Published online Jul 7, 2013. doi: 10.3748/wjg.v19.i25.4053
Revised: March 28, 2013
Accepted: April 27, 2013
Published online: July 7, 2013
Processing time: 191 Days and 5.2 Hours
AIM: To investigate the benefits of probiotics treatment in septic rats.
METHODS: The septic rats were induced by cecal ligation and puncture. The animals of control, septic model and probiotics treated groups were treated with vehicle and mixed probiotics, respectively. The mixture of probiotics included Bifidobacterium longum, Lactobacillus bulgaricus and Streptococcus thermophilus. We observed the survival of septic rats using different amounts of mixed probiotics. We also detected the bacterial population in ascites and blood of experimental sepsis using cultivation and real-time polymerase chain reaction. The severity of mucosal inflammation in colonic tissues was determined.
RESULTS: Probiotics treatment improved survival of the rats significantly and this effect was dose dependent. The survival rate was 30% for vehicle-treated septic model group. However, 1 and 1/4 doses of probiotics treatment increased survival rate significantly compared with septic model group (80% and 55% vs 30%, P < 0.05). The total viable counts of bacteria in ascites decreased significantly in probiotics treated group compared with septic model group (5.20 ± 0.57 vs 9.81 ± 0.67, P < 0.05). The total positive rate of hemoculture decreased significantly in probiotics treated group compared with septic model group (33.3% vs 100.0%, P < 0.05). The population of Escherichia coli and Staphylococcus aureus in ascites of probiotics treated group were decreased significantly compared with that of septic model group (3.93 ± 0.73 vs 8.80 ± 0.83, P < 0.05; 2.80 ± 1.04 vs 5.39 ± 1.21, P < 0.05). With probiotics treatment, there was a decrease in the scores of inflammatory cell infiltration into the intestinal mucosa in septic animals (1.50 ± 0.25 vs 2.88 ± 0.14, P < 0.01).
CONCLUSION: Escherichia coli and Staphylococcus aureus may be primary pathogens in septic rats. Probiotics improve survival of septic rats by suppressing these conditioned pathogens.
Core tip: We observed the survival of septic rats treated with different amounts of mixed probiotics. The data indicated that conditioned pathogens such as Escherichia coli and Staphylococcus aureus may be primary pathogens of septic rats in our study. Probiotics improve the survival of septic rats by suppressing the conditioned pathogens.
- Citation: Liu DQ, Gao QY, Liu HB, Li DH, Wu SW. Probiotics improve survival of septic rats by suppressing conditioned pathogens in ascites. World J Gastroenterol 2013; 19(25): 4053-4059
- URL: https://www.wjgnet.com/1007-9327/full/v19/i25/4053.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i25.4053
Sepsis is the systemic inflammatory response to infection and one of the most common causes of death in critically-illed patients[1]. Each year, more than 750000 clinical cases of death occur due to sepsis, and the mortalities from severe sepsis were 20%-30% in the period of 1979-2000 in the United States[2,3]. Microbial infection initiates and promotes systemic inflammatory responses by increasing cytokines release and neutrophils recruitment in target organs and inducing systemic inflammatory response syndrome and multiple organ dysfunction syndrome[4]. It has been demonstrated that intestinal microbes play an important role in sepsis[5]. Cecum is a pouch of large intestines connecting the terminal ileum to the ascending colon and home to a large number of anaerobic and aerobic microbes[6]. Cecal ligation and puncture (CLP) of rats produce cecal ischemia and polymicrobial infection[7]. The bacteria of colonic contents will spill into the abdomen, and produce severe peritonitis and bacteremia[8]. So the CLP has been used as a classic animal model of sepsis[9-11].
There is a complex microbial population in intestinal tract, some of which are probiotics. When administered in adequate amounts, probiotics confer a health benefit to the host[12]. The products of probiotics include mucin, organic acids, branched chain fatty acids, H2, CO2, ammonia, amines and vitamins. These products regulate host health through different pathways such as regulating energy, gene expression and cell differentiation, producing anti-inflammatory agents and keeping gut homeostasis[13,14]. The probiotics include Bifidobacteria, Lactobacilli, Enterococci, Streptococci, Propionibacteria, Bacillus, and yeasts. A variety of species of probiotics have been shown to benefit human gastrointestinal health[15-17]. However, the mechanisms of probiotics in improving survival in sepsis are unclear. In this study, we sought to address this question in a septic model of Wistar rats.
Male Wistar rats (8-10 wk old, Animal Center of Academy of Malitary Medical Sciences, China) were housed on a 12:12 h light-dark cycle under pathogen-free conditions with free access to food and water. We performed CLP, a clinically relevant animal model for human sepsis[18,19]. The animals were anesthetized by 10% chloral hydrate (3 mL/kg via intraperitoneal injection). After a midline incision was made in the abdomen, we isolated the cecum gently and placed a ligature 2.0 cm from the cecal tip using 2-0 silk suture. Ligated cecal stump was punctured by a 12-gauge needle. Colonic contents were extruded into abdominal cavity. We put back the cecum into its normal position and closed the abdomen by suturing muscle and skin, respectively. For control animals, the cecum was isolated without ligating and puncturing. The probiotic mixture consisted of three different viable strains. One dose of the probiotic mixture contained 1 × 107 CFU Bifidobacterium longum (ATCC 15697), 1 × 106 CFU Lactobacillus bulgaricus (ATCC 11842) and 1 × 106 CFU Streptococcus thermophilus (ATCC 19987). Before administration, the probiotic mixture was reconstituted in sterile water for 10 min at 37 °C. We gave probiotics to animals of treated groups through intragastric administration. The animals of control and septic model groups were treated with vehicle (sterile water). The first administration of probiotics or vehicle was started 6 h after surgery. Thereafter, it was administered once a day for 3 d.
All surviving animals were anaesthetised by 10% chloral hydrate (3 mL/kg, via intraperitoneal injection) after a 72 h period of CLP. Samples of blood and ascites were harvested for both anaerobic and aerobic microbial analysis immediately. Another portion of ascites was stored at -80 °C for DNA extraction. Then rats were killed by cervical dislocation, and colonic tissues were collected in neutral buffered formalin for histological analysis.
Serial 10-fold dilutions were made in 0.9% sterile saline. We spread 20 μL of 100-10-7 dilutions on the nonselective blood-agar (Jinzhang Co, Ltd., Tianjin, China) surface. For anaerobic incubation, the anaerobic blood-agar dishes (Jinzhang Co, Ltd., Tianjin, China) were placed in anaerobic bags (bioMérieux, France) immediately. The time of aerobic incubation was shorter (24 h) than anaerobic (48 h) at 37 °C. The colonies were determined in appropriate dilution, and total viable counts of original samples were calculated. Different colonies were separated and isolated for 2-3 times. We identified bacterial species using colony morphology and Gram’s stain. Microstation microbe analysis system (Biolog, Winooski, VT, United States) was used for advanced identification.
Bacterial genomic DNA was extracted from ascites of rats using the QIAamp DNA mini kit (Qiagen, Hilden, United States) according to the manufacturer’s protocol. We obtained 16S rRNA sequences of bacteria from the Ribosomal Database: (http://rdp.cme.msu.edu/), and designed primers for the specific bacterial strain using Primer 5.0 software package. The genomic DNA was used as template for the amplification of specimen and control standard bacterial strain through real-time polymerase chain reaction (PCR). PCR cycles were as follows: initial denaturation at 94 °C for 4 min, followed by 40 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 40 s. PCR primers were: Escherichia coli forward: 5’-CATGCCGCGTGTATGAAGAA-3’ and reverse: 5’-CGGGTAACGTCAATGAGCAAA-3’; Enterococcus faecalis forward: 5’-CAGCAGTAGGGAATCTTCGGCAATG-3’ and reverse: 5’-AGCCTCAGCGTCAGTTACAGACCAG 3’; Staphylococcus aureus forward: 5’-CGTCAGCTCGTGTCGTGAGATGTTG-3’ and reverse: 5’-GCGGTTTCGCTACCCTTTGTATTGT-3’. The real-time PCR was performed using FastStart SYBR Green Master (Roche, Basel, Switzerland) and IQ5 PCR system (BIO-RAD, Hercules, CA, United States).
The colonic tissues of at least four rats in each group were fixed in neutral buffered formalin, and processed for histological analysis. The sections of colonic tissues were stained by haematoxylin-eosin. Colonic sections were assessed for the severity of mucosal inflammation based on the following: infiltration of neutrophils and mononuclear cells into the intestinal mucosa (0, scant to normal; 1, minimal to mild; 2, mild to moderate; 3, moderate to severe; 4, severe inflammation)[20,21], and four fields of each sample were assessed. Moreover, epithelial thickness was measured under microscope (Leica, Frankfurt, Germany).
Data were expressed as the mean ± SD. All statistical analyses were performed using SPSS 17.0 software package. Survival analysis was shown in Kaplan-Meier survival curves. Survival comparisons between two subgroups were performed by the log-rank test. Differences between two groups were analysed using unpaired t test for continuous variables and the χ2 test for nominal variables. A P value of less than 0.05 was considered statistically significant.
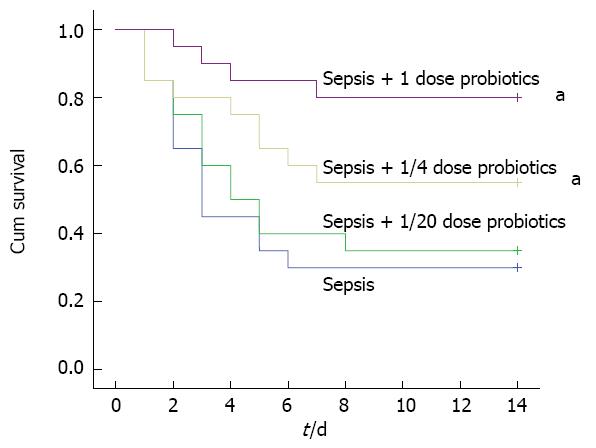
One hundred male Wistar rats were divided into five groups (control group, septic model group and three sepsis plus treatment groups) for survival analysis. We gave probiotic mixture (1, 1/4 or 1/20 doses) to animals in three treated groups by intragastric administration (once a day for 3 d). The animals of control and septic model groups were treated with vehicle only. We observed all animals for two weeks. The animals in control group survived normally. The majority of rats who had CLP showed clear signs of sepsis such as piloerection, lethargy, malaise and forming ascites. Probiotics attenuated the clinical manifestations of sepsis. Probiotics treatment also improved survival significantly and this effect was dose dependent. The survival rate was the lowest (30%, 6/20 rats) in the vehicle-treated septic model group. There was no protective effect using 1/20 dose probiotics (survival rate was 35%, 7/20 rats). However, 1 and 1/4 doses of probiotics treatment increased survival rate significantly (80%, 16/20 rats and 55%, 11/20 rats) compared with vehicle treated septic model group (P < 0.05) (Figure 1).
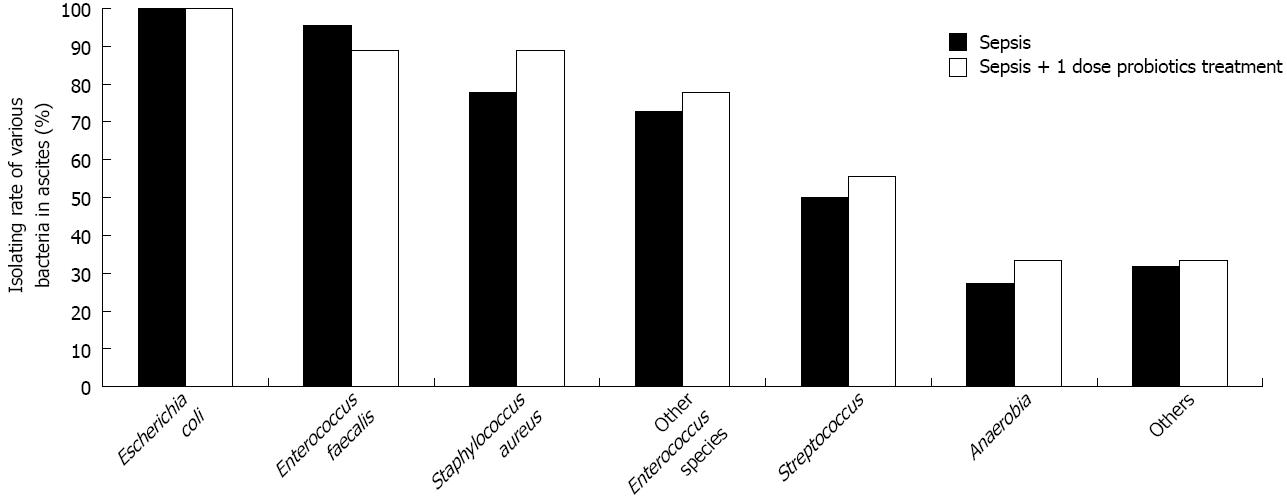
The consequence of survival analysis indicated that 1 dose probiotics treatment was more effective than other doses. Therefore, we divided 80 male Wistar rats into three groups (control group, 8 rats; septic model group, 50 rats; and sepsis plus 1 dose probiotics treated group, 22 rats). Probiotics or vehicle were given to the animals through intragastric administration (once a day for 3 d), respectively. All animals (8 rats) in the control group survived normally. Forty-four percent of animals (22 rats) were alive in septic model group, and 81.8% of animals (18 rats) were alive in probiotics treated group. We harvested samples after a 72 h period of CLP. The microbial composition of blood and ascites were analysed. No bacteria were determined in blood and ascites of control group. The bacterial spectrum of ascites (Table 1) and blood (Table 2) was lower in probiotics treated group than in septic model group. There was no statistical significance in isolating rates between two groups for all bacterial species (P > 0.05, Figure 2). However, the total viable counts of bacteria in ascites decreased significantly in probiotics treated group compared with septic model group (P < 0.05, Table 1). Similarly, the total positive rate of hemoculture decreased significantly in probiotics treated group compared with septic model group (P < 0.05, Table 2).
| Group | Bacterial spectrums in ascites | Total viable counts (Log10 cells/mL ascites) |
| Septic model group | Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, Enterococcus avium, Streptococcus viridans, Streptococcus agalactiae, Micrococcus luteus, Enterococcus gallinarum, Enterococcus durans, Enterococcus malodoratus, Streptococcus ferus, Morganella morganii ss morganii, Acinetobacter radioresistens, Streptococcus criceti, Lactobacillus reuteri, Veillonella criceti\ratti, Desulfovibrio fructosivorans, Clostridium oroticum, Lactobacillus bifermentans | 9.81 ± 0.67 |
| Probiotics treated group | Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, Enterococcus malodoratus, Morganella morganii ss morganii, Enterococcus durans, Streptococcus viridans, Prevotella dentioola, Desulfovibrio fructosivorcms, Bacterorides ovatus, Prevotella nigrescens | 5.20 ± 0.57a |
| Group | Bacterial spectrums of hemoculture | Total positive rate of hemoculture |
| Septic model group | Escherichia coli, Staphylococcus aureus, Curtobacterium pusillum, CDC group II-E subgroup A | 100% |
| Probiotics treated group | Escherichia coli, Staphylococcus aureus | 33.3%a |
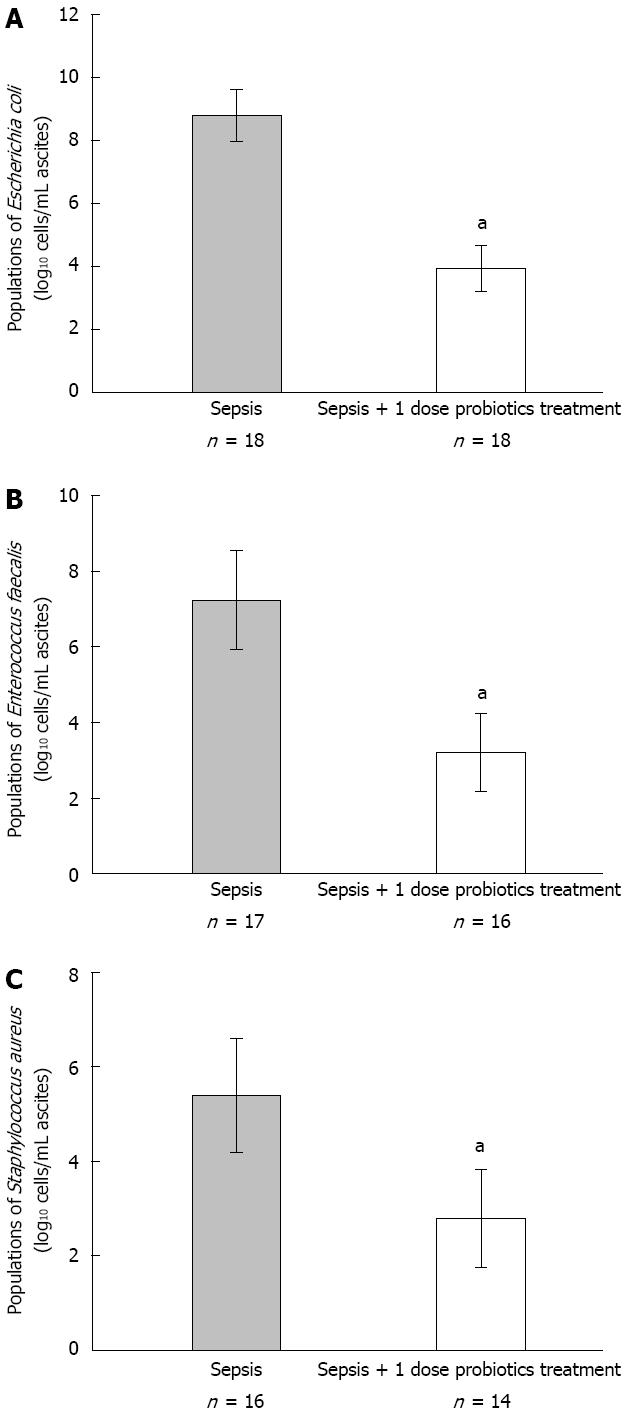
The consequence of bacterial cultivation indicated that Escherichia coli, Enterococcus faecalis and Staphylococcus aureus were predominant microbial population in ascites of sepsis. For this reason, we detected the population of these bacteria in ascites using quantitative real-time PCR. The data indicated that all population of these bacteria decreased significantly in probiotics treated group compared with septic model group (P < 0.05, Figure 3).
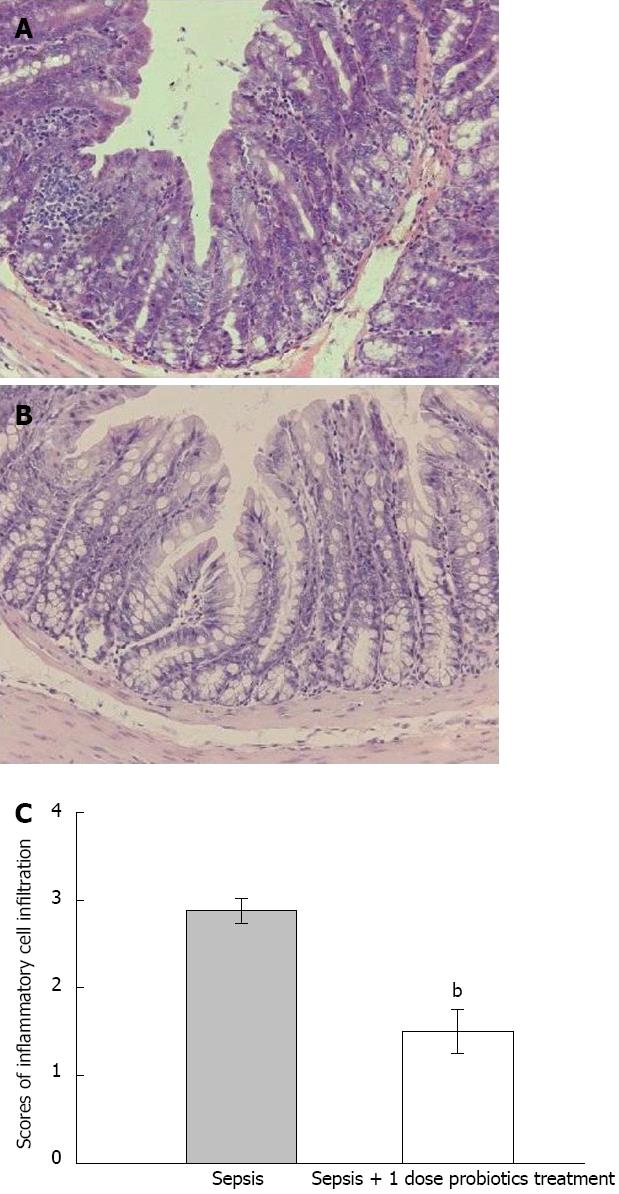
With probiotics treatment, there was a decrease in the infiltration of neutrophils and mononuclear cells into the intestinal mucosa in septic animals (P < 0.05, Figure 4). No apparent differences of epithelial cell hyperplasia were found between the rats in probiotics treated group and septic model group (data not shown).
Despite the development of antibiotics and other intensive care treatment, sepsis has a high mortality. CLP of rats is one of animal models of human sepsis. Because colonic contents are extruded into abdominal cavity, various microbes proliferate in ascites immediately. Therefore, the bacteria from feces cause polymicrobial infection, bacteremia and lethal peritonitis[22,23]. In this study, we treated the experimental septic rats with mixture of three live probiotics. We also analysed the survival of probiotics treated septic animals. It was demonstrated that probiotics improved the survival of rats with experimental sepsis and this effect was dose dependent. No protective effect was observed using the lowest concentration of probiotics (1/20 dose). However, 1 and 1/4 doses of probiotics treatment increased survival significantly compared with septic model group. Therefore, we treated septic animals in subsequent experiments using 1 dose of probiotics all the time.
Ascites culture data indicates that more pathogens grew in septic model group (109-1010 cells/mL) than in probiotic treated group (104-105 cells/mL). Both aerobes and anaerobes were detected in ascitic samples, although the majority of microbes were aerobes. Cecum contained anaerobes, facultative aerobes and aerobes. Furthermore, the amounts of anaerobes were greater than those of aerobes[24,25]. However, in our study, aerobes had been isolated frequently from septic ascitic samples such as Escherichia coli (isolating rate was 100%), Enterococcus faecalis (95.5%) and Staphylococcus aureus (77.8%). The total isolating rate of anaerobes was less than 30%. The main reason for this phenomenon was “oxygen”. When the operation of CLP was performed in experimental sepsis, the anaerobes were exposed to oxygen directly. Furthermore, some oxygen was stored in abdominal cavity of animal after operation. For these reasons, the majority of anaerobes were killed by oxygen. Thereafter, the aerobes which were minority in original colonic contents proliferated immediately. When we gave probiotics to septic rats, the bacterial spectrum of ascites and blood was lower than in the septic model group. Meanwhile, probiotics decreased total viable counts of pathogens in septic ascites significantly. In addition, the data of hemoculture showed that Escherichia coli and Staphylococcus aureus usually were detected in septic model group. Probiotics decreased the positive rate of hemoculture in septic rats.
It seemed that Escherichia coli and Staphylococcus aureus are the primary pathogens of CLP rats in septic model in our study. On one hand, we detected the population of these bacteria in ascites by quantitative real-time PCR. All population of these bacteria decreased significantly in probiotics treated group compared with septic model group. On the other hand, inflammatory response of intestinal mucosa was lessened in probiotics treated group compared with septic model group. All these data indicated that the mixture of probiotics improved the survival in a murine model of polymicrobial sepsis by suppressing the conditioned pathogens. However, the reasons for this suppression are not clear. There are two potential reasons: first, the decreased bacterial number may result from the inhibition of bacterial proliferation; second, a less bacteria infiltration or promoted bacterial killing[26-30].
Based on what had been mentioned above, we draw a conclusion that conditioned pathogens (Escherichia coli and Staphylococcus aureus) may be primary pathogens of CLP rats in septic model in our study. Probiotics (Bifidobacterium longum, Lactobacillus bulgaricus and Streptococcus thermophilus) contribute to improving the survival in an animal sepsis model by suppressing the conditioned pathogens.
Sepsis is the systemic inflammatory response to infection. Microbial infection initiates and promotes systemic inflammatory responses. A variety of species of probiotics have been shown to benefit human gastrointestinal health. However, the mechanisms of probiotics in improving sepsis are unclear. In this study, the authors sought to address this question in the septic model of Wistar rats.
Recently, more and more gastrointestinal diseases have been treated using probiotics. The probiotics and their products keep gastrointestinal tract homeostasis and regulate immune responses. For example, probiotics can regulate IgE production level and maturation of T cell in the gut.
To study the benefits of probiotics for sepsis, the authors observed the survival of cecal ligation and puncture (CLP) rats using different amounts mixed probiotics. The mixture of probiotics included Bifidobacterium longum, Lactobacillus bulgaricus and Streptococcus thermophilus. They also detected bacterial populations in ascites and blood of CLP rats using cultivation and real-time polymerase chain reaction. The data suggested that Escherichia coli and Staphylococcus aureu may be primary pathogens in the septic model. Probiotics improve survival in the septic model by suppressing the conditioned pathogens.
The results of this study suggest that probiotics improve survival in the septic model by suppressing the conditioned pathogens. This study helps to know whether probiotics can improve the clinical course of sepsis.
CLP of rats produces cecal ischemia and polymicrobial infection. The bacteria of fecal contents will spill into the abdomen, and produce severe peritonitis and bacteremia. So CLP has been used as a classic animal model of sepsis. There are complex microbial populations in intestinal tract. Some of them are probiotics. When administered in adequate amounts, probiotics confer a health benefit to the host. The products of probiotics include mucin, organic acids, branched chain fatty acids, H2, CO2, ammonia, amines and vitamins. These products regulate host health through different pathways such as regulating energy, gene expression and cell differentiation, producing anti-inflammatory agents and keeping gut homeostasis.
This study investigated the potential benefit of probiotic supplement in preventing septic death by studying the survival rates in rats treated with different doses of a probiotic mixture and then further investigated the effects of probiotics administration on the bacteria proliferation in blood and ascites in a cecal ligation and puncture sepsis model. This study shows the importance of knowing whether probiotics can improve the survival of experimental sepsis.
P- Reviewers Li XA, Misra SP S- Editor Zhai HH L- Editor Ma JY E- Editor Zhang DN
| 1. | Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2810] [Cited by in RCA: 2775] [Article Influence: 126.1] [Reference Citation Analysis (0)] |
| 2. | Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4208] [Cited by in RCA: 4300] [Article Influence: 195.5] [Reference Citation Analysis (0)] |
| 3. | Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 784] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 4. | Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24:1125-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 627] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 5. | Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28:384-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3394] [Cited by in RCA: 3546] [Article Influence: 177.3] [Reference Citation Analysis (5)] |
| 7. | Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24 Suppl 1:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 551] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 8. | Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 634] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 9. | Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 893] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 10. | Scumpia PO, Delano MJ, Kelly KM, O’Malley KA, Efron PA, McAuliffe PF, Brusko T, Ungaro R, Barker T, Wynn JL. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006;177:7943-7949. [PubMed] |
| 11. | Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1609] [Cited by in RCA: 1635] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 12. | Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics--approaching a definition. Am J Clin Nutr. 2001;73:361S-364S. [PubMed] |
| 13. | O’Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol. 2008;24:51-58. [PubMed] |
| 14. | O’Keefe SJ, Ou J, Aufreiter S, O’Connor D, Sharma S, Sepulveda J, Fukuwatari T, Shibata K, Mawhinney T. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr. 2009;139:2044-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Rossi M, Amaretti A, Raimondi S. Folate production by probiotic bacteria. Nutrients. 2011;3:118-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 410] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 16. | MacPhee RA, Hummelen R, Bisanz JE, Miller WL, Reid G. Probiotic strategies for the treatment and prevention of bacterial vaginosis. Expert Opin Pharmacother. 2010;11:2985-2995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Williams NT. Probiotics. Am J Health Syst Pharm. 2010;67:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 321] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 18. | Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 931] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 20. | Johnson-Henry KC, Nadjafi M, Avitzur Y, Mitchell DJ, Ngan BY, Galindo-Mata E, Jones NL, Sherman PM. Amelioration of the effects of Citrobacter rodentium infection in mice by pretreatment with probiotics. J Infect Dis. 2005;191:2106-2117. [PubMed] |
| 21. | Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 605] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 23. | Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3285] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 24. | Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1408] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 25. | Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599-608. [PubMed] |
| 26. | Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731-16736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2747] [Cited by in RCA: 3204] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 27. | Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 912] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 28. | Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2568] [Cited by in RCA: 2881] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 29. | Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 1132] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 30. | Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3529] [Cited by in RCA: 3527] [Article Influence: 220.4] [Reference Citation Analysis (0)] |