Published online Jul 21, 2013. doi: 10.3748/wjg.v19.i27.4432
Revised: May 15, 2013
Accepted: June 1, 2013
Published online: July 21, 2013
Processing time: 109 Days and 20.3 Hours
Positron emission tomography (PET) using 18F-fluorodeoxyglucose (18F-FDG) is an imaging modality which reflects cellular glucose metabolism. Most malignant cells accumulate and trap 18F-FDG, allowing the visualisation of increased uptake. It is hence widely used to differentiate malignant from benign lesions. “False positive” findings of hepatic lesions have been described in certain instances such as hepatic abscesses, but are rare in cases involving hepatocellular adenomas. To our knowledge, there have been only 7 reports in the English literature documenting PET-avid hepatocellular adenomas; 6 of the 7 reports were published in the last 3 years with the first report by Patel et al. We report the case of a 44-year-old Chinese female patient with a history of cervical adenocarcinoma, referred for a hepatic lesion noted on a surveillance computed tomography (CT) scan. A subsequent CT-PET performed showed a hypermetabolic lesion (standardized uptake value 7.9) in segment IVb of the liver. After discussion at a multi-disciplinary hepato-pancreato-biliary conference, the consensus was that of a metastatic lesion from her previous cervical adenocarcinoma, and a resection of the hepatic lesion was performed. Histology revealed features consistent with a hepatocyte nuclear factor-1 α inactivated steatotic hepatocellular adenoma.
Core tip: This case illustrates a unique example of a false-positive finding on the computed tomography- positron emission tomography (CT-PET) due to a hepatic adenoma mimicking a metastatic lesion in the liver. It serves to highlight that not all CT-PET findings are associated with malignancy. In equivocal cases where CT-PET findings are discordant with other imaging modalities and/or clinical/biochemical features, further evaluation with different imaging modalities or novel PET tracers may be considered. This is especially pertinent in this day and age, where the PET scan is widely used in clinical practise to differentiate benign from malignant lesions, as well as a modality in cancer surveillance and staging.
- Citation: Lim D, Lee SY, Lim KH, Chan CY. Hepatic adenoma mimicking a metastatic lesion on computed tomography-positron emission tomography scan. World J Gastroenterol 2013; 19(27): 4432-4436
- URL: https://www.wjgnet.com/1007-9327/full/v19/i27/4432.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i27.4432
Positron emission tomography (PET) using 18F-fluorodeoxyglucose (18F-FDG) is an imaging modality which reflects cellular glucose metabolism. In most malignant cells, the relatively low level of glucose-6-phosphate leads to accumulation and trapping of 18F-FDG intracellularly, which in turns results in its visualization. As such, the PET scan is widely used for differentiating benign from malignant lesions, as well as a modality in cancer surveillance and staging. With regards to lesions in the liver, computed tomography (CT)-PET imaging classically shows increased uptake for moderately/poorly- differentiated hepatocellular carcinoma (HCC) and extra hepatic metastatic disease[1]. False-positive results have generally been described in hepatic abscesses, and false-negative results described in well-differentiated HCC[2].
Hepatocellular adenoma (HCA), also known as hepatic adenoma, is a benign tumor of the liver and the second most common benign liver neoplasm after focal nodular hyperplasia[3,4]. It occurs predominantly in women within their reproductive years with a male-to- female ratio of 1:8-10[5,6]. The estimated incidence is about 1 per million in women who have never used oral contraceptives (OCP), up to a significantly higher incidence of 30-40/million in long-term OCP users[3,4].
“False positive” findings in PET/CT are rare in HCA. To our knowledge, there have been only 7 reports in the English literature documenting PET-avid HCAs; 6 of the 7 reports were published in the last 3 years with the first report in 1997 by Patel et al[7-13].
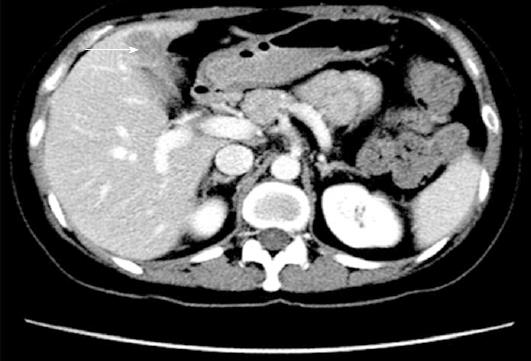
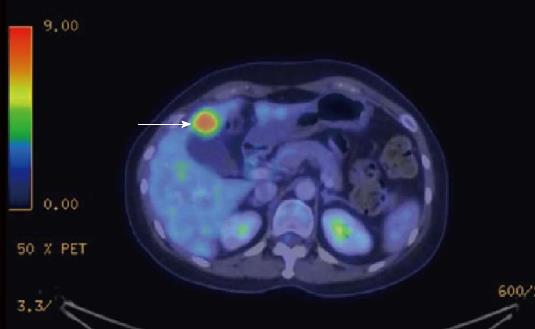
A 44-year-old female patient, with a history of International Federation of Gynecology and Obstetrics stage IB2 cervical adenocarcinoma was referred to our hepato-pancreato-biliary (HPB) service for further management of a hepatic lesion noted on her surveillance CT scan (Figure 1). Her cervical adenocarcinoma was treated with a radical hysterectomy 6 mo prior to this presentation. The hepatic lesion measured 2.3 cm × 1.8 cm and was located in segment IVb of the liver. A CT-PET was performed to further delineate the nature of the hepatic lesion and exclude extrahepatic metastases; the liver lesion appeared hypermetabolic with a standardized uptake value (SUVmax) of 7.9 (Figure 2). This was discussed at a multi-disciplinary HPB tumor conference and the consensus was that it was a solitary metastasis from the cervical adenocarcinoma without evidence of extrahepatic disease. In view of the patient’s previous surgery and the need for a lymphadenectomy, a decision was made for open surgical resection of the liver lesion with a perihilar lymphadenectomy.
On presentation, the patient was asymptomatic and recovered well from her hysterectomy. Clinical examination was largely unremarkable. Laboratory results and tumor biomarkers were within normal ranges [liver function test, complete blood count, carcinoembryonic antigen, α-fetoprotein, carbohydrate antigen (CA) 19-9, CA125]. Her hepatitis B and C virology markers were negative. She had no family history of liver cancer or OCP usage.
After exploration to exclude any peritoneal metastases, an open resection of this segment IVb hepatic lesion, cholecystectomy and perihilar lymphadenectomy was performed. A 3 cm × 2.5 cm segment IVb lesion was removed with clear margins. The patient recovered uneventfully.
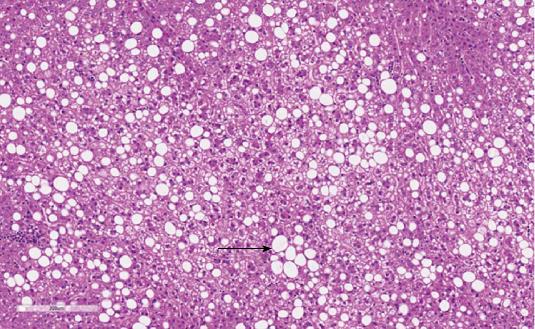
Histology revealed a well demarcated lesion composed of bland uniform hepatocytes displaying clear vacuolated macrovesicular fat globules predominantly. No bile ducts, entrapped portal tracts or ecstatic vessels were identified. No central scar was seen. Reticulin staining showed normal trabeculae without expanded thickened areas. Glypican-3 was negative. Overall features were consistent with a hepatocyte nuclear factor-1 α (HNF-1α) steatotic adenoma (Figure 3). All lymph nodes excised were also negative for malignancy.
This case illustrates a unique example of a false-positive finding on the CT-PET due to a HCA mimicking a metastatic lesion in the liver. Hepatic adenomas can be classified by their molecular phenotypes into four main groups: HNF-1α inactivation, β-catenin activating, inflammatory and unclassified[14,15]. The presence of the diffuse steatotic appearance and histological features in this case was consistent with a HNF-1α inactivated HCA. Radiological features of HNF-1α inactivated adenomas on Magnetic resonance imaging (MRI) include a diffuse signal dropout on T1-weighted chemical shift sequence, isosignal or slight hypersignal on T2-weighted images and moderate enhancement in the arterial phase, with no persistent enhancement in the portal venous and delayed phases. These appearances have been shown to have a high sensitivity and specificity for the diagnosis of steatotic adenomas[16,17].
There is an increasing importance in the role of FDG-PET in the evaluation of liver lesions to distinguish between benign and malignant. Its uses also include the surveillance, staging and monitoring of therapy for patients with cancer e.g. colorectal cancer. However, some of the limitations of PET/CT in evaluation of liver lesions are emerging[18,19]. The overall sensitivity of FDG PET/CT in detecting HCC is low with a reported range of 50%-65%, contributed mainly by the poor sensitivity in low-grade HCC[20,21].
The liver can have an unpredictable 18F-FDG uptake[19]. Glucose-6-phosphatase enzyme and glucose transporter activity have a wide variation in HCC. As a further confounder, the liver is the major producer of non-dietary glucose and a major regulator of glucose homeostasis. This limitation of PET/CT in HCC handicaps the usefulness of PET/CT in the evaluation of HCA, as one of the major concerns of HCA is its malignant potential and it harboring a foci of HCC within[6].
This case highlights that not all “hot” hepatic lesions on the PET are associated with malignancy. Benign examples of high FDG activity in the liver include focal steatosis, hepatic adenomatosis (e.g., inflammatory subtype) and infectious or inflammatory processes in the liver (e.g., liver abscess, hepatic tuberculosis). These can consequently lead to “false-positive” PET results[10,19,22,23]. Due to the limitations of CT/PET as an imaging modality, it is not possible to distinguish a PET-avid HCA from a liver abscess or a hepatic tuberculoma solely from its features on CT/PET. Other information is required to make such a distinction, such as clinical history and laboratory investigations. For example, patients with liver abscess may have signs and symptoms of infection such as fever/chills and pain; liver function test may be mildly abnormal with leukocytosis and inflammatory makers such as C-reactive protein and erythrocyte sedimentation rate may be elevated. Patients with HCA will not have such signs/symptoms of infection but may have a history of OCP usage and tend to be females in the reproductive age group. There is no evidence in the literature to suggest that CT/PET alone can distinguish PET-avid HCA from a liver abscess/tuberculoma.
Comparing our case to existing case reports of PET-avid HCAs in the literature, only 3 out of the 7 previous cases provided a detailed description of the histological findings[7-13]. Similar to our case, these 3 cases showed predominantly fatty change[8,9,11]. Of these 3 cases, only one report further classified the lesion as a HNF-1α hepatic adenoma[8].
Several hypotheses can explain the positive radiotracer uptake in such situations. In infections or inflammation, increased FDG uptake in lesions can be explained by the presence of inflammatory cells, which result in enhanced glucose metabolism or increased cell density[24-26]. However, pathological examination of the HCA in our patient revealed no evidence of inflammation. Also, none of the reported PET-avid HCAs in the literature included any of the inflammatory HCA subtype.
Radiotracer activity can increase at an early stage of malignant transformation and present as a “hot” lesion; activated β-catenin HCA has a higher malignant potential and may harbor small foci of HCC. In both our patient and the cases in the literature, none of the PET-avid HCAs were activated β-catenin mutated.
Focal fatty infiltration of the liver has been reported to be PET-avid. As a response to fat accumulation, a subacute inflammatory hepatic reaction with infiltration of activated Kupffer cells occur, resulting in a higher SUVmax than adjacent normal liver parenchymal[27,28]. Fat necrosis has been reported to have increased FDG uptake in benign lesions as well[29]. In the literature, 3 cases reported prominent fatty change but there was no mention of any inflammatory infiltrate (including our case), perhaps the fatty change itself was sufficient to evoke a PET-avid response without significant histological evidence of inflammatory infiltrate.
Lastly, hypervascularity of a HCA can induce an accumulation of glucose by the relative local increased blood flow to the lesion without a sufficient “washout”. This hypothesis was suggested for HCA without any evidence of inflammation, fatty content or malignant change[11].
In conclusion, hepatocellular adenomas are a heterogeneous group of liver benign neoplasms that are characterized by varied genetic and molecular abnormalities, pathology, tumor biology and radiological features. Positron emission tomography is increasingly utilized in cancer surveillance and work-up. This case highlights that “false-positives” can arise in case of benign liver lesions such as HCA. In equivocal cases where CT-PET findings are discordant with other imaging modalities, and/or clinical/biochemical features, further evaluation with different imaging modalities (e.g., contrast enhanced sonography or MRI with liver-specific contrast) or novel PET tracers (e.g., 11C-acetate or 18F-fluorocholine PET) should be considered[1,30-33].
P- Reviewer Gao C S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Shiomi S, Kawabe J. Clinical applications of positron emission tomography in hepatic tumors. Hepatol Res. 2011;41:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Delbeke D, Martin WH, Sandler MP, Chapman WC, Wright JK, Pinson CW. Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch Surg. 1998;133:510-55; discussion 510-515;. [PubMed] |
| 3. | Reddy KR, Schiff ER. Approach to a liver mass. Semin Liver Dis. 1993;13:423-435. [PubMed] |
| 4. | Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, Tyler CW. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644-648. [PubMed] |
| 5. | Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, Laurent C, Blanc JF, Cubel G, Trillaud H. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 6. | Farges O, Dokmak S. Malignant transformation of liver adenoma: an analysis of the literature. Dig Surg. 2010;27:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Patel PM, Alibazoglu H, Ali A, Fordham E, LaMonica G. ‘False-positive’ uptake of FDG in a hepatic adenoma. Clin Nucl Med. 1997;22:490-491. [PubMed] |
| 8. | Sumiyoshi T, Moriguchi M, Kanemoto H, Asakura K, Sasaki K, Sugiura T, Mizumo T, Uesaka K. Liver-specific contrast agent-enhanced magnetic resonance and 18F-fluorodeoxyglucose positron emission tomography findings of hepatocellular adenoma: report of a case. Surg Today. 2012;42:200-204. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Fosse P, Girault S, Hoareau J, Testard A, Couturier O, Morel O. Unusual uptake of 18FDG by a hepatic adenoma. Clin Nucl Med. 2013;38:135-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Sanli Y, Bakir B, Kuyumcu S, Ozkan ZG, Gulluoglu M, Bilge O, Turkmen C, Mudun A. Hepatic adenomatosis may mimic metastatic lesions of liver with 18F-FDG PET/CT. Clin Nucl Med. 2012;37:697-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Buc E, Dupre A, Golffier C, Chabrot P, Flamein R, Dubois A, Pezet D. Positive PET-CT scan in hepatocellular adenoma with concomitant benign liver tumors. Gastroenterol Clin Biol. 2010;34:338-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Stephenson JA, Kapasi T, Al-Taan O, Dennison AR. Uptake of 18FDG by a Hepatic Adenoma on Positron Emission Tomography. Case Reports Hepatol. 2011;2011. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Magini G, Farsad M, Frigerio M, Serra C, Colecchia A, Jovine E, Vivarelli M, Feletti V, Golfieri R, Patti C. C-11 acetate does not enhance usefulness of F-18 FDG PET/CT in differentiating between focal nodular hyperplasia and hepatic adenoma. Clin Nucl Med. 2009;34:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Walther Z, Jain D. Molecular pathology of hepatic neoplasms: classification and clinical significance. Patholog Res Int. 2011;2011:403929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Laumonier H, Bioulac-Sage P, Laurent C, Zucman-Rossi J, Balabaud C, Trillaud H. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008;48:808-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | van Aalten SM, Thomeer MG, Terkivatan T, Dwarkasing RS, Verheij J, de Man RA, Ijzermans JN. Hepatocellular adenomas: correlation of MR imaging findings with pathologic subtype classification. Radiology. 2011;261:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Katabathina VS, Menias CO, Shanbhogue AK, Jagirdar J, Paspulati RM, Prasad SR. Genetics and imaging of hepatocellular adenomas: 2011 update. Radiographics. 2011;31:1529-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Lan BY, Kwee SA, Wong LL. Positron emission tomography in hepatobiliary and pancreatic malignancies: a review. Am J Surg. 2012;204:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of primary hepatobiliary tumors, part 2jgnyan. AJR Am J Roentgenol. 2011;197:W260-W265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, Collins BT, Di Bisceglie AM. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792-797. [PubMed] |
| 21. | Wudel LJ, Delbeke D, Morris D, Rice M, Washington MK, Shyr Y, Pinson CW, Chapman WC. The role of [18F]fluorodeoxyglucose positron emission tomography imaging in the evaluation of hepatocellular carcinoma. Am Surg. 2003;69:117-124; discussion 124-126. [PubMed] |
| 22. | Jeong YJ, Sohn MH, Lim ST, Kim DW, Jeong HJ, Chung MJ, Lee CS, Yim CY. ‘Hot liver’ on 18F-FDG PET/CT imaging in a patient with hepatosplenic tuberculosis. Eur J Nucl Med Mol Imaging. 2010;37:1618-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Wang YT, Lu F, Zhu F, Qian ZB, Xu YP, Meng T. Primary hepatic tuberculoma appears similar to hepatic malignancy on F-18 FDG PET/CT. Clin Nucl Med. 2009;34:528-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Yasuda S, Fujii H, Nakahara T, Nishiumi N, Takahashi W, Ide M, Shohtsu A. 18F-FDG PET detection of colonic adenomas. J Nucl Med. 2001;42:989-992. [PubMed] |
| 25. | Kubota K, Ito K, Morooka M, Mitsumoto T, Kurihara K, Yamashita H, Takahashi Y, Mimori A. Whole-body FDG-PET/CT on rheumatoid arthritis of large joints. Ann Nucl Med. 2009;23:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Nishizawa S, Inubushi M, Kido A, Miyagawa M, Inoue T, Shinohara K, Kajihara M. Incidence and characteristics of uterine leiomyomas with FDG uptake. Ann Nucl Med. 2008;22:803-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183-190. [PubMed] |
| 28. | Kim YH, Kim JY, Jang SJ, Chung HW, Jang KS, Paik SS, Song SY, Choi YY. F-18 FDG uptake in focal fatty infiltration of liver mimicking hepatic malignancy on PET/CT images. Clin Nucl Med. 2011;36:1146-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Metser U, Miller E, Lerman H, Even-Sapir E. Benign nonphysiologic lesions with increased 18F-FDG uptake on PET/CT: characterization and incidence. AJR Am J Roentgenol. 2007;189:1203-1210. [PubMed] |
| 30. | aumonier H, Cailliez H, Balabaud C, Possenti L, Zucman-Rossi J, Bioulac-Sage P, Trillaud H. Role of contrast-enhanced sonography in differentiation of subtypes of hepatocellular adenoma: correlation with MRI findings. AJR Am J Roentgenol. 2012;199:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Bieze M, van den Esschert JW, Nio CY, Verheij J, Reitsma JB, Terpstra V, van Gulik TM, Phoa SS. Diagnostic accuracy of MRI in differentiating hepatocellular adenoma from focal nodular hyperplasia: prospective study of the additional value of gadoxetate disodium. AJR Am J Roentgenol. 2012;199:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Denecke T, Steffen IG, Agarwal S, Seehofer D, Kröncke T, Hänninen EL, Kramme IB, Neuhaus P, Saini S, Hamm B. Appearance of hepatocellular adenomas on gadoxetic acid-enhanced MRI. Eur Radiol. 2012;22:1769-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | van den Esschert JW, Bieze M, Beuers UH, van Gulik TM, Bennink RJ. Differentiation of hepatocellular adenoma and focal nodular hyperplasia using 18F-fluorocholine PET/CT. Eur J Nucl Med Mol Imaging. 2011;38:436-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |