Published online Jul 21, 2013. doi: 10.3748/wjg.v19.i27.4409
Revised: March 15, 2013
Accepted: April 10, 2013
Published online: July 21, 2013
Processing time: 196 Days and 21.7 Hours
Development of oedema and hypoproteinaemia in a liver transplant recipient may be the first signs of graft dysfunction and should prompt a full assessment. We report the novel case of a patient who, years after liver transplantation developed a functional blind loop in an incisional hernia, which manifested as oedema and hypoproteinaemia secondary to protein losing enteropathy. After numerous investigations, the diagnosis was made by flurodeoxyglucose positron emmision tomography (FDG-PET) imaging. Surgical repair of the incisional hernia was followed several months later by resolution of the protein loss, and confirmed at a post operative FDG-PET scan at one year.
Core tip: This report presents a rare case of protein losing enteropathy as a result of bacterial overgrowth in the blind loop of an incisional hernia following liver transplantation. Surgical repair of the incisional hernia in this case brought about resolution of protein loss.
- Citation: Evans JD, Perera MTP, Pal C, Neuberger J, Mirza DF. Late post liver transplant protein losing enteropathy: Rare complication of incisional hernia. World J Gastroenterol 2013; 19(27): 4409-4412
- URL: https://www.wjgnet.com/1007-9327/full/v19/i27/4409.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i27.4409
Hypoproteinaemia and peripheral oedema are common manifestations of advanced liver disease. In most patients oedema resolves after transplantation with the return of synthetic function of the graft to full capacity, the timing of which may vary from days to several months in extreme cases. Reappearance of oedema in a transplant patient is a cause for concern and may be the first indication of graft failure, which could be the result of recurrent disease or chronic rejection. Chronic graft failure culminates in synthetic failure and in some cases ensuing portal hypertension, which may lead to enteropathy. With the long standing portal hypertension, oedema of the gastric or intestinal mucosa leads to impaired lymphatic circulation and absorption of proteins further augmenting hypoalbuminaemia[1]. Therefore in those presenting with low albumin and peripheral oedema graft failure is considered the first differential diagnosis, and in this light some of the simple but rare aetiological conditions may be overlooked.
Apart from advanced liver disease, aetiology of hypoalbuminaemia ranges from malnutrition and poor protein intake to increased protein loss from body due to various reasons. The renal and gastrointestinal tracts are common sites of protein loss. So called “blind loop syndrome” refers to a segment of bowel that is by-passed in normal gastrointestinal functions, allowing overgrowth of pathogenic bacteria[2]. The case reported here illustrates a patient who presented many years after liver transplant with peripheral oedema and hypoalbuminaemia, the aetiology of which was traced to a “blind loop” located in an incisional hernia at the site of the transplant incision.
A 52-year-old female with hypertension and type II diabetes underwent orthotopic liver transplantation for hepatitis C cirrhosis. This was followed two weeks later by early re-transplantation for hepatic artery thrombosis. Over the following ten years, she suffered a number of episodes of cholangitis and required percutaneous transhepatic cholangiography and balloon dilatation of a biliary stricture on two occasions. She also developed an incisional hernia adjacent to her liver transplant scar five years after her transplants, which was managed conservatively. She had stable liver graft function throughout, and was maintained on cyclosporine based immunosuppression regime. She had mild degree of renal impairment attributed to calcineurin inhibitors and her serum creatinine was 106 mmol/L.
Twelve years following her transplant and aged 64 years she developed abdominal bloating and peripheral oedema. Her serum albumin fell to 21 g/dL and total serum protein to 50 g/dL but her other liver function tests were unremarkable. Liver biopsy demonstrated a mild degree of inflammation and fibrosis. Oesophagastroduodenoscopy (OGD) revealed mild hypertensive gastropathy but in the absence of splenomegaly or thrombocytopenia portal hypertension was deemed an unlikely cause for her hypoproteinaemia. She had no evidence of excessive renal protein loss with a negative urine dipstick for protein, an albumin creatinine ratio of 2 and 24-h urinary protein of 126 mg. She had no diarrhoea or other symptoms or signs of inflammatory bowel disease. Duodenal biopsy demonstrated normal duodenal mucosa whilst on capsule endoscopy no significant pathology was found apart from scattered angioectasia throughout the jejunum and ileum and a suggested small polypoidal mass in caecum.
Over the two years that followed, she suffered repeated episodes of oedema, bloatedness and a drop in serum albumin to levels around 20 g/dL requiring multiple albumin infusions. These increased in frequency when she required 27 units of albumin (100 mL 20% HAS) over 2 mo, and was admitted for further evaluation of the cause of her protein losing enteropathy. All biochemical investigations including serum caeruloplasmin, faecal elastase, faecal alpha-1 antitrypsin and celiac screen was normal. Colonoscopy did not identify the possible polypoid lesion showed on capsule endoscopy. Repeat OGD revealed normal villi but a few scattered pearly white spots thought to represent lymphangiectasia. Pathological analysis of duodenal biopsies revealed normal villous architecture, no intraepithelial lymphocytes but a few distended lymphatic spaces.
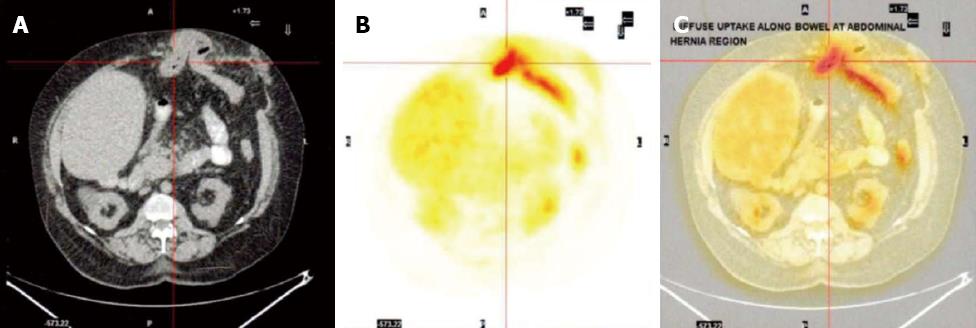
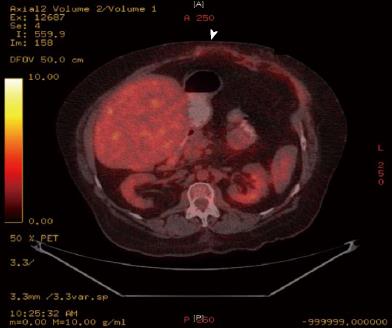
It was suggested that she was likely to have a degree of bacterial overgrowth in loop of hernia but in absence of watery diarrhoea and vitamin deficiency, the chances of her symptoms being due to protein losing enteropathy was deemed unlikely. In the absence of any other definitive aetiology for continued protein loss she was subjected to a fluorodeoxyglucose computed tomography positron emission tomography (FDG CT-PET) scan which demonstrated increased uptake in the bowel in the region of her incisional hernia indicating increased metabolic activity or infection in the blind loop of the hernia (Figure 1). A month later she underwent uncomplicated repair of her incisional hernia. Her requirement for albumin infusions decreased over the following 9 mo and at present she has been 3 mo without an albumin infusion and is maintaining a steady serum albumin level of 34 g/dL and total protein of 60 g/dL with no symptoms. A repeat CT-PET scan one year post hernia repair has demonstrated no increased uptake in the bowel around the site of her previous incisional hernia (Figure 2).
Protein losing enteropathy (PLE) is a rare condition in which protein loss into the gastrointestinal tract results in hypoproteinaemia which is manifested as peripheral oedema, ascites and sometimes pleural or pericardial effusions. The causes of PLE are numerous but can be divided broadly into mucosal injury, either erosive or non-erosive, increased central venous pressure, mesenteric lymphatic obstruction or small intestinal bacterial overgrowth (SIBO), which was the cause in this case[1]. Abdominal hernia, internal or external, have been implicated in SIBO and PLE but only a very limited number of cases are reported in the literature[3,4]. The present case is the first reported of its kind, and the learning point lies on the diagnostic dilemma. Nevertheless systematic investigations helped localise the problem and FDG-PET scan was an invaluable diagnostic tool.
SIBO can result from a number of processes and may be considered as either functional or anatomical, and in some cases a combination of both. Functional causes include hypochlorhydria, dysmotility and some immunodeficiency syndromes[5]. Anatomical situations predisposing to SIBO include resection of the ileo-caecal valve or gastro-colic and jejuno-colic fistulae in which colonic bacteria can translocate to the small bowel, or in situations where a surgical “blind loop” is created by an end to side or roux-en-y anastomosis or as part of a Billroth II gastrectomy[5,6]. A small bowel loop in an incarcerated hernia is similar to a functional blind loop in that patients often do not present with features of mechanical bowel obstruction and the classic features of intestinal obstruction are not usually present. Decreased motility of the incarcerated segment however provides the home for bacterial overgrowth due to the loss of normal mechanical clearing action usually present in the bowel. Initially an overgrowth of intestinal type bacteria occurs but with longstanding functional obstruction colonic bacteria also may translocate to the site of the obstructed bowel[7]. The exact mechanism of protein loss in SIBO is by way of a combination of mechanisms including impaired digestion and absorption of proteins and increased secretion of protein rich fluid into the intestinal loop as a result of chronic ongoing mucosal inflammation[8]. It has been proposed that the immune system plays a role in the regulation of intestinal flora[9], and the induced immunosuppressed state in this patient may have been contributory however there is no documented evidence to prove this theory in circumstances such as these.
In summary, this is an extremely rare presentation of protein-losing enteropathy as a result of an incisional hernia following liver transplantation. Incisional hernia is a common surgical complication following liver transplantation, the incidence of which increases further with re-transplantation[10]. Impaired wound healing and loss of muscle mass in the immunosuppressed patient transplanted for end stage liver disease account for this higher incidence of incisional herniae, and the majority of these are managed conservatively if they remain asymptomatic or further surgery is contraindicated[11,12]. Bacterial overgrowth in an incarcerated segment of bowel leading to blind loop syndrome and hypoalbuminaemia may mimic some features of late liver graft failure and should be considered in such situations.
P- Reviewers Hudacko R, Rosenthal P S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Umar SB, DiBaise JK. Protein-losing enteropathy: case illustrations and clinical review. Am J Gastroenterol. 2010;105:43-9; quiz 50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 2. | Stewart BA, Karrer FM, Hall RJ, Lilly JR. The blind loop syndrome in children. J Pediatr Surg. 1990;25:905-908. [PubMed] |
| 3. | Tainaka T, Ikegami R, Watanabe Y. Left paraduodenal hernia leading to protein-losing enteropathy in childhood. J Pediatr Surg. 2005;40:E21-E23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Cohen AM, Maran R, Gelvan A, Fireman Z, Lurie B. Malabsorption due to a ventral hernia. Neth J Med. 1992;41:24-27. [PubMed] |
| 5. | Singh VV, Toskes PP. Small bowel bacterial overgrowth: presentation, diagnosis, and treatment. Curr Gastroenterol Rep. 2003;5:365-372. [PubMed] |
| 6. | Machado JD, Campos CS, Lopes Dah Silva C, Marques Suen VM, Barbosa Nonino-Borges C, Dos Santos JE, Ceneviva R, Marchini JS. Intestinal bacterial overgrowth after Roux-en-Y gastric bypass. Obes Surg. 2008;18:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978-2990. [PubMed] |
| 8. | King CE, Toskes PP. Protein-losing enteropathy in the human and experimental rat blind-loop syndrome. Gastroenterology. 1981;80:504-509. [PubMed] |
| 9. | Kett K, Baklien K, Bakken A, Kral JG, Fausa O, Brandtzaeg P. Intestinal B-cell isotype response in relation to local bacterial load: evidence for immunoglobulin A subclass adaptation. Gastroenterology. 1995;109:819-825. [PubMed] |
| 10. | Vardanian AJ, Farmer DG, Ghobrial RM, Busuttil RW, Hiatt JR. Incisional hernia after liver transplantation. J Am Coll Surg. 2006;203:421-425. [PubMed] |
| 11. | Kahn J, Müller H, Iberer F, Kniepeiss D, Duller D, Rehak P, Tscheliessnigg K. Incisional hernia following liver transplantation: incidence and predisposing factors. Clin Transplant. 2007;21:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Janssen H, Lange R, Erhard J, Malagó M, Eigler FW, Broelsch CE. Causative factors, surgical treatment and outcome of incisional hernia after liver transplantation. Br J Surg. 2002;89:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |