Published online Jul 21, 2013. doi: 10.3748/wjg.v19.i27.4325
Revised: April 18, 2013
Accepted: May 8, 2013
Published online: July 21, 2013
Processing time: 162 Days and 0.7 Hours
AIM: To assess the safety and effect of the supplementation of a patented blend of dietary phytoestrogens and insoluble fibers on estrogen receptor (ER)-β and biological parameters in sporadic colonic adenomas.
METHODS: A randomized, double-blind placebo-controlled trial was performed. Patients scheduled to undergo surveillance colonoscopy for previous sporadic colonic adenomas were identified, and 60 eligible patients were randomized to placebo or active dietary intervention (ADI) twice a day, for 60 d before surveillance colonoscopy. ADI was a mixture of 175 mg milk thistle extract, 20 mg secoisolariciresinol and 750 mg oat fiber extract. ER-β and ER-α expression, apoptosis and proliferation (Ki-67 LI) were assessed in colon samples.
RESULTS: No adverse event related to ADI was recorded. ADI administration showed a significant increases in ER-β protein (0.822 ± 0.08 vs 0.768 ± 0.10, P = 0.04) and a general trend to an increase in ER-β LI (39.222 ± 2.69 vs 37.708 ± 5.31, P = 0.06), ER-β/ER-α LI ratio (6.564 ± 10.04 vs 2.437 ± 1.53, P = 0.06), terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (35.592 ± 14.97 vs 31.541 ± 11.54, P = 0.07) and Ki-67 (53.923 ± 20.91 vs 44.833 ± 10.38, P = 0.07) approximating statistical significance. A significant increase of ER-β protein (0.805 ± 0.13 vs 0.773 ± 0.13, P = 0.04), mRNA (2.278 ± 1.19 vs 1.105 ± 1.07, P < 0.02) and LI (47.533 ± 15.47 vs 34.875 ± 16.67, P < 0.05) and a decrease of ER-α protein (0.423 ± 0.06 vs 0.532 ± 0.11, P < 0.02) as well as a trend to increase of ER-β/ER-α protein in ADI vs placebo group were observed in patients without polyps (1.734 ± 0.20 vs 1.571 ± 0.42, P = 0.07).
CONCLUSION: The role of ER-β on the control of apoptosis, and its amenability to dietary intervention, are supported in our study.
Core tip: Active dietary intervention, a mixture of phytoestrogens and insoluble fibers, was administered in patients with sporadic colonic adenomas in a randomized, double-blind placebo-controlled trial. Dietary supplementation induced an increase in estrogen receptor (ER)-β protein and the ER-β/ER-α ratio with a general trend towards an increase in epithelial proliferation and apoptosis. These results, even if limited by the small number of subjects and short period of dietary supplementation, suggest the possibility of an interaction with epithelial apoptosis by means of mediating ER-β levels.
- Citation: Principi M, Di Leo A, Pricci M, Scavo MP, Guido R, Tanzi S, Piscitelli D, Pisani A, Ierardi E, Comelli MC, Barone M. Phytoestrogens/insoluble fibers and colonic estrogen receptor β: Randomized, double-blind, placebo-controlled study. World J Gastroenterol 2013; 19(27): 4325-4333
- URL: https://www.wjgnet.com/1007-9327/full/v19/i27/4325.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i27.4325
There is considerable evidence, both observational and interventional, to suggest that estrogens may have a chemopreventive activity for colorectal cancer (CRC). Women have a lower rate of colonic adenomas and cancers than men before menopause but the differences progressively lessen after menopause[1]. The mortality from CRC has been decreasing progressively in women compared to men since 1950[2] and this decrease is correlated with the time frames of increasing use of hormone replacement therapy (HRT), as confirmed by controlled trials[3,4].
Any chemopreventive effect of estrogens for CRC would likely be mediated by the interaction of circulating estrogens with estrogen receptors (ERs) in the colonic epithelium[5]; the presence of these receptors has been investigated by different methods, in many papers since the 1980s[6-8]. There are two distinct types of estrogen receptors (ERs) in human tissues. ER-α is primarily expressed in the breast and endometrium, whereas ER-β is found in a wide variety of tissues that are traditionally considered non-hormonal, including the colon. ER-α and ER-β activation may lead to biologically opposite patterns, as demonstrated in cancer cell lines (HC 11, LoVo)[9,10]. Thus, it is reasonable to hypothesize that estrogens could have dissimilar biological consequences in different tissues according to the amount and type of ERs[11]; the ER-β/ER-α ratio has therefore been developed to measure this phenomenon[12]. There is a substantial body of evidence suggesting that the level of ER-β expression itself, and/or the ER-β/ER-α ratio, is related to colonic carcinogenesis in both humans and animal models of CRC. ER-β is abundantly expressed in the normal colon but its expression is progressively decreased in adenomas and CRC in relation to the disease aggressiveness[13-15]. Similarly, familial adenomatous polyposis shows progressively lowered ER-β levels and a reduced ER-β/ER-α ratio in pre-neoplastic and neoplastic tissue[14]. Finally, the results of different studies suggest that some herbal supplements may exert significant and potentially beneficial effects on decreasing the amount of precancerous lesions by inducing apoptosis in the large intestine[16-19]. In an adenomatous polyposis coliMin/+ (ApcMin/+) mouse model, dietary supplementation significantly counteracted the intestinal tumorigenesis and increased the ER-β expression in the colon[20]. Moreover, observational studies suggest that phytoestrogens intake in whole grain, as well as enterolignans and silibinin, may be associated with a decreased incidence of advanced colon adenomas and cancers in both men and women[21-24].
On these bases, we conducted a randomized, double-blind placebo-controlled study to determine whether short term administration of dietary phytoestrogens and insoluble fibers, (silymarin, secoisolariciresinol diglycoside from flaxseed and insoluble fibers from oat extract, (Eviendep® CMD Pharma Limited. A Nestle Health Science Company, London, United Kingdom) can selectively alter ERs expression in the normal appearing colonic mucosa of patients undergoing surveillance colonoscopy after a previous polypectomy. Epithelial proliferation and apoptosis were assessed at the same time.
The expression of these biomarkers was evaluated in two steps: firstly analysis after administration of the active dietary intervention (ADI, Eviendep) vs the placebo, and then a further subdivision into 4 subgroups: ADI vs placebo, with, or without polyp recurrence.
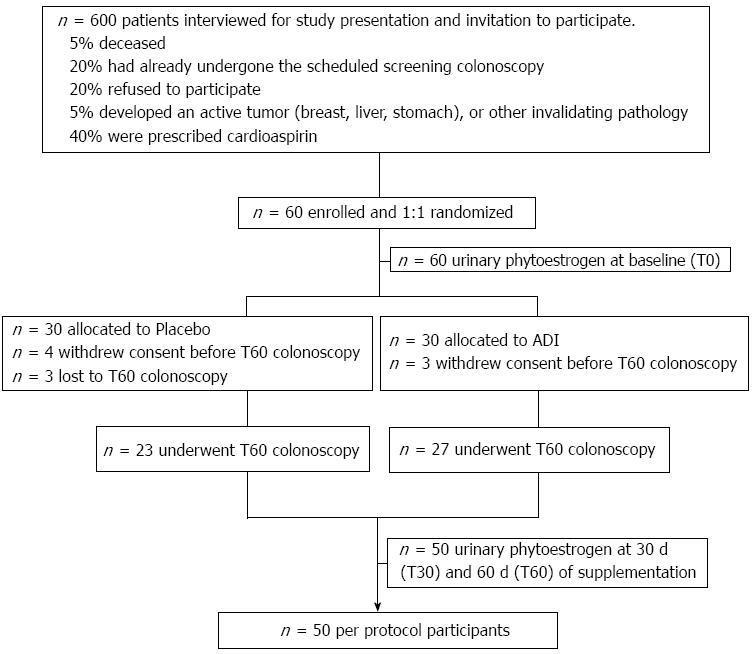
The study was approved by the Ethics Committee of the University Hospital of Bari (No. 1410). All patients signed informed consent, in accordance with the Helsinki Declaration, revision 1983. The study was registered at Clinical Trial.gov (ID: NCT01402648). In accordance with the CONSORT statement flowchart, the study design and flow is described in Figure 1.
All subjects with endoscopic records, males or post-menopausal females (defined as the absence of a menstrual cycle for at least 2 years prior to enrollment) aged at least 50 years, who had undergone a previous endoscopic polypectomy and were enrolled in follow-up for surveillance colonoscopy to be performed 3 or 5 years later, according to standard guidelines, were screened for study eligibility. Six hundred patients, potentially eligible by chart-review, were contacted by the EC authorized Clinical Investigators by telephone-interview to explain the study and invite them to participate.
Inclusion criteria were based on biochemical evaluation of blood samples i.e., hemoglobin ≥ 12.0 g/dL; platelets ≥ 120000/mm3; international normalized ratio ≤ 1.5; alanine aminotranferease or aspartate aminotransferase ≤ 1.5 times the upper limit of normal values (ULN); alkaline phosphatase ≤ 1.5 times ULN; bilirubin ≤ 1.5 times ULN; blood urea nitrogen ≤ 40 mg/dL and normal blood pressure or controlled hypertension.
The following were exclusion criteria: chronic inflammatory bowel disease, intestinal and/or extra-intestinal malignant neoplasms, acute or chronic renal disease, anemia, coagulation disorders or a body mass index (BMI) > 30 kg/m2, anti-cancer treatment and/or systemic corticosteroids within 6 mo of enrollment; anticoagulants or platelet anti-aggregants and antibiotics within 30 d of enrollment, HRT, selective estrogen receptor modulator, e.g., tamoxifene and related compounds or other supplemented phytoestrogens, aspirin and nonsteroidal antiinflammatory drugs within the previous 6 mo.
Sixty consenting, eligible patients, out of 600 interviewed, were randomly allocated to placebo (PL) or ADI (Eviendep) in a 1:1 ratio at baseline (T0), i.e., 60 d in advance of their scheduled surveillance colonoscopy (T60). The period of 60 d of supplementation was chosen as adequate for complete turnover of the colonic epithelial cells (migration from the crypt and release into the lumen) approximately eight times and, therefore, for the reliability of the biological evaluation[25].
ADI was provided as a sachet composed of 750 mg insoluble and indigestible oat fiber (cellulose, hemicellulose and lignin), 50 mg flaxseed dry extract (containing 20% secoisolariciresinol diglycoside), and 175 mg milk thistle extract (70% silymarin by UV and 30% silibinin by high performance liquid chromatography). PL sachets contained maltodextrins (910 mg), and 100% of ADI excipients. The ADI and PL were provided by the Sponsor in identical boxes and sachets, and labeled with the protocol code and the allocated participant number. The ADI and PL were stored and distributed, according to the assigned treatment group, to the participants through the Hospital Pharmacy. The patients and investigators were blinded to assignment. There were no dietary restrictions.
Computer-generated randomization, study monitoring, database acquisition and statistical analysis were conducted through a contract with medical trial analysis (Ferrara, Italy), an independent Clinical Research Organization.
Safety was assessed by vital signs (heart rate, blood pressure); and blood homeworks at baseline (T0), and after 30 d (T30) and 60 d (T60).
Phytoestrogen intake, and compliance were assessed by urinary measurements i.e., enterolignans enterodiol (ED) and enterolactone (EL), at T0, T30 and T60[19,20]. A few d before colonoscopy, all patients were asked to avoid fruit and vegetable intake and received bowel cleansing with PEG 4000 (1120 g/4 L water solution).
The number, location and size of all visualized polyps were recorded during endoscopy. A standard protocol for polyp removal was followed. Diminutive polyps (0.5 cm) were ablated with electrocoagulation, whereas all polyps > 0.5 cm in size were removed and submitted for histological assessment. Eight biopsies of the normal appearing sigmoidal mucosa were collected from all participants; seven of the biopsies were frozen in liquid nitrogen for biochemical and biologic endpoint analyses and one was well-oriented on blotting paper and then fixed in 4% formalin for immunostaining. The polyps were classified as hyperplastic or dysplastic by a gastrointestinal pathologist.
Colonic biopsies were assessed for ER-β and ER-α mRNA by reverse transcriptase (RT)-polymerase chain reaction (PCR), and protein by Enzyme-Linked ImmunoSorbant Assay. Total RNA was extracted from biopsies using the RNA easy mini kit (Qiagen), according to the manufacturer’s instructions. RNA concentration and quality were assessed by spectrophotometric readings at 260 and 280 nm. ER-β and ER-α cDNA were generated by Reverse Transcription of 0.5 μg of total RNA in a 20 μL reaction volume (iScript Select cDNA Synthesis Kit, Biorad), according to the manufacturer’s instructions. Two rounds of amplification (PCR-1 and PCR-2) were performed. The outer and inner primers used to detect ER-β and ER-α mRNAs at exon 3 have been previously described[26]. PCR-1 was performed in 50 μL final volume (iTaq DNA Polymerase Kit-Biorad) containing 2 μL cDNA and 40 pmol of outer primers through a denaturation step (95 °C for 3 min), 30 cycles (94 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s) and a final primer extension step (72 °C for 10 min). For PCR-2, PCR-1-derived ER-β and ER-α were diluted tenfold, and a 3 μL aliquot was amplified with 40 pmol of inner primers as per PCR1 conditions. β-actin cDNA (0.5 μL) served as internal control. DNA fragments were separated on a 2% agarose gel stained with ethidium bromide and size assessed by comparison with 100 bp DNA marker using Molecular Imager ChemiDOC XRS+ (Biorad). After normalization to the fluorescent β-actin PCR band intensity, ER-β and ER-α mRNA were expressed as arbitrary units of fluorescence.
For the extraction of proteins, colonic biopsies were homogenized in lysis buffer [100 mmol/L Tris-HCl (pH 7.5), 300 mmol/L NaCl, 4 mmol/L EDTA, 2% NP40, 0.5% Na deoxycholate, 1 mmol/L sodium orthovanadate and a protease inhibitor cocktail-Roche]. Supernatants were collected after centrifugation (13000 g for 25 min) and the total protein concentration was measured by the Bradford method (Biorad). ER-β and ER-α were measured using QuantiSir specific gene knockdown quantification specific kits (Epigentek, Brooklyn, NY, United States), following the manufacturer’s instructions. ERs content was normalized to GAPDH and expressed as optical densities (A) by computer-assisted densitometry.
Human urine spot samples (fasting) were extracted and measured by mass spectroscopy using a waters quattro premier mass spectrometer and waters acquity equipment. The lower and upper limit of quantitation (LLOQ and ULOQ) were 0.5 ng/mL and 2000 ng/mL for EL and ED, respectively. Two full calibration lines prepared in surrogate matrix (phosphate buffered saline; PBS), corresponding to five different EL and ED concentrations over the LLOQ and ULOQ, served as quality control (QC) samples. Duplicate QC samples prepared in the calibrated range were run together with the clinical samples. The concentration of ED and EL (ng/mL) and the coefficient of variation [CV (%) = SD of results/mean of results × 100] were measured.
Immunohistochemistry evaluated ERs expression, cell proliferation by Ki-67 and apoptosis by both terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) and caspase-3. In all cases, IHC data was expressed as labeling index (LI), i.e., the percent of immunostained colonocytes over the total counted cells along the length of 10 well oriented crypts. A further evaluation was based on the intensity of IHC staining divided into weak, moderate and strong. Both LI and intensity staining were calculated by two independent observers (MPS, ST) in a blinded fashion. Diagnostic agreement was revealed by a weighted k statistics coefficient > 0.8.
IHC was performed only on the sections of biopsies taken from non-adenomatous mucosa[4,14]. After permeabilization and antigen retrieval under shaking conditions in TBS buffer with TWEEN 0.025% for ER-β (15 min) and by microwave irradiation in citric buffer at pH 6.0 for ER-α (3 cycles of 5 min), the slides were covered with 5% goat serum for 30’ at room temperature (RT) to block non-specific binding. The slides were incubated with primary antibodies diluted 1:50 in PBS, at 4 °C overnight (anti-ER-β: Novocastra Menarini, Milano, Italy and anti-ERα: Santa Cruz, CA, United States). Negative controls were obtained by dipping slides in PBS without primary antibodies. Reactions were detected by a polymer-based visualization kit (EnVision, Dako, Glostrup, Denmark). Slides were then incubated with the chromogen 3, 3-diaminobenzidine-tetrahydrochloride (DAB, Vector laboratories) for 40 min at RT, and Harris hematoxylin (Sigma) served for nuclear counterstaining.
Ki-67 expression was evaluated by monoclonal antibody (clone MIB-1, Dako, Glostrup, Denmark). The sections were treated in a microwave oven twice for 5 min in citrate buffer (pH 6.0) at high power (750 W) before primary antibody incubation for 1 h at RT. The secondary peroxidase-conjugated antibody (EnVision, Dako, Glostrup, Denmark) was applied to the sections. Peroxidase activity was visualized by diaminobenzidine chromogen (DAB, Vector laboratory) and counterstained with hematoxylin.
TUNEL was investigated by the in situ cell death detection kit, Roche. In brief, sections were treated with 0.1 mol/L citrate buffer (pH 6.0) cooled to an internal temperature of sub-boiling in the microwave oven at 350 W for 10 min, incubated with TUNEL probe at 37 °C for 1 h and counterstained with TOPRO 3 (Invitrogen Molecular Probes), diluted at 1:5000. All sections were observed at × 400 magnification by confocal microscopy (Leica TSC SP2 confocal laser scanning microscope).
Caspase-3 was detected with a methodological approach allowing evaluation of both its exclusive expression and co-expression with ER-β. In detail, a rabbit polyclonal antibody from cell signaling (clone D 175) was used. Antigen retrieval was performed by rocking the slides in TBS buffer with TWEEN 0.025% for 10 min followed by microwave irradiation in citric buffer at pH 6.0 for 10 min at 750 watts; slides were incubated for 1 h at room temperature in 1% BSA blocking solution and then dipped in a mixture of the two primary antibodies (ER-β 1:50, Caspase-3 1:30) at 4 °C overnight. The sections were covered with the secondary antibody, Alexa 555 fluorescent-conjugated Goat anti-Rabbit (Invitrogen), diluted to 1:100 in PBS. TOPRO-3 (Invitrogen-Molecular Probes) diluted in PBS and incubated for 10’ at room temperature, providing nuclear counterstaining. Cells were counted at × 400 magnification, by confocal microscopy (Leica TSC SP2 confocal laser scanning microscope).
Sample size estimation was based on the assumption of an equivalence margin between the study arms of 0.05 and an actual difference (meanADI - meanPL) of 1, SDADI = 2 and SDPL = 2. Sixty patients were required to complete the study for an 80% powered study and a two-tailed 0.05 α error. The non parametric, two sided Wilcoxon Rank Sums test weighted ERs and biomarkers expression in study groups. ANOVA, χ2 or t test were used to compare demographics and all the other functional parameters. Spearman’s correlation was applied to assess relationships among ERs and the other biomarkers with the common diet and the allocated supplements. All analyses were performed using SAS version 8.2 (Statistical Analysis Software, Cary, NC). Diagnostic agreement was tested by calculating the weighted k statistics coefficient interpreted in accordance with the benchmarks of Landis and Koch. A value below 0.4 indicated poor agreement, a value between 0.4 and 0.8 moderate to good agreement, and a value of more than 0.8 excellent agreement[26].
A flow diagram of the study is shown in Figure 1. A total of 60 eligible consenting subjects were identified from 600 interviewed potential participants. They were enrolled and randomly assigned 1:1 to placebo or ADI. The groups were matched for age, gender, BMI, and season of enrollment; this last character displayed the absence of biases in phytoestrogen intake.
Ten out of 60 subjects dropped out, as reported in Figure 1. The final per protocol population consisted of 50 participants who completed the 60 d treatment with dietary supplements and underwent surveillance colonoscopy.
In 21 subjects (42%) polyps were found; the majority of these (58%) were electrocoagulated while the remainders were processed for histological examination. None showed high grade dysplasia.
No adverse events or biochemical modifications were reported in either the ADI or the placebo group.
Urinary enterolignan levels (ED, EL and ED + EL: ng/mL) were comparable between the study groups at baseline (Table 1). ADI treatment induced a significant increase in all urinary enterolignan levels at T30 and T60.
| Placebo (n = 23) | ADI (n = 27) | P value | |
| ED + ELT0 | 697.8 ± 963.3 | 410.3 ± 336.9 | 0.270 |
| ED + ELT30 | 472.4 ± 475.5 | 3300.1 ± 1535.5 | < 0.001 |
| ED + ELT60 | 116.0 ± 235.2 | 327.5 ± 313.1 | < 0.001 |
At T60 an expected fall in urinary lignans occurred in both groups due to the dietary restriction of fruits, vegetables and the colonoscopy bowel cleansing.
The effect of ADI on ER-β and ER-α protein and mRNA content at T60 is shown in Table 2. ADI induced a statistically significant increase of ER-β protein. ER-β mRNA levels were higher in the ADI than placebo group but the difference did not reach significance. ER-α protein and mRNA content were similar between the two groups; the ER-β/ER-α protein ratio showed a trend to an increase in the ADI group.
| Placebo (n = 23) | ADI (n = 27) | P value | |
| ER-β protein | 0.768 ± 0.10 | 0.822 ± 0.08 | 0.04 |
| ER-β mRNA | 0.994 ± 0.99 | 1.266 ± 1.24 | 0.10 |
| ER-β protein | 0.510 ± 0.11 | 0.490 ± 0.12 | 0.50 |
| ER-β mRNA | 0.139 ± 0.28 | 0.230 ± 0.24 | 0.20 |
| ER-β/ER-α protein | 1.571 ± 0.42 | 1.734 ± 0.20 | 0.07 |
The median value of ER-β and ER-α proteins, referred to all 50 patients enrolled, were 0.82 and 0.47 A, respectively. The patients distribution around these numbers was different for the two proteins. The ADI group showed a spread above the value of ER-β in a higher percentage as compared to the placebo group (62.96% vs 34.78%; P < 0.05) while, on the contrary, in the ADI group the median value of ER-α protein decreased as compared to the placebo group (66.7% vs 34.8%; P = 0.02).
Analysis of agreement between the observers provided κ = 0.87 (95%CI = 0.76-0.98). The assessment of intensity staining did not affect the results of LI, as the percentage of weakly stained cells was less than 10% and the results were referred only to moderate-strong intensity.
Table 3 reports the effect of ADI on the LI of ER-β, ER-α, Ki-67, TUNEL and caspase-3. The ADI treated patients had a trend towards an increase in ER-β LI (P = 0.06), the ER-β/ER-α LI ratio (2.5 fold compared to PL; P = 0.06), TUNEL (P = 0.07) and Ki-67 (P = 0.07) as compared to the placebo group, although none of these differences reached statistical significance.
| LI | Placebo (n = 23) | ADI (n = 27) | P value |
| ER-β | 37.708 ± 5.31 | 39.222 ± 2.69 | 0.06 |
| ER-β | 20.416 ± 10.71 | 16.481 ± 10.67 | 0.20 |
| ER-β/ER-α | 2.437 ± 1.53 | 6.564 ± 10.04 | 0.06 |
| TUNEL | 31.541 ± 11.54 | 35.592 ± 14.97 | 0.07 |
| Caspase-3 | 29.717 ± 7.98 | 32.937 ± 13.54 | 0.10 |
| Ki-67 | 44.833 ± 10.38 | 53.923 ± 20.91 | 0.07 |
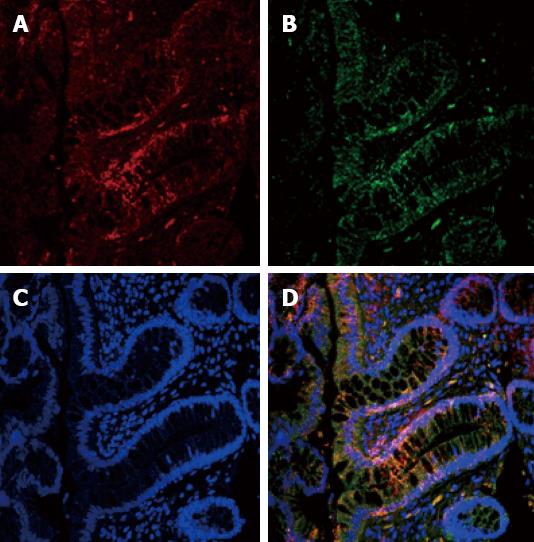
Spearman correlation showed that, in the ADI group, the ER-β LI was directly correlated with the TUNEL LI (r = 0.406, P = 0.03) and caspase-3 LI (r = 0.529, P < 0.004). The relationship between ER-β and apoptosis was confirmed by the IHC evidence of ER-β caspase 3 co-localization, as clearly shown in Figure 2. In the PL group these correlations showed the same direction but did not reach statistical significance (r = 0.379, P = 0.07 and r = 0.134, P = 0.55, respectively).
The expression of biomarkers in the normal mucosa of patients with (recurrent) and without (non-recurrent) polyps, was assessed. Table 4 shows that in patients without recurrence, there was a significant increase of the ER-β pattern (mRNA, protein and LI) and a decrease of ER-α protein in the ADI vs the placebo group. An increased ER-β/ER-α protein ratio (P = 0.01, not shown in the table) was also observed.
| Non- recurrent | ER-βprotein | ER-βmRNA | ER-βLI | ER-βprotein | ER-βmRNA | ER-βLI | TUNEL LI | Caspase-3 LI | Ki-67 LI |
| ADI (median) | 0.805 | 2.278 | 47.533 | 0.423 | 0.295 | 15.333 | 43.777 | 39.444 | 55.33 |
| SD | 0.131 | 1.192 | 15.472 | 0.062 | 0.26 | 9.76 | 17.243 | 15.63 | 20.41 |
| PL (median) | 0.773 | 1.105 | 34.875 | 0.532 | 0.159 | 16.750 | 31.375 | 29.53 | 45.31 |
| SD | 0.13 | 1.07 | 16.67 | 0.11 | 0.32 | 8.86 | 12.93 | 9.13 | 11.29 |
In patients with recurrence, a higher ER-β protein (P = 0.04) and a lower ER-α LI (P = 0.02), were also demonstrated in the ADI group.
There are substantial observational human data to suggest the possibility that ERs expression might be a marker of colon cancer risk. The ApcMin/+ mouse model of intestinal neoplasia has been used to study this association. Barone et al[20] reported that the ER-β level and ER-β/ER-α ratio was substantially lower in the normal small intestinal mucosa of ApcMin/+ mice than in syngenic wild type mice, and there was a consequent decrease of intestinal apoptotic activity. This fall was regulated by silymarin and an insoluble fibers (consisting of 6% lignin, known to be intestinally converted to the active enterolignans, enterodiol and EL) mixture. In this combination, silymarin is a potent ER-β agonist[27,28] and lignans have a substantial phytoestrogenic activity[29]. The silymarin/lignan combination also markedly decreased the number and size of intestinal tumors in ApcMin/+ mice[20]. Moreover, it was demonstrated that oophorectomy in female ApcMin/+ mice led to an increased number of polyps, which could be abolished by the administration of estrogens[3] or a ER-β selective agonist[4] like coumestrol. Similarly, in intact male ApcMin/+ mice, ER-β up-regulation via administration of a dietary ER-β selective agonist can reduce the number of adenomas with high grade dysplasia[22]. Giroux et al[31] addressed this relationship directly, showing that ER-β deficiency induced by ER-β knock-out in female ApcMin/+ mice led to enhanced small intestine tumorigenesis.
The current study was designed to investigate whether a similar dietary supplement could modify ERs levels in humans. ADI (Eviendep® CMD Pharma Limited, United Kingdom), a dietary supplement containing a patented combination of insoluble fibers and dietary phytoestrogens (silymarin and lignans) can substantially increase phytoestrogen levels in humans. We determined whether ADI affects the balance of expression of the ERs, as well as between proliferation and apoptosis, in the colonic mucosa of men and post-menopausal women undergoing surveillance colonoscopy for previous adenomas. The expression of these parameters was evaluated in two study steps, firstly analysis of the situation after active treatment vs placebo, and then a further analysis of the same biomarkers in 4 subgroups: supplement vs placebo, with, or without recurrence.
In the first step, in comparison with the placebo group, the ADI group showed a mild but significant increase in the mean ER-β protein content (P = 0.04), as well as a trend towards increase of the ER-β/ER-α ratio (P = 0.07), while ER-α protein (P = 0.5) and the mRNA content (P = 0.2) were similar between the two groups. In our study, we found that ADI induced a statistically significant increase of ER-β protein, whilst ER-β mRNA levels were higher than in placebo group but the difference did not reach significance. Moreover, both the protein and mRNA were significantly increased in the subjects without the finding of polyps at endoscopy. A possible explanation of our result may be due to an increased synthesis as well as a reduced degradation of ER-β protein. Indeed, the transcription, synthesis and degradation of ER protein are processes subjected to mechanisms of complex control through highly regulated adjustment systems. Degradation, in particular, takes place in the cell by a cytosolic complex, the proteasome. It is mediated by ubiquitin that binds to the ligand binding domain of the receptor (ubiquitination)[32,33]. It is possible that these mechanisms may be different in the colonic mucosa prepared or not the development of polyps. Furthermore, TUNEL (P = 0.07) and Ki-67 (P = 0.07) approximated statistical significance. Finally, correlation tests suggested a link between ER-β and apoptosis, thus confirming previous experiences[20]. Moreover, it has been shown that ER-βin vitro can up-regulate Lo Vo cells, which in turn activate the caspase 3 and 8 to induce apoptosis in Lo Vo cells, i.e., transient transfected cellular elements used with the aim of evaluating a relationship between ERs and apoptosis[9]. Therefore, in the context of our randomized, double-blind placebo-controlled study, short-term exposure to ADI increased ER-β without substantially affecting ER-α in the normal colonic mucosa of patients who had had a previous polypectomy, regardless of whether they had adenomas at T60 surveillance colonoscopy. The overall increase in the ER-β/ER-α ratio in the ADI group was associated with an increased apoptotic rate in the normal appearing colonic mucosa. Indeed, the real goal was the assessment of biomarkers (estrogen receptors and indicators of proliferation/apoptosis) after a short period of diet. Merely for diet duration, highly significant results were not in our expectations, although a trend of our dietary supplementation to modify the biomarkers is clear from our study.
The second step led us to conclude that ADI administration was unable to affect polyp recurrence, but it should be remembered that the period of administration was not designed for this purpose. Of course, we failed to prove ADI clinical effects since the aim of our study could not be to demonstrate that our dietary supplementation had a chemopreventive effect on the growth of intestinal polyps. Indeed, a long time is needed for the development of these lesions (years), whilst the duration of the diet was very short (2 mo). However, we demonstrated that ADI significantly reduces ER-α and increases ER-β independently of polyp recurrence. Moreover, ADI induces apoptosis through TUNEL and caspase 3 expression. To our knowledge this is the first demonstration that ER-β expression can be modified in humans. If loss of ER-β is a marker of risk for CRC, high levels of ER-β, induced by ADI, might have chemoprotective effects and the mixture of sylimarin and lignans could increase ER-β signaling by increasing and activating the receptor signaling pathway.
As far as the safety of ADI treatment is concerned, there were no adverse events nor changes in blood chemistry. Moreover, the absence of an ER-α expression (mRNA, protein and immunochemically stained cells) increase after ADI supplementation empirically supported this evidence.
As expected, ADI substantially increased phytoestrogen levels, as indirectly measured by urinary enterolignan (ED and EL) levels. Although total urinary enterolignans were decreased in both the ADI and placebo groups at T60 due to dietary restriction of fruits and vegetables prior to colonoscopy, they were significantly increased (8 fold) at T30 in the ADI group. This assay was conducted to demonstrate the adherence to the diet of the subjects as well as to avoid that external dietary factors could affect our results. This evaluation could be an indirect index of phytoestrogen level increase in ADI group. Additionally, literature evidences showed that a high estrous cycle duration, increased corticosterone and 17 β-estradiol levels with an overexpression of receptors ER-α and ER-β in animal model[34] thus suggesting that increased estrogen levels may raise the expression of specific receptors.
Our results suggest that dietary supplements may be able to modulate ER-β, a potentially important biomarker of colon cancer risk. Additional studies of this possibility seem warranted. In conclusion, the role of ER-β in the control of apoptosis and its amenability to dietary intervention are supported by our study. Our data could encourage further, perhaps larger, studies to be undertaken with the aim of demonstrating the secondary chemopreventive potential of ADI against CRC.
Studies in the adenomatous polyposis coli (ApcMin/+) mouse model suggest that estrogen receptor β (ER-β) expression is lower in the intestinal mucosa as compared to APC wild type mice, while dietary supplementation significantly counteracted the intestinal tumorigenesis by increasing ER-β expression. This plot is a start-up to apply in human studies as well.
Estrogens may have chemopreventive activity for colorectal cancer (CRC), of which sporadic adenoma is a direct precursor. This study assessed the safety and effect of supplementation of a patented blend of dietary phytoestrogens and insoluble fibers, active dietary intervention (ADI), on ER-β expression and on cellular proliferation and apoptosis in patients with recurrent sporadic colonic adenomas. The study was not designed to obtain a chemoprevention for the short diet duration.
ADI is an innovative, brand new mixture which has proved safe in humans and effective in increasing ER-β expression, that may offset intestinal carcinogenesis in patients with a positive history of recurrence for sporadic adenomas
In the future authors would like to analyze the possibility of prolonging treatment with ADI for longer than in this study (i.e., 2 mo) and to perform endoscopy before and after treatment, compatibly with the compliance of enrolled patients. This could help to increase the strength and to enhance the statistical significance of the results already obtained; the treatment may be shown to play a role in the prophylaxis of adenoma recurrence.
ERs, estrogen receptors, α and β, are widespread in human tissue. ER-α is primarily expressed in the breast and endometrium, whereas ER-β is present in a wide variety of tissues that are traditionally considered non-hormonal, including the colon. ER-α and ER-β activation may lead to biologically opposite patterns, as demonstrated in cancer cell lines
The protective role of estrogen is a well-known topic in the literature, the interaction of a modified diet (ADI) with the expression of the receptors is an issue that may pose in the future of the implications on chemoprevention of CRC. The work is well structured methodologically, certainly a larger sample could provide greater and more extensive statistical significance
P- Reviewers Chan WK, Kawai H, Langdon S S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | Koo JH, Leong RW. Sex differences in epidemiological, clinical and pathological characteristics of colorectal cancer. J Gastroenterol Hepatol. 2010;25:33-42. [PubMed] [DOI] [Full Text] |
| 2. | Rennert G, Rennert HS, Pinchev M, Lavie O, Gruber SB. Use of hormone replacement therapy and the risk of colorectal cancer. J Clin Oncol. 2009;27:4542-4547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Woodson K, Lanza E, Tangrea JA, Albert PS, Slattery M, Pinsky J, Caan B, Paskett E, Iber F, Kikendall JW. Hormone replacement therapy and colorectal adenoma recurrence among women in the Polyp Prevention Trial. J Natl Cancer Inst. 2001;93:1799-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Solimando R, Bazzoli F, Ricciardiello L. Chemoprevention of colorectal cancer: a role for ursodeoxycholic acid, folate and hormone replacement treatment. Best Pract Res Clin Gastroenterol. 2011;25:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147:4831-4842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Francavilla A, Di Leo A, Polimeno L, Conte D, Barone M, Fanizza G, Chiumarulo C, Rizzo G, Rubino M. Nuclear and cytosolic estrogen receptors in human colon carcinoma and in surrounding noncancerous colonic tissue. Gastroenterology. 1987;93:1301-1306. [PubMed] |
| 7. | Jassam N, Bell SM, Speirs V, Quirke P. Loss of expression of oestrogen receptor beta in colon cancer and its association with Dukes’ staging. Oncol Rep. 2005;14:17-21. [PubMed] |
| 8. | Di Leo A, Messa C, Cavallini A, Linsalata M. Estrogens and colorectal cancer. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:1-12. [PubMed] |
| 9. | Hsu HH, Cheng SF, Wu CC, Chu CH, Weng YJ, Lin CS, Lee SD, Wu HC, Huang CY, Kuo WW. Apoptotic effects of over-expressed estrogen receptor-beta on LoVo colon cancer cell is mediated by p53 signalings in a ligand-dependent manner. Chin J Physiol. 2006;49:110-116. [PubMed] |
| 10. | Helguero LA, Faulds MH, Gustafsson JA, Haldosén LA. Estrogen receptors alfa (ERalpha) and beta (ERbeta) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene. 2005;24:6605-6616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen receptor beta: an overview and update. Nucl Recept Signal. 2008;6:e003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61:632-640. [PubMed] |
| 13. | Di Leo A, Barone M, Maiorano E, Tanzi S, Piscitelli D, Marangi S, Lofano K, Ierardi E, Principi M, Francavilla A. ER-beta expression in large bowel adenomas: implications in colon carcinogenesis. Dig Liver Dis. 2008;40:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Barone M, Scavo MP, Papagni S, Piscitelli D, Guido R, Di Lena M, Comelli MC, Di Leo A. ERβ expression in normal, adenomatous and carcinomatous tissues of patients with familial adenomatous polyposis. Scand J Gastroenterol. 2010;45:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. Eur J Cancer. 2003;39:1251-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 250] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin). Carcinogenesis. 2005;26:1450-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Kohno H, Suzuki R, Noguchi R, Hosokawa M, Miyashita K, Tanaka T. Dietary conjugated linolenic acid inhibits azoxymethane-induced colonic aberrant crypt foci in rats. Jpn J Cancer Res. 2002;93:133-142. [PubMed] |
| 18. | Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26:4457-4498. [PubMed] |
| 19. | Singh RP, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res. 2008;14:7773-7780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Barone M, Tanzi S, Lofano K, Scavo MP, Pricci M, Demarinis L, Papagni S, Guido R, Maiorano E, Ingravallo G. Dietary-induced ERbeta upregulation counteracts intestinal neoplasia development in intact male ApcMin/+ mice. Carcinogenesis. 2010;31:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Egeberg R, Olsen A, Loft S, Christensen J, Johnsen NF, Overvad K, Tjønneland A. Intake of wholegrain products and risk of colorectal cancers in the Diet, Cancer and Health cohort study. Br J Cancer. 2010;103:730-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Kuijsten A, Hollman PC, Boshuizen HC, Buijsman MN, van ‘t Veer P, Kok FJ, Arts IC, Bueno-de-Mesquita HB. Plasma enterolignan concentrations and colorectal cancer risk in a nested case-control study. Am J Epidemiol. 2008;167:734-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Milder IE, Kuijsten A, Arts IC, Feskens EJ, Kampman E, Hollman PC, Van ‘t Veer P. Relation between plasma enterodiol and enterolactone and dietary intake of lignans in a Dutch endoscopy-based population. J Nutr. 2007;137:1266-1271. [PubMed] |
| 24. | Rajamanickam S, Velmurugan B, Kaur M, Singh RP, Agarwal R. Chemoprevention of intestinal tumorigenesis in APCmin/+ mice by silibinin. Cancer Res. 2010;70:2368-2378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | LIPKIN M. CELL REPLICATION IN THE GASTROINTESTINAL TRACT OF MAN. Gastroenterology. 1965;48:616-624. [PubMed] |
| 26. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] |
| 27. | Cavallini A, Messa C, Pricci M, Caruso ML, Barone M, Di Leo A. Distribution of estrogen receptor subtypes, expression of their variant forms, and clinicopathological characteristics of human colorectal cancer. Dig Dis Sci. 2002;47:2720-2728. [PubMed] |
| 28. | Seidlová-Wuttke D, Becker T, Christoffel V, Jarry H, Wuttke W. Silymarin is a selective estrogen receptor beta (ERbeta) agonist and has estrogenic effects in the metaphysis of the femur but no or antiestrogenic effects in the uterus of ovariectomized (ovx) rats. J Steroid Biochem Mol Biol. 2003;86:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | El-Shitany NA, Hegazy S, El-Desoky K. Evidences for antiosteoporotic and selective estrogen receptor modulator activity of silymarin compared with ethinylestradiol in ovariectomized rats. Phytomedicine. 2010;17:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Begum AN, Nicolle C, Mila I, Lapierre C, Nagano K, Fukushima K, Heinonen SM, Adlercreutz H, Rémésy C, Scalbert A. Dietary lignins are precursors of mammalian lignans in rats. J Nutr. 2004;134:120-127. [PubMed] |
| 31. | Giroux V, Lemay F, Bernatchez G, Robitaille Y, Carrier JC. Estrogen receptor beta deficiency enhances small intestinal tumorigenesis in ApcMin/+ mice. Int J Cancer. 2008;123:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA. 1999;96:1858-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 452] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 33. | Reid G, Denger S, Kos M, Gannon F. Human estrogen receptor-alpha: regulation by synthesis, modification and degradation. Cell Mol Life Sci. 2002;59:821-831. [PubMed] |
| 34. | Amorim JP, Chuffa LG, Teixeira GR, Mendes LO, Fioruci BA, Martins OA, Mello W, Anselmo-Franci JA, Pinheiro PF, Martinez M. Variations in maternal care alter corticosterone and 17beta-estradiol levels, estrous cycle and folliculogenesis and stimulate the expression of estrogen receptors alpha and beta in the ovaries of UCh rats. Reprod Biol Endocrinol. 2011;9:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |