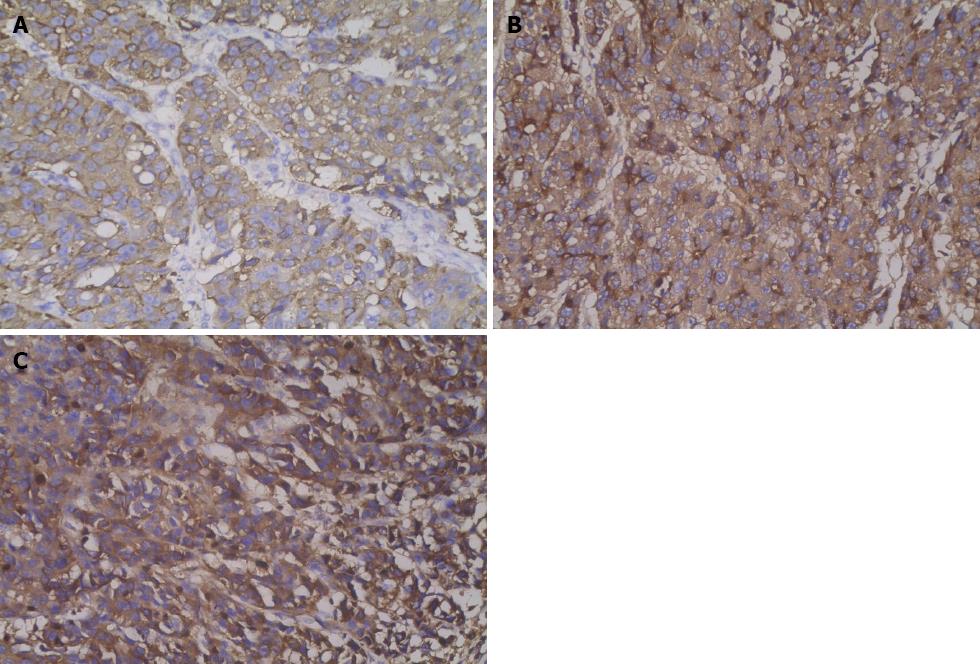
The distal gastrectomy resected a 2.0-cm gastric hepatoid adenocarcinoma at the lesser curvature, which infiltrated the muscularis propria (pT2) with no vascular invasion (V0). Additionally, a regional lymph node assessment revealed 16 lymph nodes with no cancer metastasis (pN0). However, there were several necrotic nodules in the left lateral lobe of the liver, but no cancer was detected. The histopathologic examination showed poorly differentiated adenocarcinoma with hepatoid features. The carcinoma was composed of polygonal tumor cells with abundant eosinophilic cytoplasm and round nuclei occasionally exhibiting obvious nucleoli with high mitotic activity. The tumor cells were arranged mainly in a trabecular pattern, whereas a smaller area of glandular formations was also observed (Figure 3A). Immunohistochemistry (IHC) revealed that the neoplastic cells were diffusely positive for alpha-1 antitrypsin (AAT), alpha-1 antichymotrypsin (ACT), cytokeratins 8, 18, 19 and carcinoembryonic antigen (CEA). Interestingly, many neoplastic cells showed cytoplasmic staining for AFP (Figure 4A). These IHC findings combined with the morphological features described above supported a diagnosis of HAS.
The patient recovered soon after surgery with no major complications. The total hospital stay for the surgical procedures was 15 d. Adjuvant chemotherapy with capecitabine was recommended, and the patient completed six cycles of chemotherapy. The patient’s serum AFP levels returned to normal within one month post-surgery. No progress has been observed after reexamination with CT, and the serum AFP levels were stable at 20 mo post-surgery.
A 54-year-old Chinese male presented to a local doctor with a one-month history of retrosternal pain. An endoscopy revealed an elevated tumor at the esophagogastric junction. Furthermore, a gastric biopsy discovered a poorly differentiated adenocarcinoma. The patient was referred to our hospital on March 3, 2011 for further examination. Upon physical examination, mild abdominal tenderness was detected under the xiphoid. A laboratory investigation showed that the patient’s serum AFP levels were elevated to 858.1 ng/mL. Furthermore, the results of additional laboratory tests, including routine blood and liver function tests, were all within normal limits. In addition, hepatitis B surface antigen and antibody and hepatitis C antibody were all negative. Upper gastrointestinal radiological studies showed an irregular structure, filling defect and wall stiffness at the esophagogastric junction. A CT scan showed thickening of the wall of the esophagogastric junction; however, no metastasis was observed. AFP-producing gastric cancer was diagnosed, and a total gastrectomy with lymph node dissection was performed on March 12, 2011.
The surgically resected specimen showed an elevated tumor (4.0 cm in maximal diameter) at the gastroesophageal junction, with central ulceration and surface erosion. Light microscopic examination revealed that the tumor invasion was limited to the area from the mucosa to the proper muscle layer. The tumor was diagnosed as HAS based on the pathological and IHC findings (Figure 3B). However, no cancer metastasis was detected in the 23 lymph nodes examined. The tumor was diagnosed as stage IB (pT2N0M0) according to the American Joint Committee on Cancer guidelines[6].
Postoperatively, the patient underwent a regimen of six cycles of oxaliplatin plus fluorouracil adjuvant chemotherapy. The serum AFP levels decreased to 8.2 ng/mL within the first two months, but they increased again to 99.9 ng/mL at 5 mo post-surgery. Furthermore, the CT scan showed lung metastases. Therefore, a chemotherapy regimen with paclitaxel and capecitabine was administered. However, after two cycles of second-line chemotherapy, the serum AFP levels increased to 8431 ng/mL, and the lung metastases progressed. Therefore, the patient declined additional therapy and was discharged. A careful follow-up of this patient revealed growth of the lung metastases; the patient died 18 mo post-surgery.
A 61-year-old woman was admitted to the clinic of our hospital on January 27, 2012 with epigastric pain that had persisted for one-month. The patient had no relevant medical history. A systematic review disclosed a weight loss of 5 kg in 1 mo but no hematemesis, hematochezia or melena. A physical examination revealed mild upper abdominal tenderness. A laboratory investigation showed a hematocrit of 27% with a hemoglobin concentration of 8.5 g/dL; the mean corpuscular volume was 70.8 fL, and the red cell distribution width was 18%. In addition, the AFP levels were > 50000 ng/mL. Additional tumor markers were normal. Furthermore, the hepatitis B and C panel was negative.
A gastroduodenoscopy revealed a large gastric antrum ulcer, and a CT scan showed wall thickening in the stomach, massive lymph node swelling around the lesser curvature of the stomach, head of pancreas, portal and splenic vein tumor thrombosis and multiple low density nodules in the spleen.
A histopathologic examination of the antral ulcer biopsy showed a moderately differentiated adenocarcinoma with hepatoid features (Figure 3C). The results from the IHC study revealed the expression of AFP, AAT and ACT in tumor cells (Figure 4B and C). The final diagnosis in this case was advanced stage HAS with lymph node metastases. The patient underwent chemotherapy with oxaliplatin plus S-1. However, no remission was observed in the stomach tumor. The AFP levels were > 50000 ng/mL after four cycles of chemotherapy. Meanwhile, the patient had grade 4 myelosuppression and hepatic dysfunction (Child-Pugh class B). Therefore, further chemotherapy was terminated. The patient died 8 mo after admission.
HAC is a rare but important type of extrahepatic tumor that has a morphologic similarity to HCC[1]. HAC was first reported as an AFP-producing tumor in 1970[7]. Kodama et al[8] described two histological types of AFP-producing gastric carcinoma: the medullary type and the well-differentiated papillary or tubular type. The two types of gastric carcinomas were occasionally found to coexist in a single tumor. Ishikura et al[9,10] proposed the term “hepatoid adenocarcinoma of the stomach” for primary gastric carcinomas that are characterized by both hepatoid differentiation and the production of large amounts of AFP. The reported incidence of AFP producing gastric cancer includes 1.3%-15% of all gastric cancer cases[11]. Later, Ishikura et al[12] reported on primary AFP-negative gastric carcinomas with characteristic histologic features mimicking hepatocellular carcinoma. Moreover, Nagai et al[13] reported that 46% of HAS demonstrated negative AFP levels by IHC staining and that microscopically hepatoid features, regardless of AFP-staining, were more important for a positive prognosis. Currently, the diagnosis of HAS is not dependent on whether AFP is produced, but it is based on the characteristic histological features.
Similar to most hepatic cellular carcinoma, HAC can produce AFP, AAT and ACT. Furthermore, specific well-differentiated HAC can secret bile[14]. Hepatoid carcinoma has a tubular adenocarcinomatous component, but the relationship between the tubular adenocarcinomatous and the hepatoid component remains unclear. A recent analysis by Kumashiro et al[15] compared the cellular phenotypes of 23 cases of hepatoid adenocarcinoma of the stomach and showed tubular adenocarcinomatous components with 69 cases of non-hepatoid adenocarcinoma of the stomach. The results suggested that hepatoid adenocarcinoma was derived from an adenocarcinoma with an intestinal phenotype and that its hepatoid component was related to reduced CDX2 expression levels.
Hepatoid adenocarcinoma of the stomach frequently occurs in older people. The average patient age is 63.5 years, and the male-to-female ratio is 2.32:1. The most common location is the antrum (60.2%), followed by the cardia and fundus. Moreover, epigastric pain and generalized fatigue caused by anemia are the most common symptoms[11]. In most patients, metastases to the liver and/or lymph nodes are detected preoperatively[11].
The diagnosis of HAS depends on the recognition of the characteristic histological features[13]. Histopathologically, the tumor is composed of two closely related areas, including hepatoid-like foci and adenocarcinomatous. Tumor cells in the hepatoid foci resemble the morphology of HCC, and immunohistochemically can be positive for AFP, AAT and ACT. Additionally, polyclonal CEA staining shows a “canalicular” pattern, which is characteristic of hepatoid features. The adenocarcinomatous component may be well or poorly differentiated, often with clear cells and a papillary pattern[10,16]. In differential diagnosis, additional AFP-producing gastric tumors and metastasizing germ cell tumors should be excluded[17]. HCC should be excluded particularly when HAS metastasizes to the liver[18]. Generally, in HCC, neighboring cirrhotic lesions are found, and tumor cells are positive for Hep Par-1 antibody, a sensitive and specific immunohistochemical marker for hepatocyte differentiation, whereas in metastatic HAS Hep Par-1 is often negative, and neighboring cirrhotic lesions are rare[16]. A recent finding indicated that the immunostaining of the palate, lung and nasal epithelium carcinoma-associated protein could be used as a novel marker to distinguish HAS from primary HCC[19].
In terms of treatment, there are few data in the literature specifically pertaining to HAS. The disease is treated similarly to common gastric adenocarcinoma. Radical surgery is the optimal treatment for HAS and may effectively prolong survival. Additionally, the liver metastases should undergo simultaneous resections[20]. Adjuvant chemotherapy and radiotherapy should be given according to current gastric cancer indications, despite the fact that no specific data on the adjuvant treatment of HAS are available. For patients with metastasis, chemotherapy is the primary treatment modality[20]. It has been reported that a better prognosis might be associated with good performance and active treatment, including palliative gastrectomy and chemotherapy[21]. Palliative chemotherapy is recommended to all patients with advanced gastric cancer and good performance statuses[22], although there is no standard chemotherapy protocol for HAS.
According to the literature, the prognosis of HAS is poorer than that of ordinary gastric adenocarcinoma. Nagai et al[13] reported that hepatoid adenocarcinoma had a poorer prognosis compared with AFP-producing non-hepatoid adenocarcinoma. However, the reasons for the poor prognosis are not clearly understood. One possibility is that hepatoid adenocarcinoma produces AAT and/or ACT as well as AFP. AAT and ACT have immunosuppressive and protease-inhibitory properties that enhance invasiveness[11].
The three patients in our study had an average age of 57 years, and their serum AFP levels were elevated. Two patients were found in the advanced stage with lymph nodes and/or liver metastases at the time of diagnosis, and the third patient developed lung metastasis seven months postoperatively. Two patients underwent surgery. Although all three patients had received chemotherapies, their outcomes were not satisfactory. In the first case, the patient who underwent curative gastrectomy and adjuvant chemotherapy showed a relatively good prognosis.
In conclusion, HAS is a relatively rare tumor with unique hepatoid features and a poor prognosis. Aggressive therapies, including surgery and chemotherapy, may yield good survival outcomes.
P- Reviewers Liaw CC, Tran T S- Editor Wen LL L- Editor A E- Editor Li JY
| 1. | Terracciano LM, Glatz K, Mhawech P, Vasei M, Lehmann FS, Vecchione R, Tornillo L. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol. 2003;27:1302-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol. 2013;19:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 118] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Yano T, Ishikura H, Wada T, Kishimoto T, Kondo S, Katoh H, Yoshiki T. Hepatoid adenocarcinoma of the pancreas. Histopathology. 1999;35:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Ishikura H, Ishiguro T, Enatsu C, Fujii H, Kakuta Y, Kanda M, Yoshiki T. Hepatoid adenocarcinoma of the renal pelvis producing alpha-fetoprotein of hepatic type and bile pigment. Cancer. 1991;67:3051-3056. [PubMed] |
| 5. | Gardiner GW, Lajoie G, Keith R. Hepatoid adenocarcinoma of the papilla of Vater. Histopathology. 1992;20:541-544. [PubMed] |
| 6. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 7. | Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med. 1970;78:1277-1278. [PubMed] |
| 8. | Kodama T, Kameya T, Hirota T, Shimosato Y, Ohkura H, Mukojima T, Kitaoka H. Production of alpha-fetoprotein, normal serum proteins, and human chorionic gonadotropin in stomach cancer: histologic and immunohistochemical analyses of 35 cases. Cancer. 1981;48:1647-1655. [PubMed] |
| 9. | Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer. 1985;56:840-848. [PubMed] |
| 10. | Ishikura H, Kirimoto K, Shamoto M, Miyamoto Y, Yamagiwa H, Itoh T, Aizawa M. Hepatoid adenocarcinomas of the stomach. An analysis of seven cases. Cancer. 1986;58:119-126. [PubMed] |
| 11. | Inagawa S, Shimazaki J, Hori M, Yoshimi F, Adachi S, Kawamoto T, Fukao K, Itabashi M. Hepatoid adenocarcinoma of the stomach. Gastric Cancer. 2001;4:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Ishikura H, Kishimoto T, Andachi H, Kakuta Y, Yoshiki T. Gastrointestinal hepatoid adenocarcinoma: venous permeation and mimicry of hepatocellular carcinoma, a report of four cases. Histopathology. 1997;31:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72:1827-1835. [PubMed] |
| 14. | Luo HF, Wang HJ, Tan G, Wang ZY. Hepatoid adenocarcinoma in stomach: a case report. Zhongde Linchuang Zhongliuxve Zazhi. 2011;10:297-299. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Kumashiro Y, Yao T, Aishima S, Hirahashi M, Nishiyama K, Yamada T, Takayanagi R, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach: histogenesis and progression in association with intestinal phenotype. Hum Pathol. 2007;38:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Gao YB, Zhang DF, Jin XL, Xiao JC. Preliminary study on the clinical and pathological relevance of gastric hepatoid adenocarcinoma. J Dig Dis. 2007;8:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Jalle T, Gérard C, Lada PE, Sagan C, Gournay J, Arnaud JP, Paineau J, Hamy A. Hepatoid adenocarcinoma of the stomach. A case report. Ann Chir. 2006;131:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Lu CC, De-Chuan C, Lee HS, Chu HC. Pure hepatoid adenocarcinoma of the stomach with spleen and lymph-node metastases. Am J Surg. 2010;199:e42-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Sentani K, Oue N, Sakamoto N, Arihiro K, Aoyagi K, Sasaki H, Yasui W. Gene expression profiling with microarray and SAGE identifies PLUNC as a marker for hepatoid adenocarcinoma of the stomach. Mod Pathol. 2008;21:464-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Zhang JF, Shi SS, Shao YF, Zhang HZ. Clinicopathological and prognostic features of hepatoid adenocarcinoma of the stomach. Zhonghua Yixve Zazhi (Engl). 2011;124:1470-1476. [PubMed] |
| 21. | Baek SK, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic characteristics and treatment outcomes of hepatoid adenocarcinoma of the stomach, a rare but unique subtype of gastric cancer. BMC Gastroenterol. 2011;11:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Gálvez-Muñoz E, Gallego-Plazas J, Gonzalez-Orozco V, Menarguez-Pina F, Ruiz-Maciá JA, Morcillo MA. Hepatoid adenocarcinoma of the stomach - a different histology for not so different gastric adenocarcinoma: a case report. Int Semin Surg Oncol. 2009;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |