Published online Jul 21, 2013. doi: 10.3748/wjg.v19.i27.4422
Revised: May 15, 2013
Accepted: May 18, 2013
Published online: July 21, 2013
Processing time: 146 Days and 12.2 Hours
Unlike hepatic haemorrhage following blunt abdominal trauma, spontaneous abdomen bleeding is rare, even in the presence of a hepatocellular adenoma (HA) or carcinoma. However, the diagnosis of a tumour underlying a haematoma after liver trauma is unusual, especially when it occurs more after two years after the accident. Here, we report a case of a ruptured HA due to blunt abdominal trauma. A 36-year-old woman was admitted to our hospital with sudden onset of upper abdominal pain. Her medical history revealed a blunt abdominal trauma two years prior. Initial abdominal computed tomography scan revealed a large haematoma measuring more than 16 cm in diameter in the right lobe of the liver. Magnetic resonance imaging showed haemorrhagic areas and some regions with hepatocyte hyperplasia, suggesting HA. The patient underwent right hepatic lobectomy, and a histopathological examination confirmed a diagnosis of HA. In conclusion, it is important to consider that abdominal trauma may hide old, asymptomatic and not previously detected injuries, as in the case reported.
Core tip: This paper clarifies that surgical liver diseases should be evaluated by experts at specialized centers. In addition, experts should pay attention to unusual situations as reported. Asymptomatic liver tumors are more common than imagined, even when presented underlying other acute disease, such as blunt trauma.
- Citation: Cotta-Pereira RL, Valente LF, De Paula DG, Eiras-Araújo AL, Iglesias AC. Rupture of a hepatic adenoma in a young woman after an abdominal trauma: A case report. World J Gastroenterol 2013; 19(27): 4422-4426
- URL: https://www.wjgnet.com/1007-9327/full/v19/i27/4422.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i27.4422
Hepatocellular adenoma (HA) is rare, benign lesion occasionally found in young women with a long-term history of oral contraceptive use[1-3]. However, there are other predisposing factors, such anabolic androgenic steroids (AAS) use[4], diabetes mellitus, beta-thalassemia and glycogen storage disease[5-7].
The majority of patients with HA are asymptomatic, but the occurrence of large and multiple adenomas is frequently associated with complications. The most important complications of HA are haemorrhage and malignant transformation into hepatocellular carcinoma (HCC), but the underlying pathophysiology is not fully known. Some data suggest that HA patients with beta-catenin mutations are more likely to undergo malignant transformation[8-10]. Symptomatic patients usually present with right upper quadrant pain secondary to HA bleeding, which can present as internal haemorrhage with necrotic changes (mostly observed in adenomas > 4 cm) or spontaneous rupture that causes subcapsular haematoma and possible haemoperitoneum[11].
In clinical practice, ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) are used to determine the diagnosis, but it is difficult to accurately distinguish between HA and other lesions, such as focal nodular hyperplasia (FNH). In such cases, a liver biopsy is sometimes necessary to establish a diagnosis[11,12]. It is important to emphasise that the presence of HA in trauma situations is quite rare[13].
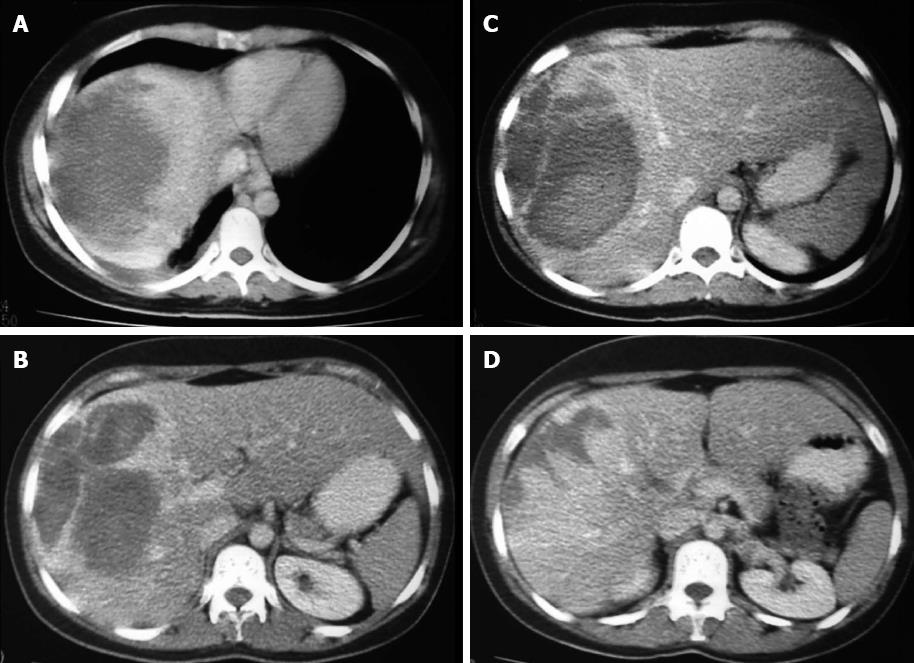
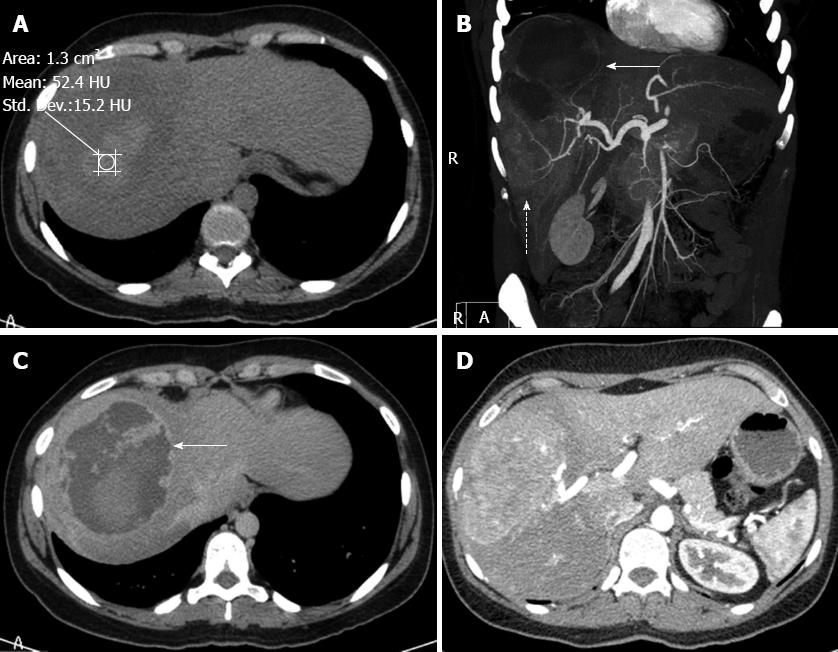
A 36-year-old woman visited our hospital for evaluation of her abdominal discomfort and anaemia. Her past medical history revealed a fall down the stairs of her building two years prior. At that time, the patient was admitted to a tertiary hospital and underwent laboratory blood tests, US and CT (Figure 1). The diagnosis was haematoma after liver trauma, and a conservative treatment approach was proposed. No surgery or drainage was required. The patient was discharged after seven days and was referred for follow-up care. Two years after the accident, the patient had non-specific abdominal pain in the right hypochondrium and symptomatic anaemia. Upon physical examination of the patient at our hospital, tenderness in the upper right quadrant and a palpable mass were detected. CT revealed a large mass with areas of low attenuation in segments VI, VII and VIII of the right lobe of the liver (Figure 2A). Laboratory exams revealed slight alterations of liver function (alanine aminotransferase: 132.30 IU/L, aspartate aminotransferase: 37.40 IU/L) and elevated alkaline phosphatase (20049 IU/L), but the levels of gamma-glutamyl transferase, total and fractionated bilirubin, cholinesterase, glycaemia and serum electrolytes were all within normal limits. The results of coagulation tests were entirely normal, as were the alpha-fetoprotein serum levels. Hepatitis virus markers, including hepatitis B and C, were negative.
A CT scan revealed a large, complex lesion in the right lobe of the liver with two distinct components. The upper component was moderately hyperdense with an attenuation coefficient of 52.4 UH and was not enhanced on a contrast-enhanced CT scan, suggesting liquefaction due to bleeding (Figure 2A and C). The lower component of the lesion was solid, markedly hypervascular and nourished via calibrous branches of the right hepatic artery (Figure 2B and D). There was no evidence of traumatic damage to the right thoracic-abdominal wall. The cutaneous, subcutaneous, muscular and osseous planes had normal anatomy and densities. These features suggest the possibility of a pre-existing hepatic lesion associated with bleeding, which may or may not have been facilitated by the traumatic event. The CT images also showed signs of cirrhosis. These data, along with the age and sex of the patient, were suggestive of a diagnosis of bleeding HA.
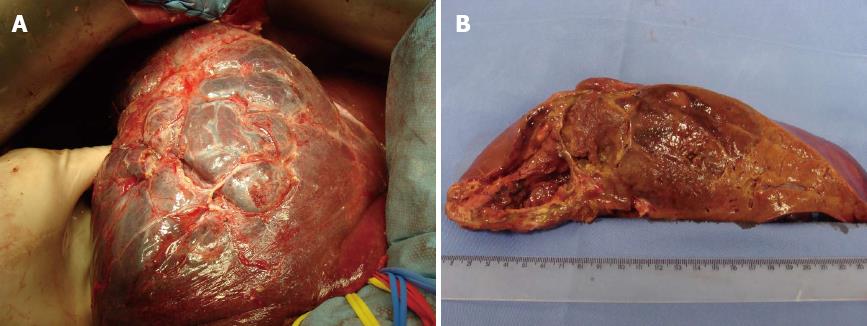
We identified a heterogeneous mass with soft tissue density occupying almost the entire right lobe of the liver (Figure 3). The patient underwent right hepatectomy, and the surgical specimen was sent for pathological analysis. There were no postoperative complications, and the patient was discharged five days after surgery.
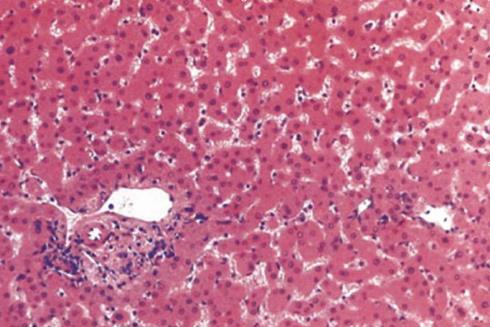
Histopathology examination revealed an HA with focal areas of haemorrhage (Figure 4). The lesion showed a heterogeneous pattern, indicating the existence of haemorrhagic areas in the tumour. The presence of a heterogeneous liver mass with internal haemorrhage and/or haemoperitoneum was suggestive of HA.
The treatment of blunt liver trauma has changed in the last several years, and conservative management is often prioritised over surgical treatment, even in patients with severe hepatic trauma[14,15]. The use of helical CT in the diagnosis and management of trauma injuries allows the option for nonsurgical treatments[15]. The American Association for the Surgery of Trauma (AAST) developed a CT-based liver injury grading system that allows physicians to select the best treatment option based on the imaging parameters[14-17]. Nonoperative treatment and detection of complications is based on clinical signs [e.g., pain, meteorism, pulse, tension, urine, ventilation and biochemical tests (e.g., haematocrit, haemoglobin and hepatic constants)]. This follow-up can take place in the intensive care unit and includes blood gas analysis and intra-abdominal pressure management[17]. Still, delayed laparotomies and late complications can occur, and the decreased rates of morbidity and mortality indicate that conservative options are better, even for patients with major liver trauma[14-19]. In our article, we point to an unusual event, which is the possibility of misdiagnosis following trauma.
Hepatic masses can exist prior to blunt abdominal trauma[20], and hepatic incidentalomas are not uncommon. As previously reported, the patient described herein already had a large adenoma when the trauma occurred, which resulted in rupture of the mass. Because she was seen at a centre that does not specialise in hepatic surgery, the correct diagnosis was not made, which could have been fatal[20]. The imaging diagnosis was a hepatic lesion (laceration/haematoma) due to trauma. Admittedly, adenomas may rupture spontaneously[13,18], and their propensity to haemorrhage is explained by their histological features.
Adenomas consist of large plates or cords of dilated sinusoids with poor connective tissue support. Classically, HA is a soft, well-demarcated tumour with little or no fibrous capsule and is composed of hepatocyte plates that are only mildly thickened and irregular. The tumour parenchyma is supplied by thin-walled arteries with minimal connective tissue[10-14,18]. Therefore, trauma to an adenoma most likely enhances their tendency to haemorrhage. Furthermore, it should be remembered that HA greater than 5 cm the indication for surgery should be given because of the high risk of tumor-related complications[21].
Currently, CT is the most frequently used modality in evaluating patients with liver injuries; it is a key element in the initial evaluation of haemodynamically stable patients who have suffered abdominal traumas[1-3]. Dramatic advances in multi-detector technology have significantly improved the accuracy of CT and dynamic contrast-enhanced CT, which can identify imaging characteristics of intrahepatic lesions, especially for rare changes caused by uncommon disease entities[1,4]. Emphasis should be laid on detecting the haemoperitoneum, then localising the source of bleeding and finally detecting the primary cause.
Spontaneous hepatic bleeding not related to trauma or anticoagulant therapy is a rare condition. This situation can be caused by underlying liver disease. Common causes are non-traumatic are HA or HCC. CT scans may be able to characterise and localise bleeding and to identify the underlying liver lesion[12]. Therefore, it is important to note that seemingly harmless blunt abdominal trauma may be very damaging when it is associated with an underlying hepatic lesion.
In conclusion, we note the importance of referring patients with liver trauma and possible hepatic disorders to specialised centres for the appropriate treatment.
P- Reviewers Hinz S, Keese M S- Editor Wen LL L- Editor A E- Editor Ma S
| 1. | Kerlin P, Davis GL, McGill DB, Weiland LH, Adson MA, Sheedy PF. Hepatic adenoma and focal nodular hyperplasia: clinical, pathologic, and radiologic features. Gastroenterology. 1983;84:994-1002. [PubMed] |
| 2. | Molina EG, Schiff ER. Benign solid lesions of the liver. Schiff’s diseases of the liver. 8th ed. Philadelphia: Lippincott-Raven 1999; 1245-1267. |
| 3. | Edmondson HA, Henderson B, Benton B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 335] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Barnes AD, Hattis RP, Toot PJ. Oral contraceptives and six benign liver tumors-the mestranol question. West J Med. 1984;140:954-955. [PubMed] |
| 5. | Socas L, Zumbado M, Pérez-Luzardo O, Ramos A, Pérez C, Hernández JR, Boada LD. Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: a report of two cases and a review of the literature. Br J Sports Med. 2005;39:e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Leese T, Farges O, Bismuth H. Liver cell adenomas. A 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg. 1988;208:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 168] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010;12:509-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 8. | Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 535] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 9. | Torbenson M, Kannangai R, Abraham S, Sahin F, Choti M, Wang J. Concurrent evaluation of p53, beta-catenin, and alpha-fetoprotein expression in human hepatocellular carcinoma. Am J Clin Pathol. 2004;122:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Anthony PP. Tumors and tumor-like lesions of the liver and biliary tract: Aetiology epidemiology and pathology. Pathology of the liver. 4th ed. London: Churchill Livingstone 2002; 711-775. |
| 11. | Marini P, Vilgrain V, Belghiti J. Management of spontaneous rupture of liver tumours. Dig Surg. 2002;19:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Chiche L, Dao T, Salamé E, Galais MP, Bouvard N, Schmutz G, Rousselot P, Bioulac-Sage P, Ségol P, Gignoux M. Liver adenomatosis: reappraisal, diagnosis, and surgical management: eight new cases and review of the literature. Ann Surg. 2000;231:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 147] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Erdogan D, Busch OR, van Delden OM, Ten Kate FJ, Gouma DJ, van Gulik TM. Management of spontaneous haemorrhage and rupture of hepatocellular adenomas. A single centre experience. Liver Int. 2006;26:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Nguyen BN, Fléjou JF, Terris B, Belghiti J, Degott C. Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol. 1999;23:1441-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 271] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Kaplan U, Hatoum OA, Chulsky A, Menzal H, Kopelman D. Two weeks delayed bleeding in blunt liver injury: case report and review of the literature. World J Emerg Surg. 2011;6:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Yoon W, Jeong YY, Kim JK, Seo JJ, Lim HS, Shin SS, Kim JC, Jeong SW, Park JG, Kang HK. CT in blunt liver trauma. Radiographics. 2005;25:87-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Moore EE, Shackford SR, Pachter HL, McAninch JW, Browner BD, Champion HR, Flint LM, Gennarelli TA, Malangoni MA, Ramenofsky ML. Organ injury scaling: spleen, liver, and kidney. J Trauma. 1989;29:1664-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 628] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 18. | Fabre A, Audet P, Vilgrain V, Nguyen BN, Valla D, Belghiti J, Degott C. Histologic scoring of liver biopsy in focal nodular hyperplasia with atypical presentation. Hepatology. 2002;35:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Létoublon C, Arvieux C. Nonoperative management of blunt hepatic trauma. Minerva Anestesiol. 2002;68:132-137. [PubMed] |
| 20. | Suárez-Peñaranda JM, de la Calle MC, Rodrìguez-Calvo MS, Muñoz JI, Concheiro L. Rupture of liver cell adenoma with fatal massive hemoperitoneum resulting from minor road accident. Am J Forensic Med Pathol. 2001;22:275-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |