Published online Jul 7, 2013. doi: 10.3748/wjg.v19.i25.4031
Revised: April 1, 2013
Accepted: April 18, 2013
Published online: July 7, 2013
Processing time: 139 Days and 2.8 Hours
AIM: To identify clinical and pathological differences between serum immunoglobulin G4 (IgG4)-positive (SIP) and IgG4-negative (SIN) type 1 autoimmune pancreatitis (AIP) in South Korea.
METHODS: AIP was diagnosed by the international consensus diagnostic criteria. The medical records and pathology were retrospectively reviewed and IgG4-positive cells were counted in a high power field (HPF). Type I AIP was defined as a high serum level of IgG4 or histological finding. SIN type 1 AIP was defined as a histological evidence of type 1 AIP and a normal serum IgG4 level. The clinical and pathological findings were compared between the two groups. The analysis was performed using Student’s t test, Fischer’s exact test and Mann-Whitney’s U test. A P value of < 0.05 was considered statistically significant. As repeated comparison was made, P values of less than 5% (P < 0.05) were considered significant.
RESULTS: Twenty five patients with definite type 1 AIP (19 histologically and six serologically diagnosed cases) were enrolled. The mean tissue IgG4 concentrations were significantly higher in SIP than SIN group (40 cells per HPF vs 18 cells per HPF, P = 0.02). Among eight SIN patients, the tissue IgG4 concentrations were less than 15 cells per HPF in most of cases, except one. The sensitivity of serum IgG4 was 68% (17 SIP and eight SIN AIP). Other organ involvement was more frequently associated with SIP than SIN AIP (59% vs 26%, P = 0.016). However, the relapse rate and diffuse swelling of the pancreas were not associated with serum IgG4 level. The concentrations of IgG4-positive cells per HPF were higher in SIP than SIN AIP (40 vs 18, P = 0.02).
CONCLUSION: The sensitivity of serum IgG4 was 68% in type 1 AIP. High serum IgG4 level was associated with other organ involvement and tissue IgG4 concentration but did not affect the relapse rate in type 1 AIP.
Core tip: Type 1 autoimmune pancreatitis (AIP) is one of the immunoglobulin G4 (IgG4)-related diseases and serum IgG4 is known as a useful diagnostic marker. However, the sensitivity of serum IgG4 is variable. The sensitivity of serum IgG4 was not sufficient (68%) in definite type 1 AIP. The demographic findings were not different between SIP and SIN type 1 AIP, but other organ involvement was significantly more common in SIP than in SIN type 1 AIP. High serum IgG4 level was associated with other organ involvement and tissue IgG4 concentration, but did not affect the relapse rate in type 1 AIP.
- Citation: Paik WH, Ryu JK, Park JM, Song BJ, Park JK, Kim YT, Lee K. Clinical and pathological differences between serum immunoglobulin G4-positive and -negative type 1 autoimmune pancreatitis. World J Gastroenterol 2013; 19(25): 4031-4038
- URL: https://www.wjgnet.com/1007-9327/full/v19/i25/4031.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i25.4031
Autoimmune pancreatitis (AIP) is a type of chronic pancreatitis with irregular narrowing of the pancreatic duct and systemic fibroinflammatory disease. AIP is characterized by a remarkable response to steroid therapy. According to a multicenter nationwide study in Korea, the prevalence of AIP was 2.0% among 814 patients with chronic pancreatitis[1]. An early report from Japan that proposed the term lymphoplasmacytic sclerosing pancreatitis (LPSP) described some specific morphological features of AIP, such as diffuse lymphoplasmacytic infiltration with marked interstitial fibrosis and obliterative phlebitis[2].
The Japan Pancreas Society proposed diagnostic criteria for the first time in 2002, and the characteristic features of AIP were defined as the elevation of serum immunoglobulin G4 (IgG4) and LPSP on pathology[3]. However, emerging evidence suggests the presence of two AIP types that have different clinical profiles and outcomes. In 2003, a Mayo clinic group found two distinct histological patterns, which were designated LPSP and idiopathic duct-centric chronic pancreatitis (IDCP)[4]. IDCP was characterized by inflammatory infiltrates that were denser in the lobules than in interlobular fibrotic areas.
Recently, the expert panel in the international consensus study has agreed that there are two distinct histopathological types of AIP[5]. Type 1 AIP has dense periductal lymphoplasmacytic infiltrate with storiform fibrosis and obliterative phlebitis, whereas type 2 is distinguished from type 1 by granulocyte epithelial lesions, less prominent lymphoplasmacytic infiltrate, and less prominent storiform fibrosis. Recently, international consensus diagnostic criteria (ICDC) for AIP were developed based on the agreement of an international panel of experts and ICDC include both types 1 and 2 AIP[6].
According to the ICDC, the radiological imaging and the response to steroids are common features of both types 1 and 2 AIP. However, typical serological abnormalities, such as serum IgG4 elevation and other organ involvement, can be seen only in type 1. Thus, for a definitive diagnosis of type 2 AIP, histological confirmation is always necessary. Type 2 AIP is associated with inflammatory bowel disease and affects younger patients without a gender predilection[7]. Both types of AIP respond to steroid very well, but type 2 AIP has a lower relapse rate than type 1 AIP[7].
Although elevation of serum IgG4 is the one of the characteristic features in type 1 AIP, the sensitivity of serum IgG4 is variable. The initial Japanese study reported that the sensitivities of IgG4 were 90.9%[8]. However, other studies reported the sensitivity of IgG4 as approximately 70.0%[9-12]. The problem of the previous studies was that there was no clear classification of AIP type because the study was performed before the concept of type 2 AIP was established. If the study population had included more type 2 AIP, the sensitivity of IgG4 would have been low. However, a recent multicenter study also showed that the sensitivity was only 63.0% among histologically proven type 1 AIP[13]. Type 1 AIP is considered as the pancreatic manifestation of IgG4-related systemic disease in which tissue infiltration of IgG4-positive plasma cells is a characteristic feature[14-16]. However, the reason for the variable level of serum IgG4, the relation between serum level and tissue concentration of IgG4, and clinical significance of serum IgG4 level in type 1 AIP is unknown and remains an interesting issue.
The aim of this study was to find clinical and pathological differences between serum IgG4-positive (SIP) and serum IGg4-negative (SIN) type 1 AIP in Korea.
From January 2005 to May 2011, all patients with AIP were retrospectively reviewed at the Seoul National University Hospital. The diagnosis of AIP was based on the ICDC[6] and patients without available serum IgG4 level were excluded. Patients with definite AIP were enrolled. The institutional review board of Seoul National University Hospital approved the study.
The histology was obtained before steroid therapy in all cases. If the histology was available, type 1 AIP was defined as LPSP and type 2 as IDCP. The serum IgG4 level was obtained before steroid therapy and tissue acquisition. If tissue was not obtained, type 1 AIP was also defined if the serum IgG4 level was higher than upper limit of normal value (134 mg/dL). If the patients had no or unclear pathological findings and serum IgG4 level was normal, the patients were classified as indeterminate type and excluded in this study.
Pancreatic imaging was categorized as diffuse or segmental swelling by computed tomography (CT) scan. The presence of extrapancreatic lesions included sclerosing cholangitis, sclerosing sialoadenitis, lymphadenopathy, retroperitoneal fibrosis, and ulcerative colitis. Sclerosing cholangitis was defined as the presence of benign stricture of the bile duct on cholangiography. The stricture of only lower bile duct was not included in sclerosing cholangitis. The presence of sialoadenitis, lymphadenopathy and retroperitoneal fibrosis was determined based on CT findings.
Steroid therapy was done at 0.6 mg/kg per day of prednisolone for one month and gradually tapered to a maintenance dose over three months. Steroid maintenance therapy (5 mg/d) was administered for 6 mo to prevent relapse. Relapse was defined as a recurrence of symptoms with the development of pancreatic or extrapancreatic abnormal findings on imaging studies.
Surgically resected or core biopsied specimens were reviewed by a specialist pathologist without any clinical information. Fine needle aspiration specimens were not considered as available histology and not reviewed. All specimens were stained with anti-IgG4 antibody for immunohistochemical examination. The number of IgG4-positive plasma cells was counted in a high power field (HPF). In surgical specimens, LPSP was defined with at least three of the four characteristic features which are (1) dense infiltration of plasma cells and lymphocytes, particularly periductal; (2) peculiar storiform fibrosis; (3) venulitis with lymphocytes and plasma cells often leading to obliteration of the affected veins; and (4) abundant (> 10 cells per HPF) IgG4-positive plasma cells. In biopsy specimens, AIP was considered with lymphoplasmacytic infiltration with fibrosis and abundant (> 10 cells per HPF) IgG4-positive plasma cells.
Statistical analysis was done with statistical software (SPSS version 19.0 for Windows, SPSS Inc, Chicago, IL, United States; MedCalc version 11.5.0.0, MedCalc Software, Mariakerke, Belgium). The data were compared between two groups. The analysis was performed using Student’s t test, Fischer’s exact test and Mann-Whitney’s U test. A P value of < 0.05 was considered statistically significant. As repeated comparisons were made, P values of less than 5% (P < 0.05) were considered significant.
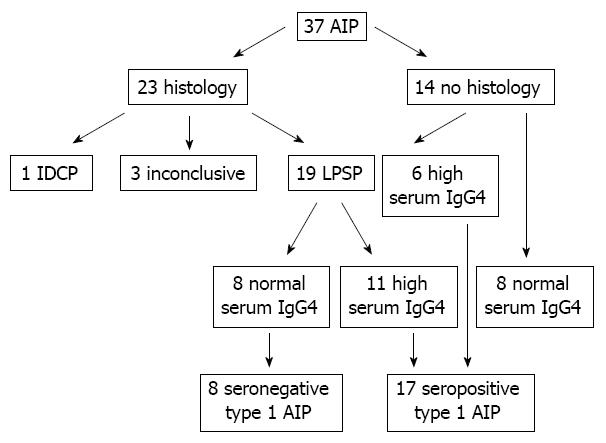
Thirty seven patients with AIP were enrolled and histology was available for 23 patients (Figure 1). Among 23 patients with histology, 19 patients showed typical finding of type 1 AIP and were confirmed as type 1 AIP. Only one patient was histologically confirmed as a type 2 AIP and had a history of ulcerative colitis. The pathological diagnosis was inconclusive in three cases among eight core biopsies. One type 2 and three SIN AIP patients with inconclusive pathology were excluded from this study. Among 19 patients with type 1 AIP, 11 patients had high serum IgG4 level and eight patients had normal levels. Among 14 patients without histology, six patients had elevated serum IgG4 levels (146, 213, 250, 279, 300 and 4000 mg/dL) and were included in type 1 AIP. Another eight patients with normal serum IgG4 levels were classified as indeterminate AIP and excluded from this study. Ultimately, 17 patients with SIP type 1 AIP and eight patients with SIN type 1 AIP were enrolled in this study. The median age was 61 years (range, 33-84 years) and males were predominant (72%). The sensitivity of serum IgG4 was 68.0%.
The mean age of the two groups was similar (62 years vs 60 years in SIP and SIN type 1 AIP) and there was no difference in sex between two groups (Table 1). The diffuse type of AIP seemed to be more common in the SIP than in the SIN group (47% vs 31%) but the difference was not significant (P = 0.39). The median serum IgG4 level was 312 mg/dL (normal range, 145-4000 mg/dL) in the SIP group and was 33 mg/dL (normal range, 6-75 mg/dL) in the SIN group and the difference was significant (P = 0.03). The patients of the SIP group were more likely to have other organ involvement than those of the SIN group (59% vs 26%, P = 0.016). Among the SIP group, sclerosing cholangitis was the most common (four cases) and sialoadenitis was also common (three cases) as other organ involvement. Retroperitoneal fibrosis, mediastinal lymphadenitis and lacrimal gland also represented other organ involvements. Among the SIN group, one patient had retroperitoneal fibrosis. Only one patient with sclerosing cholangitis was pathologically confirmed as an other organ involvement and other patients were diagnosed with only image and steroid responsiveness. The surgical resection rate was higher in the SIN than in the SIP group (75% vs 26%, P = 0.018). The mean follow up duration was not different between the two groups (30 mo vs 16 mo in SIP and SIN groups, P = 0.075). All patients, except those who received surgical resection, received steroid treatment and the response rate was 100% in both SIP and SIN groups. The relapse rate was not different between the two groups (36% vs 25% in SIP and SIN group, P = 0.80). The mean interval from steroid treatment and relapse was not different between the two groups (14 mo vs 11 mo in SIP and SIN groups, P = 0.82).
| Variables | SIP | SIN | P value |
| Patients | 17 | 8 | |
| Mean age, yr | 62 (33-84) | 60 (42-72) | 0.359 |
| Sex (M/F) | 13:4 | 5:3 | 0.172 |
| Diffuse type | 8 (47) | 3 (31) | 0.390 |
| Median serum IgG4 (mg/dL) | 312 (145-4000) | 33 (6-75) | 0.030 |
| Other organ involvement | 10 (59) | 1 (26) | 0.016 |
| Histologic examination | |||
| Resection | 5 (26) | 6 (75) | 0.018 |
| Biopsy | 6 (32) | 2 (25) | |
| Not done | 6 (32) | ||
| Mean follow up, mo | 30 | 16 | 0.075 |
| Relapse | 6 (35) | 2 (25) | 0.850 |
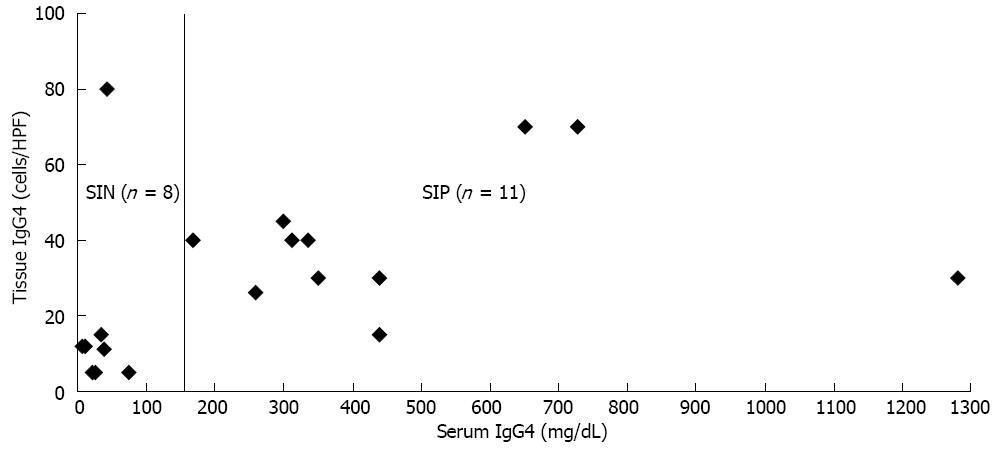
Among the 25 patients with type 1 AIP, 19 patients had tissue specimens, which included 11 SIP and 8 SIN groups. The mean tissue IgG4 concentrations were significantly higher in the SIP than the SIN group (40 cells per HPF vs 18 cells per HPF, P = 0.02). Among eight SIN patients, the tissue IgG4 concentrations were less than 15 cells per HPF in most of cases, except one (Figure 2). Among 11 SIP patients, the tissue IgG4 concentrations were more than 25 cells per HPF, except for one case (15 cells per HPF). However, there was no linear correlation between serum and tissue IgG4 concentration among the 11 SIP patients.
The clinical features of eight patients with SIN type 1 AIP are summarized in Table 2. Three cases were typical diffuse type AIP. However, surgical resection was done in two cases because serum IgG4 was normal and the possibility of malignancy could not be excluded in the early period (2005). For four cases with segmental type, surgical resections were performed because the possibility of malignancy could not be excluded by imaging at that time. Only one patient had retroperitoneal fibrosis and experienced disease relapse. Six patients who received surgical resection could be confirmed as type 1 AIP with LPSP (level 1 criterion) and level 1/2 parenchymal imaging. One patient (case 3) had level 1 parenchymal imaging and level 2 histology. The other patient (case 4) could be diagnosed as type 1 AIP with level 1 ductal imaging, level 2 histology and response to steroids.
| Patients | Age/sex | Tissue | Image | OOI | Serum IgG4 (mg/dL) | Tissue IgG4 in HPF | Relapse |
| Case 1 | 72/F | Resection | Diffuse | No | 75 | 5 | No |
| Case 2 | 42/M | Resection | Diffuse | No | 26 | 5 | Yes |
| Case 3 | 71/F | Biopsy | Diffuse | RF | 33 | 15 | Yes |
| Case 4 | 61/M | Biopsy | Tail | No | 39 | 11 | No |
| Case 5 | 61/M | Resection | Body | No | 43 | 80 | No |
| Case 6 | 51/M | Resection | Tail | No | 21 | 5 | No |
| Case 7 | 66/M | Resection | Head | No | 6 | 12 | No |
| Case 8 | 53/F | Resection | Body | No | 11 | 12 | No |
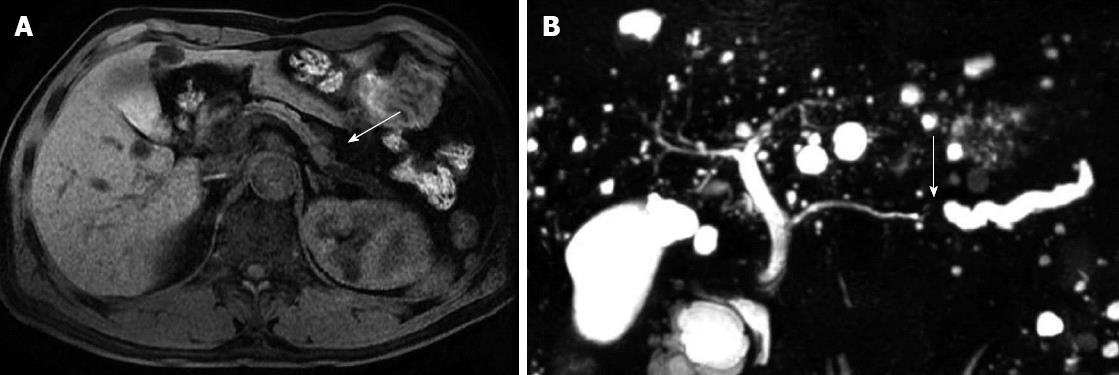
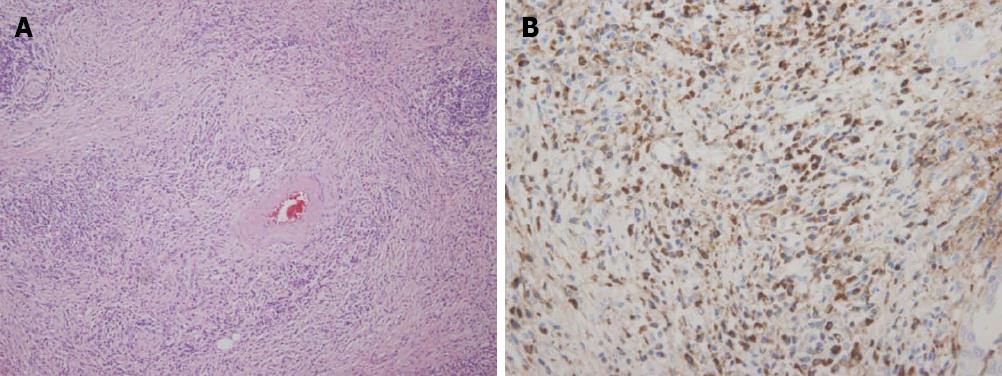
One patient had a relatively high tissue IgG4 concentration (80 cells per HPF) despite a low serum IgG4 level (43 mg/dL). He was 61-year-old male and a mass was detected incidentally at the body of the pancreas. Magnetic resonance image (MRI) findings also showed a slightly exophytic mass of iso-attenuation at the body of the pancreas with distal parenchymal atrophy and an abrupt cutting of the pancreatic duct was noticed with upstream ductal dilatation (Figure 3). Image findings were compatible with pancreatic cancer and a distal pancreatectomy was performed. Gross pathological finding showed a 1.3 cm × 1.2 cm × 3 cm sized white solid mass with uncertain margins. Microscopy showed dense periductal lymphoplasmacytic infiltration, storiform fibrosis and obliterative phlebitis (Figure 4A). The IgG4 immunohistochemistry also showed dense infiltration (80 cells per HPF) (Figure 4B). After the operation, he did not develop any symptoms or signs of recurrence for 3 years of follow-up.
IgG4-related disease was recognized as a systemic disease since 2003[17] and AIP was proposed as one of the IgG4-related sclerosing diseases in 2006[18]. Since then, two histopathological subtypes, LPSP and IDCP, have been recognized[19]. Type 1 AIP is now considered as the pancreatic manifestation of an IgG4-related systemic fibroinflammatory diseases involving the salivary gland, bile duct, and retroperitoneum. Thus, serum IgG4 is a useful marker for the diagnosis of type 1 AIP and most diagnostic criteria of AIP include serum IgG4 elevation as one of the criteria[6,9,20]. However, the sensitivity of serum IgG4 is variable and different among countries. If the study population includes more type 2 AIP, the sensitivity of IgG4 may be low because serum IgG4 is not usually elevated in type 2 AIP. A recent international multicenter study, which enrolled 713 patients with AIP from eight countries, reported that sensitivity of serum IgG4 was only 63% among 204 patients with histologically proven type 1 AIP[13]. The relatively low sensitivity of serum IgG4 can make the diagnosis of AIP in the clinical setting confusing. In our study, four patients with segmental type AIP underwent unnecessary surgical resection.
We questioned why the serum IgG4 test was not sufficiently sensitive if type 1 AIP is a type of IgG4-related systemic disease. Therefore, we conducted our study to analyze the clinical and pathological differences between SIP and SIN type 1 AIP. Unfortunately, there have been few studies concerning the normal serum IgG4 AIP[21,22]. One study included 58 AIP patients including 13 normal serum IgG4 AIP[21] but histology was available in only 14 cases (six cases among 13 SIN AIP). Another study included 27 patients with AIP, including seven SIN AIP[22]. Histology was not available in any cases because endoscopic ultrasonography guided fine needle aspiration was performed in 26 cases using 22 gauge needle, not to diagnose AIP, but to exclude pancreatic malignancy. Thus, it could not be determined that all of the enrolled patients were really type 1 AIP in both studies. To exclude possible type 2 AIP, our study enrolled 19 patients with histologically proven type 1 AIP and six patients who were clinically diagnosed as type 1 AIP with elevated serum IgG4 levels. Of course, there is the possibility of type 2 AIP despite the elevated serum IgG4 level among six patients, because serum IgG4 elevation was detected in 23% among 47 patients with histologically proven type 2 AIP according to a recent study[13]. However, the possibility might be very low, because the serum IgG4 level was relatively high (213, 250, 279, 300, 4000 mg/dL), except in one case (146 mg/dL) and type 2 AIP was reported to be relatively rare in Asian countries, including South Korea[13]. In addition, five patients had other organ involvement, which is rarely be seen in type 2 AIP[7].
The surgical resection rate was higher in the SIN than the SIP group. One reason could be a difficult diagnosis of AIP. If the lesion is in the body/tail and serum IgG4 is normal, the clinicians would not suspect the possibility of AIP and would not hesitate to perform a surgical resection. Another reason might be selection bias of this study, because we excluded eight patients with normal serum IgG4 and no histology. The eight patients received steroid treatment and their steroid responsiveness was 100%. One patient experienced a relapse.
In this study, the clinical profiles of type 1 AIP were similar to another recent multicenter study including 327 Asian patients[12]. The higher mean age (over 60 year), male predominance, common other organ involvement, especially sclerosing cholangitis and frequent relapse, are common features of Asian patients of AIP that are similar to our study. Interestingly, the important clinical difference between SIP and SIN type 1 AIP was the frequency of other organ involvement. Other organ involvement was significantly more common in SIP than SIN type 1 AIP (59% vs 26%). Only one patient among the SIN group had retroperitoneal fibrosis. This result implies that other organ involvement can affect the serum IgG4 level. Mikulicz’s disease refers to idiopathic symmetrical swelling of the lacrimal, submandibular gland and is an IgG4-related systemic disease. A recent study reported that the serum IgG4 level is very high (894 mg/dL) in Mikulicz’s disease and significantly higher in patients with extrasalivary gland involvement[23]. More frequent other organ involvement in our SIP type 1 AIP is similar to the results of previous studies[21,22,24].
Another reason for variable serum IgG4 levels may be the number of IgG4-positive plasma cells in the tissue. As expected, the mean tissue IgG4 concentration was significantly low in SIN compared with SIP type 1 AIP. All patients in the SIP group had high IgG4 concentrations (over 25 cells per HPF), except one case (15 cells per HPF). However, the patients in the SIN group had very low IgG4 concentrations (below 15 cells per HPF), except one case (80 cells per HPF). The data might lead us to conclude that the IgG4 concentration of pancreatic tissue can influence the sensitivity of serum IgG4 in type 1 AIP. However, the serum level of IgG4 had no correlation with tissue IgG4 concentration in SIP type 1 AIP. Thus the serum level may be influenced by not only tissue concentration, but also other factors, such as the size of the involved pancreas and other organ involvement. We think that this is the first study to investigate the correlation between serum and tissue IgG4 concentration in type 1 AIP.
Other possible clinical roles of serum IgG4, other than as a diagnostic marker, are uncertain and are a interesting issue in type 1 AIP. The clinical use of serum IgG4 may be relevant in three settings: monitoring of therapy, monitoring for disease relapse and prediction of relapse. The large multicenter study in Japan reported that IgG4 levels failed to normalize in 115/182 (63%) of the patients treated with steroids[25]. The study suggested that serial IgG4 levels are helpful in identifying early relapse. However, only 30% of patients with persistent IgG4 elevation relapsed, whereas relapse was also seen in 10% of patients with normal IgG4 levels. The results regarding the value of initial serum IgG4 levels in predicting relapse vary among studies, some reporting higher relapse rate in patients with elevated serum IgG4 levels[22,26], whereas others failed to observe any association[7,27-29]. To clarify the role of serum IgG4 in predicting relapse, type 2 AIP should be excluded in the normal serum IgG4 group, because type 2 AIP is known for rare relapse[7]. The positive study might include some patients with type 2 AIP. In our study, the relapse rate was similar between the two groups of type 1 AIP. Thus, our data supports the view that initial serum IgG4 levels cannot predict relapse in type 1 AIP.
In conclusion, the sensitivity of serum IgG4 was not sufficient (68%) for definite diagnosis of type 1 AIP. The demographic findings were similar between SIP and SIN type 1 AIP, but other organ involvement was significantly more common in SIP than SIN type 1 AIP. High serum IgG4 level was associated with other organ involvement and tissue IgG4 concentration, but did not affect the relapse rate in type 1 AIP.
Type 1 autoimmune pancreatitis (AIP) is one of the immunoglobulin G4 (IgG4)-related diseases and serum IgG4 is known as a useful diagnostic marker. However, the sensitivity of serum IgG4 is variable. AIP is a type of chronic pancreatitis with irregular narrowing of the pancreatic duct and systemic fibroinflammatory disease and is characterized by a remarkable response to steroid therapy.
IgG4-related disease was recognized as a systemic disease in 2003 and AIP was proposed as one of the IgG4-related sclerosing diseases in 2006. Two histopathological subtypes, lymphoplasmacytic sclerosing pancreatitis and idiopathic duct-centric chronic pancreatitis, have been recognized, and type 1 AIP is now considered as the pancreatic manifestation of an IgG4-related systemic fibroinflammatory diseases involving the salivary gland, bile duct, and retroperitoneum. Thus, serum IgG4 is a useful marker for the diagnosis of type 1 AIP and most diagnostic criteria of AIP include serum IgG4 elevation as one of the criteria. However, the sensitivity of serum IgG4 is variable and different among countries.
The sensitivity of serum IgG4 was not sufficient (68%) for defining type 1 AIP. The demographic findings were similar between serum IgG4-positive (SIP) and serum IgG4-negative (SIN) type 1 AIP, but other organ involvement was significantly more common in SIP than SIN type 1 AIP. High serum IgG4 level was associated with other organ involvement and tissue IgG4 concentration, but did not affect the relapse rate in type 1 AIP.
The authors compared the clinical and pathological differences between serum IgG4-positive and IgG4-negative type 1 autoimmune pancreatitis and demonstrated that the sensitivity of serum IgG4 was 68% in type 1 AIP. The high serum IgG4 level was associated with other organ involvement and tissue IgG4 concentration, but did not affect the relapse rate in type 1 AIP.
P- Reviewers Ishida M, Kamisawa T, Zhang XC S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Ryu JK, Lee JK, Kim YT, Lee DK, Seo DW, Lee KT, Kim HG, Kim JS, Lee HS, Kim TN. Clinical features of chronic pancreatitis in Korea: a multicenter nationwide study. Digestion. 2005;72:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 413] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Pearson RK, Longnecker DS, Chari ST, Smyrk TC, Okazaki K, Frulloni L, Cavallini G. Controversies in clinical pancreatology: autoimmune pancreatitis: does it exist? Pancreas. 2003;27:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 382] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 5. | Zhang L, Chari S, Smyrk TC, Deshpande V, Klöppel G, Kojima M, Liu X, Longnecker DS, Mino-Kenudson M, Notohara K. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas. 2011;40:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1059] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 7. | Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010;139:140-148; quiz e12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Kawa S, Hamano H. Assessment of serological markers for the diagnosis of autoimmune pancreatitis. J Jpn Pancreas Soc. 2003;17:607-610. |
| 9. | Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-1016; quiz 934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 657] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 10. | Choi EK, Kim MH, Lee TY, Kwon S, Oh HC, Hwang CY, Seo DW, Lee SS, Lee SK. The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas. 2007;35:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Ryu JK, Chung JB, Park SW, Lee JK, Lee KT, Lee WJ, Moon JH, Cho KB, Kang DW, Hwang JH. Review of 67 patients with autoimmune pancreatitis in Korea: a multicenter nationwide study. Pancreas. 2008;37:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Kamisawa T, Kim MH, Liao WC, Liu Q, Balakrishnan V, Okazaki K, Shimosegawa T, Chung JB, Lee KT, Wang HP. Clinical characteristics of 327 Asian patients with autoimmune pancreatitis based on Asian diagnostic criteria. Pancreas. 2011;40:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, Werner J, Bergmann F, Lerch MM, Mayerle J. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 984] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 15. | Zhang L, Smyrk TC. Autoimmune pancreatitis and IgG4-related systemic diseases. Int J Clin Exp Pathol. 2010;3:491-504. [PubMed] |
| 16. | Sah RP, Chari ST. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol. 2011;23:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 17. | Kamisawa T, Egawa N, Nakajima H. Autoimmune pancreatitis is a systemic autoimmune disease. Am J Gastroenterol. 2003;98:2811-2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006;41:613-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 19. | Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas. 2010;39:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 20. | Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, Park SW, Shimosegawa T, Lee K, Ito T. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 21. | Kamisawa T, Takuma K, Tabata T, Inaba Y, Egawa N, Tsuruta K, Hishima T, Sasaki T, Itoi T. Serum IgG4-negative autoimmune pancreatitis. J Gastroenterol. 2011;46:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Matsubayashi H, Sawai H, Kimura H, Yamaguchi Y, Tanaka M, Kakushima N, Takizawa K, Kadooka M, Takao T, Hebbar S. Characteristics of autoimmune pancreatitis based on serum IgG4 level. Dig Liver Dis. 2011;43:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Himi T, Takano K, Yamamoto M, Naishiro Y, Takahashi H. A novel concept of Mikulicz’s disease as IgG4-related disease. Auris Nasus Larynx. 2012;39:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Hamano H, Arakura N, Muraki T, Ozaki Y, Kiyosawa K, Kawa S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol. 2006;41:1197-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 25. | Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, Okumura F, Nishikawa T, Kobayashi K, Ichiya T. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 486] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 26. | Frulloni L, Scattolini C, Falconi M, Zamboni G, Capelli P, Manfredi R, Graziani R, D’Onofrio M, Katsotourchi AM, Amodio A. Autoimmune pancreatitis: differences between the focal and diffuse forms in 87 patients. Am J Gastroenterol. 2009;104:2288-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, Okumura F, Miyabe K, Yoshida M, Sano H. Clinical significance of extrapancreatic lesions in autoimmune pancreatitis. Pancreas. 2010;39:e1-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 29. | Kubota K, Iida H, Fujisawa T, Yoneda M, Inamori M, Abe Y, Kirikoshi H, Saito S, Ohshiro H, Kakuta Y. Clinical factors predictive of spontaneous remission or relapse in cases of autoimmune pancreatitis. Gastrointest Endosc. 2007;66:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |