Published online Jul 7, 2013. doi: 10.3748/wjg.v19.i25.3980
Revised: April 10, 2013
Accepted: May 16, 2013
Published online: July 7, 2013
Processing time: 134 Days and 7 Hours
AIM: To investigate the effect of polydatin (PD), a resveratrol glucoside, on mast cell degranulation and anti-allergic activity.
METHODS: After the rats were orally sensitized with ovalbumin (OVA) for 48 d and underwent PD treatment for 4 d, all the rats were stimulated by 100 mg/mL OVA for 24 h and then sacrificed for the following experiments. The small intestines from all the groups were prepared for morphology examination by hematoxylin and eosin staining. We also used a smooth muscle organ bath to evaluate the motility of the small intestines. The OVA-specific immunoglobulin E (IgE) production and interleukin-4 (IL-4) levels in serum or supernatant of intestinal mucosa homogenates were analyzed by enzyme-linked immunosorbent assay (ELISA). Using toluidine blue stain, the activation and degranulation of isolated rat peritoneal mast cells (RPMCs) were analyzed. Release of histamine from RPMCs was measured by ELISA, and regulation of PD on intracellular Ca2+ mobilization was investigated by probing intracellular Ca2+ with fluo-4 fluorescent dye, with the signal recorded and analyzed.
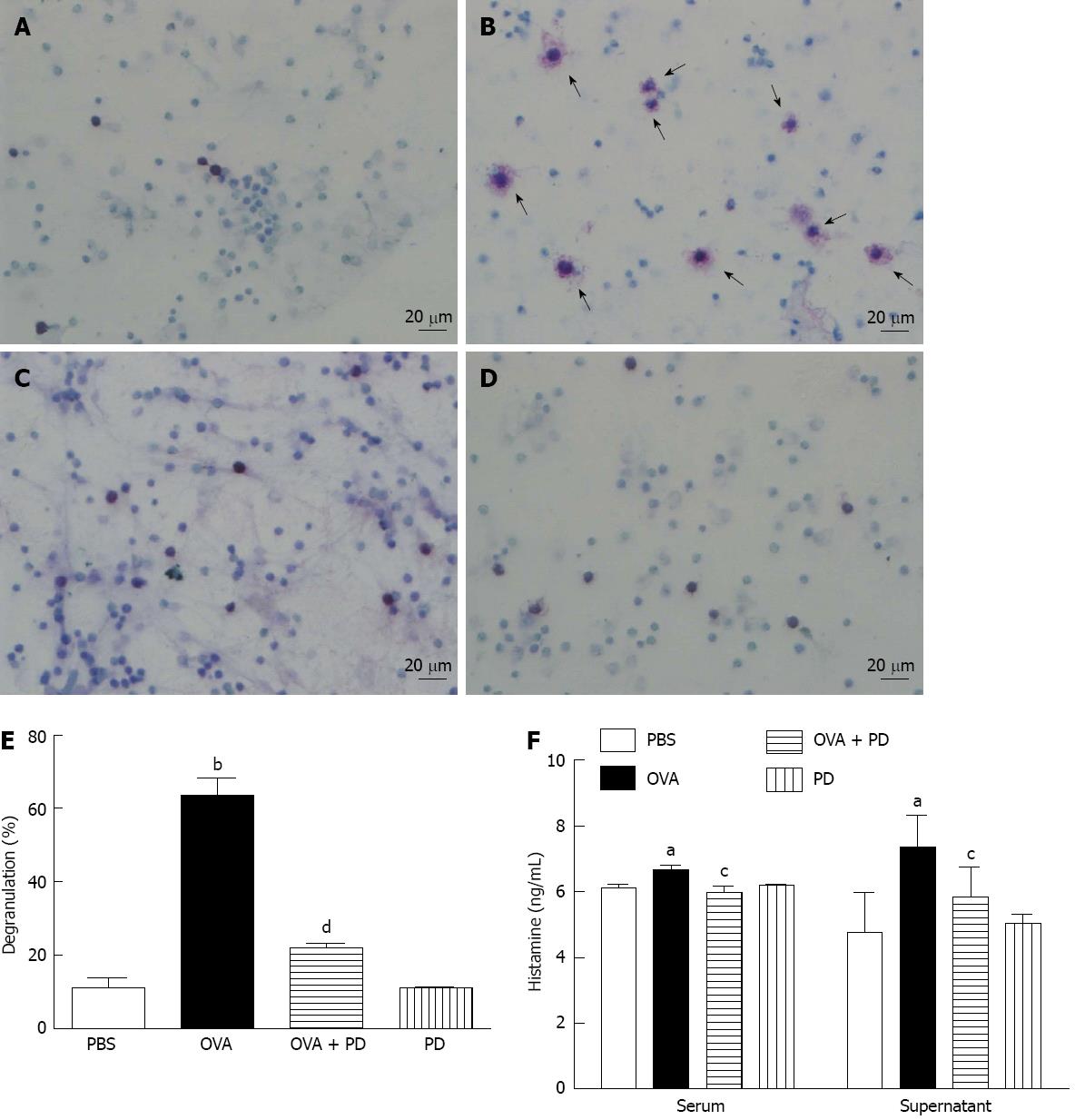
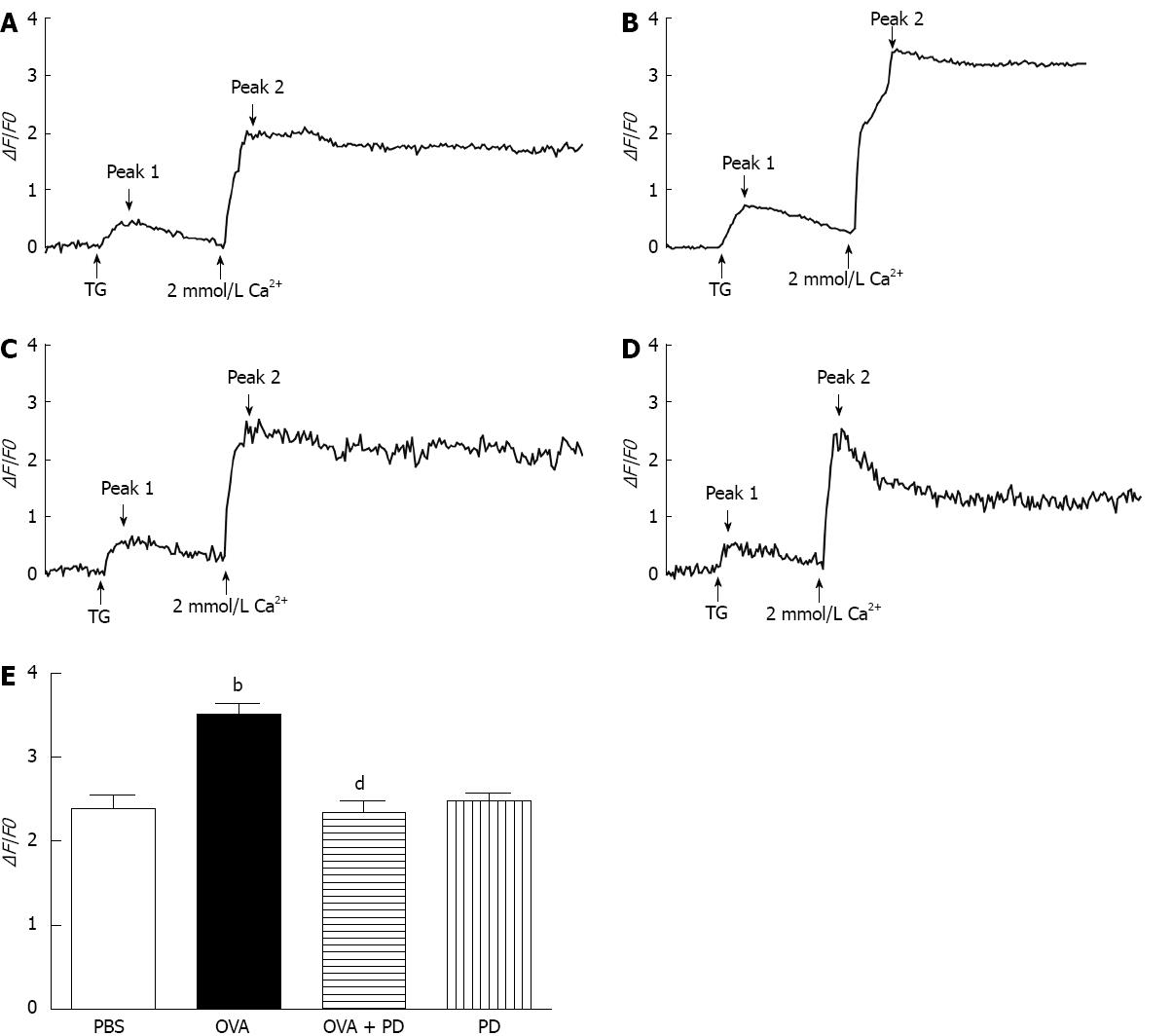
RESULTS: We found that intragastric treatment with PD significantly reduced loss of mucosal barrier integrity in the small intestine. However, OVA-sensitization caused significant hyperactivity in the small intestine of allergic rats, which was attenuated by PD administration by 42% (1.26 ± 0.13 g vs OVA 2.18 ± 0.21 g, P < 0.01). PD therapy also inhibited IgE production (3.95 ± 0.53 ng/mL vs OVA 4.53 ± 0.52 ng/mL, P < 0.05) by suppressing the secretion of Th2-type cytokine, IL-4, by 34% (38.58 ± 4.41 pg/mL vs OVA 58.15 ± 6.24 pg/mL, P < 0.01). The ratio of degranulated mast cells, as indicated by vehicles (at least five) around the cells, dramatically increased in the OVA group by 5.5 fold (63.50% ± 15.51% vs phosphate-buffered saline 11.15% ± 8.26%, P < 0.001) and fell by 65% after PD treatment (21.95% ± 4.37% vs OVA 63.50% ± 15.51%, P < 0.001). PD mediated attenuation of mast cell degranulation was further confirmed by decreased histamine levels in both serum (5.98 ± 0.17 vs OVA 6.67 ± 0.12, P < 0.05) and intestinal mucosa homogenates (5.83 ± 0.91 vs OVA 7.35 ± 0.97, P < 0.05). Furthermore, we demonstrated that administration with PD significantly decreased mast cell degranulation due to reduced Ca2+ influx through store-operated calcium channels (SOCs) (2.35 ± 0.39 vs OVA 3.51 ± 0.38, P < 0.01).
CONCLUSION: Taken together, our data indicate that PD stabilizes mast cells by suppressing intracellular Ca2+ mobilization, mainly through inhibiting Ca2+ entry via SOCs, thus exerting a protective role against OVA-sensitized food allergy.
Core tip: In the present study, we have demonstrated for the first time that polydatin has the capacity for preventing pathogenesis of food allergy, which is dependent on regulation of Ca2+ mobilization via store-operated calcium channels in mast cells.
-
Citation: Yang B, Li JJ, Cao JJ, Yang CB, Liu J, Ji QM, Liu ZG. Polydatin attenuated food allergy
via store-operated calcium channels in mast cell. World J Gastroenterol 2013; 19(25): 3980-3989 - URL: https://www.wjgnet.com/1007-9327/full/v19/i25/3980.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i25.3980
Food allergy (FA) is an adverse reaction mediated by immunoglobulin E (IgE) or non-IgE antibodies[1], which involves an abnormal response by the immune system to specific proteins in foods[2]. FA has been recognized as a worldwide health problem, especially in western countries, which is due to the severity of the reactions and its dramatic increase over the past three decades[3-5]. The majority of food allergies worldwide are caused by “eight main food allergens”, including peanuts, tree nuts, eggs, milk, fish, crustacean shellfish, wheat, and soy[6]. It has been suggested that 25% of infants[7], 8% of children[3-5], and 2%-5% of adults[8] suffer from FA. However, the current understanding about the etiology of food allergies remains poor, and no effective treatment is available except the preventative measure of avoiding the offending food in the diet.
Mast cells play an essential role in the development of intestinal inflammatory disorders during food allergy. Cross-linking of the high-affinity IgE receptor (FcεRI) on mast cells by allergens results in degranulation, leukotriene generation, and cytokine synthesis. Degranulated mast cells release inflammatory mediators, including histamine and Th2 cytokines[9], which cause abnormal gut contractions and intestinal mucosa damage, which in term then result in abdominal pain, cramps, vomiting, and/or diarrhea[10]. It has been known that IgE-dependent mast cell degranulation relies on intracellular Ca2+ signaling[11,12]. Cytoplasmic Ca2+ mainly comes from the stored Ca2+ in the endoplasmic reticulum (ER) and extracellular Ca2+ through store-operated calcium channels (SOCs)[13]. Therefore, modulation of Ca2+ mobilization is a potential therapeutic strategy for stabilizing mast cells upon FcεRI activation and potentially offering a novel treatment method for allergic diseases.
Polydatin (PD), also known as polygoni cuspidati radix, is a natural component isolated from Polygonum cuspidatum. It has been determined as a resveratrol glucoside with a 3,4,5-trihydroxystilben-3-D-mono-D-glucoside molecular structure. Previous studies have demonstrated that PD has a therapeutic effect on the treatment of allergic diseases. Using the passive cutaneous anaphylaxis (PCA) model in mice, Lim et al[14] showed that PD reduced mast cell degranulation by suppressing phosphorylation of Syk and mitogen-activated protein kinases. On the other hand, Yuan et al[15] found that PD alleviated PCA in mice by stabilizing mast cells via the inhibition of Ca2+ release-activated Ca2+ channels. However, the therapeutic effect of PD on food allergies has not yet been determined.
In this study, we established ovalbumin (OVA)-induced food allergic models and evaluated the therapeutic effect of PD on food allergy. Furthermore, we explored the effect of PD on mast cell degranulation and found that the underlying mechanism was related to Ca2+ mobilization. Our research presented here is the first to reveal that PD can inhibit food allergy by suppressing mast cell degranulation via regulation of SOCs.
Four-week old female Brown-Norway (BN) rats were purchased from Vital River Laboratories (Beijing, China) and housed in groups of four per cage in a controlled environment with a photoperiod of 12 h light to 12 h dark and a temperature of 20 ± 2 °C. Sanitary controls were performed for all major rodent pathogens, with the results of these tests being uniformly negative. All the animal experimental procedures were approved by the Animal Care and Use Committee of Shenzhen University and carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (publication No. 85-23, revised 1996).
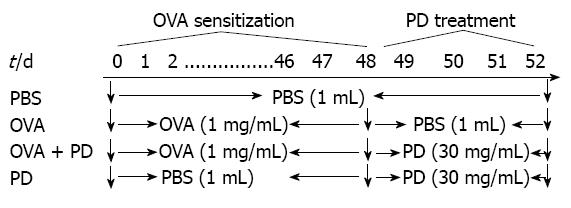
Forty-eight Brown-Norway rats were randomly divided into four groups: control group (n = 12), OVA group (n = 12), OVA + PD group (n = 12), and PD group (n = 12). Each group received phosphate-buffered saline (PBS), OVA, or PD, as shown in Figure 1[16]. The control group received 1 mL PBS (0.1 mol/L) daily by gavage administration for 52 d, while the OVA group was orally treated with 1 mg OVA (1 mg/mL) for the first 48 d and 1 mL PBS (0.1 mol/L) from days 48 to 52. The OVA + PD group received PD (150 mg/mL × 1 mL daily per rat) oral treatment daily from days 49 to 52 after OVA sensitization. The PD group was not challenged by OVA. All the groups of rats were stimulated by 100 mg/mL OVA for 24 h at the end of day 52 and then sacrificed for the following experiments.
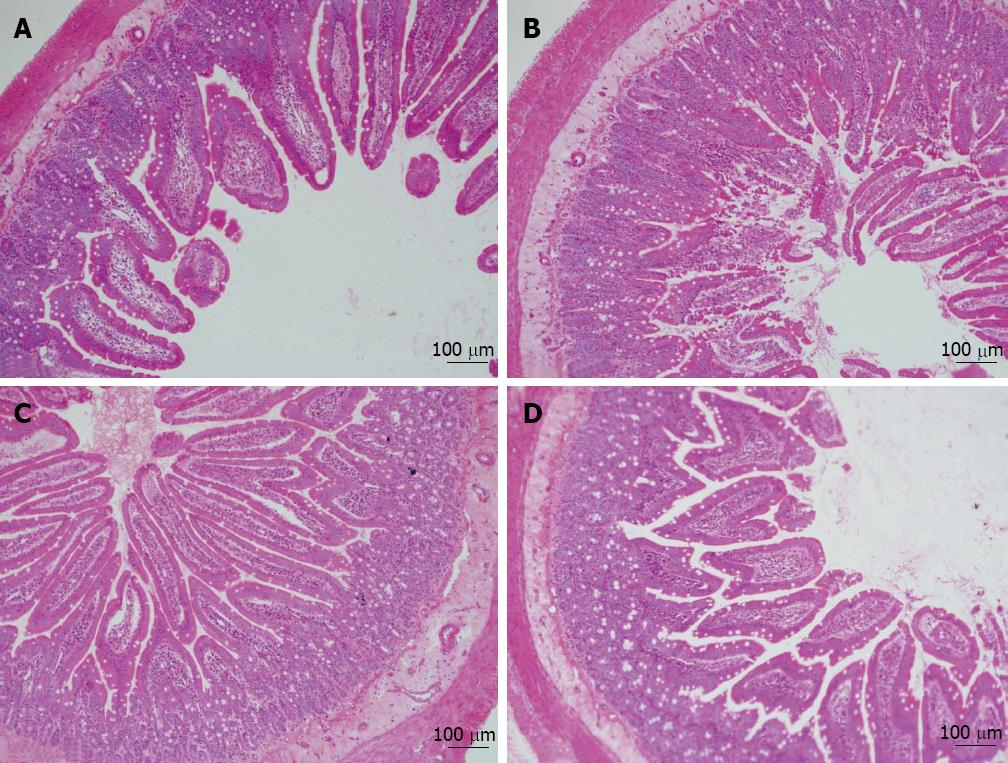
The jejunal parts of the small intestine were isolated from the rats and embedded with paraffin. Sections (7 μm) were prepared and subjected to hematoxylin and eosin (HE) staining as previously reported[17].
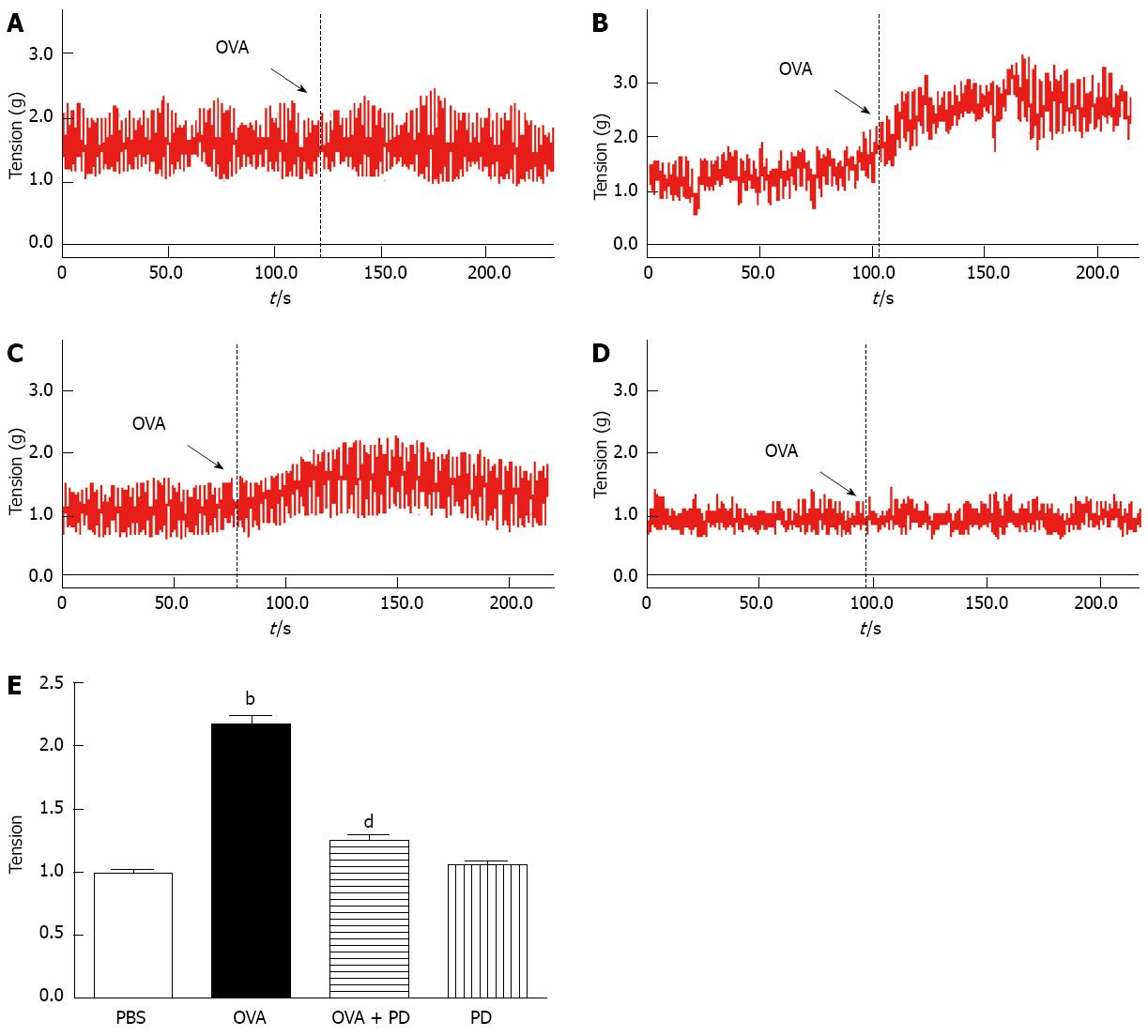
The tension of smooth muscle contractility was measured as previously reported[18]. Briefly, 2 cm long segments of the small intestine from the upper part of the jejunum to the lower part of the ileum were cut and mounted by hanging from triangle hooks. The hooks were connected to transducers from the upper end, and were inserted through the gut lumen from the lower end, allowing the circular muscle to contract. The tissue segments were incubated in chambers containing 20 mL Tyrode’s solution (136 mmol/L NaCl, 5.4 mmol/L KCl, 1.0 mmol/L MgCl2, 0.33 mmol/L NaH2PO4, 1.8 mmol/L NaCl, 10.0 mmol/L glucose and 5.0 mmol/L HEPES), which was kept at 37 °C and constantly aerated with a mixture of 95% oxygen and 5% carbon dioxide. The initial tension load was set at 1.0 g, from which the segments spontaneously relaxed over time. The segments were allowed to stabilize for 30 min before they were stimulated with 1 mg/mL OVA for 3 min. R0 was defined as the mean basal tension, when the segments were under rest conditions. ΔR denotes (R1-R0), where R1 is the contract tension when the segments were stimulated with OVA.
Typical mast cells in rat small intestine tissue or peritoneal lavage solution (RPLS) were stained with toluidine blue stain as previously described[19]. Briefly, 200 L RPLS was air dried on cromolyn sodium pretreated slides and then covered with several drops of staining solution (toluidine blue stain dissolved in 70% ethanol). After 90 s, the staining solutions were washed away quickly with running tap water and the stained cells were examined and counted under a light microscope (Olympus, Japan).
The contents of interleukin-4 (IL-4) (eBioscience Inc., CA, United States) and histamine (R & D Inc., MN, United States) in RPLS and serum were assayed by commercial enzyme-linked immunosorbent assay (ELISA) kits using paired antibodies according to the manufacturer’s instructions. Serum IgE levels were also checked using a commercial ELISA kit (BD Pharmingen, CA, United States), following the manufacturer’s instructions.
The BN rats were sacrificed after being anaesthetized by ether inhalation in air. Rat peritoneal mast cells (RPMCs) were obtained by peritoneal lavage and purified by density gradient fractionation as described previously[20,21]. Isolated RPMCs preparations contained > 98% mast cells and at least 98% of these cells were viable, as checked by metachromatic staining in 0.05% toluidine blue.
Intracellular Ca2+ signal was measured as described previously with minor modification[22]. RPMCs or RBL-2H3 cells were incubated with 5 μmol/L Ca2+ fluorescent probe fluo-4 AM (Invitrogen, CA, United States) for 30 min at room temperature. After washing with Tyrode’s solution three times, the dye inside the cells was allowed to de-esterify for 30 min at 37 °C. It has been determined that nearly 95% of the fluorescent dye was retained in the cytoplasm. Fluorescent images of Ca2+ were obtained using an Olympus 1000 confocal microscope with a 40 × oil immersion lens (NA 1.3) (Olympus, Japan). The fluo-4 signal was excited at 488-nm and emitted at > 505 nm. Frame-scan images were acquired at a sampling rate of 15 ms per frame and 20 s per interval.
Image data were analyzed off-line using fv10-asw.2.1 software. A selected image from each image set was used as a template for designating the region of interest (ROI) within each cell. The integrated intracellular Ca2+ concentration was determined by calculating ΔF/F0. F0 was defined as the mean basal fluorescence intensity of the dye recorded during the first 5-10 scanning frames, when the cells were under rest conditions. ΔF denotes (F-F0), where F is the temporal fluorescence intensity. The ΔF/F0 values within each ROI were plotted as a function of time (typical time-courses of Ca2+ response to thapsigargin or DNP-BSA stimulation in single RBL-2H3 cells). The amplitude of the Ca2+ response within each cell was quantified as the highest ΔF/F0 level reached during the measurement period, which was averaged over all cells within each group.
Data are presented as mean ± SE. When two comparisons were obtained, Student’s unpaired two tailed t test was used. When multiple comparisons were obtained, the analyses consisted of one-way analysis of variance for repeated measures and Student-Newman-Keuls multiple comparison test. A value of P < 0.05 was considered to be statistically significant.
In the present study, 1 mg OVA was used to sensitize BN rats orally and establish a food allergy model as previously described[16,23]. Loss of mucosal barrier integrity is a leading cause of food allergy[24]. Thus, we isolated jejunal fractions from the small intestine and checked tissue damage by HE staining. As shown in Figure 2, the results revealed that the intestinal mucosae were severely injured in the OVA group: the intestinal villi were eroded, and the swelling, shedding, and numbers of intestinal villi were significantly reduced. The morphological abnormality of the small intestine caused by OVA-sensitization was significantly attenuated by PD treatment.
The correlation between intestinal allergy and smooth muscle motility has been indicated by the fact that exposure to luminal allergen induces a state of proximal small intestinal hyperreactivity[25,26]. In order to evaluate the effects of PD on intestine motility, 2 cm intestine segments from each group were prepared, and the intestinal contraction tension was detected by smooth muscle organ bath. In response to 1 mg/mL OVA, intestinal segments isolated from OVA-allergic rats had a significant higher tension than the PBS group. The elevation of tissue tension caused by OVA sensitization was significantly attenuated by PD treatment by approximately 42% (Figure 3).
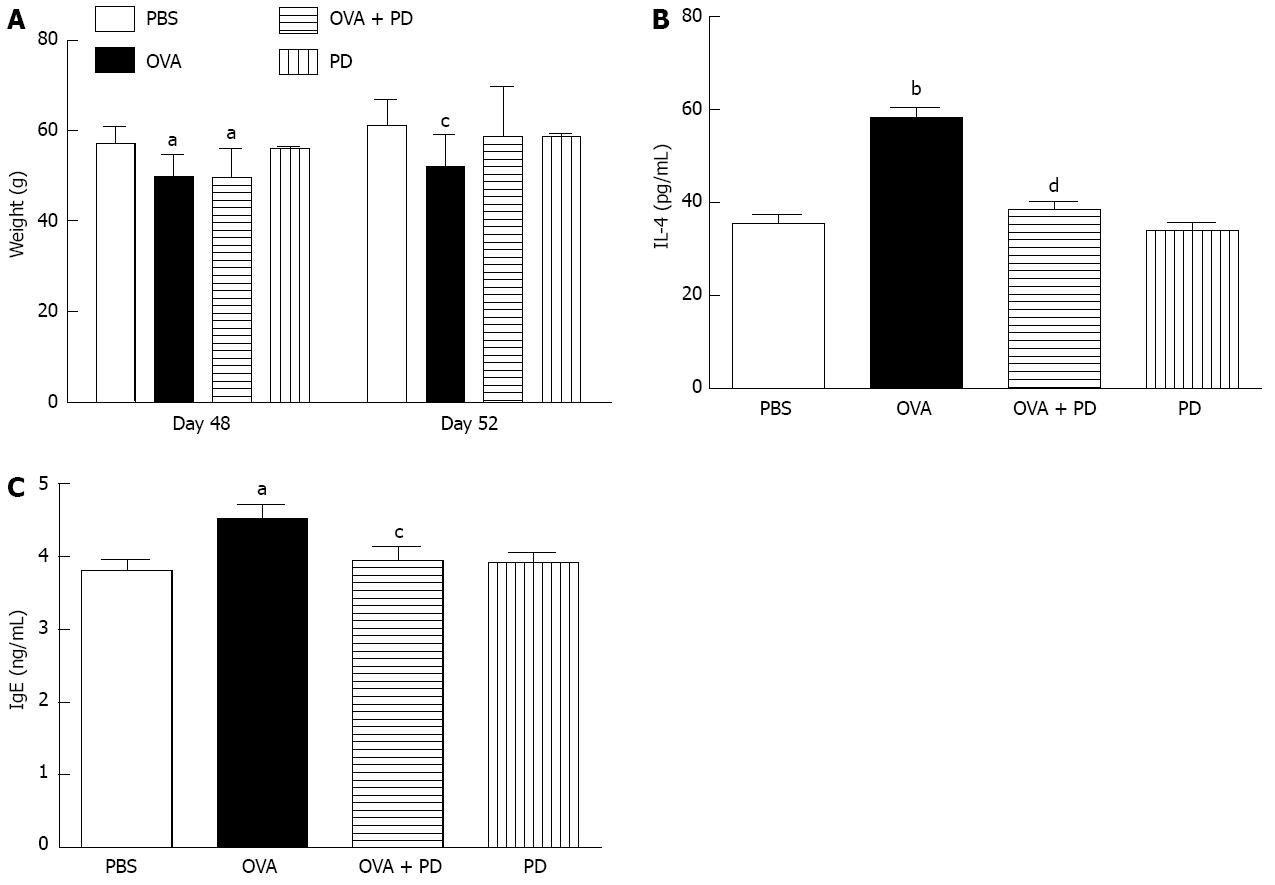
The body weight of all rats in each group was monitored on days 0, 48 and 52. We found that basal body weight levels on day 0 were similar in all four groups. Compared to the PBS group, OVA sensitization significantly reduced body weight on day 48 (Figure 4A, left panel). After being treated with PD for 4 d, there was no significant difference between the PBS and OVA + PD groups (Figure 4A, left panel), which indicates that PD administration could maintain the body weight of an allergic rat at a normal level. The cytokine levels in the supernatant of the intestine mucosa were measured by ELISA. The results showed that the concentration of IL-4 in the OVA-challenged group was significantly higher than in the control group (58.15 ± 6.24 pg/mL vs 35.51 ± 5.48 pg/mL) (Figure 4B). Treatment with PD reduced the enhancement of IL-4 by 34%. Meanwhile, ELISA analysis showed that the concentration of OVA-specific IgE in serum was enhanced by 1.2 fold in the OVA group and PD therapy returned it to a normal level (Figure 4C).
Mast cell degranulation and histamine release are major factors in food allergy. The number and morphology of the mast cells in rat small intestine tissues (data not shown) or RPLS were examined by toluidine blue stain. In the OVA group, the number of mast cells was significantly increased and the cell size was much bigger, with more shrink on the cell membrane, bubbles in the cytoplasm, and degranulation vehicles around the cells (Figure 5A-D). In vivo administration with PD for 4 d reversed OVA-challenge-induced damage in mast cells. The ratio of degranulated mast cell, as indicated by vehicles (at least five) around the cells, dramatically increased in the OVA group by 5.5 fold and fell by 65% after PD treatment (Figure 5E). PD mediated attenuation of mast cell degranulation was further confirmed by decreased histamine levels. It was found that histamine release in both serum and RPLS was significantly increased in the OVA-induced food allergic group, which was attenuated by PD therapy by approximately 11% and 20% respectively (Figure 5F).
Rapid translocation of Ca2+ has been well-known to be essential for mast cell degranulation[27]. In a food allergic model, we also found that mast cell activation is related to stimulation of Ca2+ mobilization (data not published). To explore the underlying mechanism for the inhibitory effect of PD on mast cell degranulation, we isolated RPMCs and monitored intracellular Ca2+ with fluo-4 (5 mol/L). Using a standard Ca2+ add-back assay, in which intracellular Ca2+ stores were depleted by thapsigargin (TG), a sarcoplasmic/endoplasmic reticulum calcium ATPase (Ca2+ pump) blocker, in Ca2+-free extracellular solution, after which the extracellular Ca2+ concentration was returned to 2 mmol/L[28]. Using this protocol, TG elicited two Ca2+ peaks, where the first one represented the ER Ca2+ release, and the second represented Ca2+ entry through activated SOCs. As shown in the first peaks in Figure 6A-D, when the cells were in Ca2+-free solution, the TG-evoked Ca2+ amplitude was similar in all the groups, suggesting the amounts of Ca2+ released from ER are nearly the same. In the presence of 2 mmol/L extracellular Ca2+, the TG-evoked Ca2+ influx was dramatically enhanced in OVA-sensitized RPMC by 1.5 fold, while PD treatment reduced the Ca2+ entry to normal level. The results indicate that PD attenuated OVA-induced Ca2+ influx elevation through SOCs.
The effect of resveratrol, a structural and functional analog of PD, on the regulation of intracellular Ca2+ signaling has been reported by several groups, although the results vary in different cell types[29,30]. Furthermore, a previous study in our lab identified PD as a novel mast cell stabilizer in passive cutaneous anaphylaxis mice[15]. There are two major findings in the present study. Firstly, using an in vivo food allergic model, we demonstrated that PD has therapeutic effects against food allergy by decreasing antigen-stimulated mast cell degranulation. Secondly, it was showed that PD suppressed Ca2+ mobilization by inhibiting Ca2+ entry through SOCs, which were the major contributors to PD-induced mast cell stabilization.
Food allergy is an immunological adverse reaction caused by food, which encompasses a range of disorders including IgE-mediated anaphylaxis, food protein-induced enterocolitis syndrome, and food-induced eosinophilic gastrointestinal disorders. Allergens from eggs seem to be one of the most frequent causes of food allergic reaction reported[31]. Thus, in this study, we used OVA to sensitize rats and establish a food allergic model. The allergic animal exhibited abnormal intestinal morphology and increased smooth muscle contractility, enhanced Th2 cytokine levels (IL-4), and OVA-specific IgE concentration. Our results are in line with other published data, as IL-4 has been reported to be the hallmark Th2-type cytokine with multiple immunological functions, including directing Th2 cell differentiation, triggering Ig class switching to IgE in B cells, driving mast cell expansion in intestines[32], and inducing an exaggerated contractile response in intestinal smooth muscle[33]. Furthermore, mast cell activation and degranulation, which was due to Ca2+ mobilization via SOCs, was also demonstrated.
Mast cells are the main effector cells in the pathogenesis of multiple allergic diseases, including asthma, allergic rhinitis, gastrointestinal allergy, and cutaneous anaphylaxis. The majority of mast cell studies have addressed their predominant role in acute allergic reactions (immediate hypersensitivity) and more recently, their roles in late-phase allergic reactions[34,35]. Therefore, developing new drugs capable of stabilizing mast cells would be valuable for treating diseases attributable to type I hypersensitivity reactions. Using the RBL-2H3 mast cell line, intensive research have been focused on looking for promising drugs to inhibit mast cell activation and degranulation, among which PD showed some latent effects[15]. In the present study, following administration with PD, the mast cell-dependent food allergic rats did not have apparent anaphylactic symptoms (data not shown) and had a marked decrease in Th2 responsiveness after oral challenge with OVA. We have found that PD significantly reduced IgE production by inhibiting Th2 cytokines release. On the other hand, PD showed the potential to block mast cell degranulation by decreasing Ca2+ influx via SOCs.
The importance of calcium influx in mast cell activation and degranulation has been well recognized[36]. The degranulation of mast cells is Ca2+ dependent, and an increase in intracellular Ca2+ characterized by Ca2+ entry through SOCs is essential for granules release[13,27,37]. In this study, we found that PD treatment significantly attenuated FcεRI-elicited intracellular Ca2+ increase, indicating that PD stabilized mast cells by suppressing Ca2+ mobilization. Furthermore, we found that PD inhibited Ca2+ entry through SOCs. Multiple mechanisms are involved in the regulation of SOC activity. It has recently been discovered that two subunits, STIM1 and Orai1, play a vital role in both the signaling and the permeation mechanisms for Ca2+ influx through SOCs. Overexpression of STIM1 together with Orai1 caused a dramatic increase in store-operated Ca2+ entry in RBL cells[38]. The mechanism underlying PD-mediated inhibition of SOCs activity remains unclear.
In summary, the present study established PD as a novel mast cell stabilizer, with the capacity for preventing pathogenesis of food allergy and perhaps other mast cell-dependent allergic diseases through stabilizing mast cells. The underlying mechanism for PD-induced stabilization of mast cells is related to the inhibition of Ca2+ mobilization upon FcεRI activation.
The prevalence of food allergy has increased dramatically during the last three decades, but currently there is no satisfactory therapy except avoidance of the allergen in the diet. However, the offending foods causing an allergic effect are usually essential nutrients to human health. Therefore, to develop a new drug for food allergies is extremely important. Polydatin is a natural component isolated from Polygonum cuspidatum, and has been demonstrated to be effective in the treatment of allergic diseases.
Polydatin is a sort of natural biological material and has been used as a medicine for many diseases. In the area of treatment of allergic diseases with polydatin, the research hotspot is how this product could stabilize mast cells and reduce allergic reactions in passive cutaneous anaphylaxis animals. The therapeutic effect of polydatin on food allergy has not yet been determined.
In previous studies in other laboratories and author’s group, the extract of polydatin has been identified as a novel mast cell stabilizer in passive cutaneous anaphylaxis mice, in addition to its other new therapeutic effects, such as anticancer activity. Using an ovalbumin-sensitization mouse model, the authors are the first group to demonstrate that polydatin could attenuate food allergy by reducing mast cell degranulation. Furthermore, it was shown that polydatin suppressed Ca2+ mobilization by inhibiting Ca2+ entry through store-operated calcium channels, which were the major contributors to polydatin -induced mast cell stabilization.
The current results suggest that polydatin is a potential therapeutic drug that could be used in food allergy therapy.
Food allergy mediated by immunoglobulin E (IgE) or non-IgE reaction, is an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food. It encompasses a range of disorders including IgE-mediated anaphylaxis, food protein-induced enterocolitis syndrome, and food-induced eosinophilic gastrointestinal disorders; Polydatin, also known as polygoni cuspidate radix, is a natural component isolated from Polygonum cuspidatum. It has been determined as a resveratrol glucoside with a 3,4,5-trihydroxystilben-3-D-mono-D-glucoside molecular structure. It is traditionally used in South Korea, China, and Japan as a folk remedy for menoxenia, skin burns, gallstones, hepatitis, inflammation, and osteomyelitis.
The authors investigated the effect of polydatin, a resveratrol glucoside, on mast cell degranulation and anti-allergic activity. This is a paper with some interesting value.
P- Reviewer Chang C S- Editor Gou SX L- Editor Rutherford A E- Editor Li JY
| 1. | Montalto M, Santoro L, D’Onofrio F, Curigliano V, Gallo A, Visca D, Cammarota G, Gasbarrini A, Gasbarrini G. Adverse reactions to food: allergies and intolerances. Dig Dis. 2008;26:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, Fiocchi A, Chiang W, Beyer K, Wood R. ICON: food allergy. J Allergy Clin Immunol. 2012;129:906-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 3. | Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007;62:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Poulos LM, Waters AM, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005. J Allergy Clin Immunol. 2007;120:878-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | Lin RY, Anderson AS, Shah SN, Nurruzzaman F. Increasing anaphylaxis hospitalizations in the first 2 decades of life: New York State, 1990 -2006. Ann Allergy Asthma Immunol. 2008;101:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1227] [Cited by in RCA: 1057] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 7. | Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, Dean T. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006;117:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Ramesh S. Food allergy overview in children. Clin Rev Allergy Immunol. 2008;34:217-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Lorentz A, Schwengberg S, Sellge G, Manns MP, Bischoff SC. Human intestinal mast cells are capable of producing different cytokine profiles: role of IgE receptor cross-linking and IL-4. J Immunol. 2000;164:43-48. [PubMed] |
| 10. | Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805-819; quiz 820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 823] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 11. | MacGlashan D. IgE receptor and signal transduction in mast cells and basophils. Curr Opin Immunol. 2008;20:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Eiseman E, Bolen JB. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992;355:78-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 401] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, Beaven MA. Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J Immunol. 2008;180:2233-2239. [PubMed] |
| 14. | Lim BO, Lee JH, Ko NY, Mun SH, Kim JW, Kim do K, Kim JD, Kim BK, Kim HS, Her E. Polygoni cuspidati radix inhibits the activation of Syk kinase in mast cells for antiallergic activity. Exp Biol Med (Maywood). 2007;232:1425-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Yuan M, Li J, Lv J, Mo X, Yang C, Chen X, Liu Z, Liu J. Polydatin (PD) inhibits IgE-mediated passive cutaneous anaphylaxis in mice by stabilizing mast cells through modulating Ca2+ mobilization. Toxicol Appl Pharmacol. 2012;264:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Knippels LM, Penninks AH, Spanhaak S, Houben GF. Oral sensitization to food proteins: a Brown Norway rat model. Clin Exp Allergy. 1998;28:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Chen XW, Lau KW, Yang F, Sun SS, Fung MC. An adjuvant free mouse model of oral allergenic sensitization to rice seeds protein. BMC Gastroenterol. 2011;11:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Kadowaki H, Yamamoto T, Kageyama-Yahara N, Kurokawa N, Kadowaki M. The pathophysiological roles of COX-1 and COX-2 in the intestinal smooth muscle contractility under the anaphylactic condition. Biomed Res. 2008;29:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Ghannadan M, Baghestanian M, Wimazal F, Eisenmenger M, Latal D, Kargül G, Walchshofer S, Sillaber C, Lechner K, Valent P. Phenotypic characterization of human skin mast cells by combined staining with toluidine blue and CD antibodies. J Invest Dermatol. 1998;111:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Swindle EJ, Metcalfe DD, Coleman JW. Rodent and human mast cells produce functionally significant intracellular reactive oxygen species but not nitric oxide. J Biol Chem. 2004;279:48751-48759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Yang C, Mo X, Lv J, Liu X, Yuan M, Dong M, Li L, Luo X, Fan X, Jin Z. Lipopolysaccharide enhances FcepsilonRI-mediated mast cell degranulation by increasing Ca2+ entry through store-operated Ca2+ channels: implications for lipopolysaccharide exacerbating allergic asthma. Exp Physiol. 2012;97:1315-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | De Jonge F, De Laet A, Van Nassauw L, Brown JK, Miller HR, van Bogaert PP, Timmermans JP, Kroese AB. In vitro activation of murine DRG neurons by CGRP-mediated mucosal mast cell degranulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G178-G191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Jia XD, Li N, Wu YN, Yang XG. Studies on BN rats model to determine the potential allergenicity of proteins from genetically modified foods. World J Gastroenterol. 2005;11:5381-5384. [PubMed] |
| 24. | Untersmayr E, Bakos N, Schöll I, Kundi M, Roth-Walter F, Szalai K, Riemer AB, Ankersmit HJ, Scheiner O, Boltz-Nitulescu G. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J. 2005;19:656-658. [PubMed] |
| 25. | Liu HY, Whitehouse WM, Giday Z. Proximal small bowel transit pattern in patients with malabsorption induced by bovine milk protein ingestion. Radiology. 1975;115:415-420. [PubMed] |
| 26. | Liu HY, Giday Z, Moore BF. Possible pathogenetic mechanisms producing bovine milk protein inducible malabsorption: a hypothesis. Ann Allergy. 1977;39:1-7. [PubMed] |
| 27. | Fewtrell C, Sherman E. IgE receptor-activated calcium permeability pathway in rat basophilic leukemia cells: measurement of the unidirectional influx of calcium using quin2-buffered cells. Biochemistry. 1987;26:6995-7003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Alvarez DF, King JA, Townsley MI. Resistance to store depletion-induced endothelial injury in rat lung after chronic heart failure. Am J Respir Crit Care Med. 2005;172:1153-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Zhao KS, Jin C, Huang X, Liu J, Yan WS, Huang Q, Kan W. The mechanism of Polydatin in shock treatment. Clin Hemorheol Microcirc. 2003;29:211-217. [PubMed] |
| 30. | Campos-Toimil M, Elíes J, Orallo F. Trans- and cis-resveratrol increase cytoplasmic calcium levels in A7r5 vascular smooth muscle cells. Mol Nutr Food Res. 2005;49:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Poulsen LK, Hansen TK, Nørgaard A, Vestergaard H, Stahl Skov P, Bindslev-Jensen C. Allergens from fish and egg. Allergy. 2001;56 Suppl 67:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, Chatila TA, Oettgen HC. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol. 2013;6:740-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 33. | Akiho H, Deng Y, Blennerhassett P, Kanbayashi H, Collins SM. Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology. 2005;129:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Swedin L, Ellis R, Neimert-Andersson T, Ryrfeldt A, Nilsson G, Inman M, Dahlén SE, Adner M. Prostaglandin modulation of airway inflammation and hyperresponsiveness in mice sensitized without adjuvant. Prostaglandins Other Lipid Mediat. 2010;92:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Fish SC, Donaldson DD, Goldman SJ, Williams CM, Kasaian MT. IgE generation and mast cell effector function in mice deficient in IL-4 and IL-13. J Immunol. 2005;174:7716-7724. [PubMed] |
| 36. | Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | Sanchez-Miranda E, Ibarra-Sanchez A, Gonzalez-Espinosa C. Fyn kinase controls FcepsilonRI receptor-operated calcium entry necessary for full degranulation in mast cells. Biochem Biophys Res Commun. 2010;391:1714-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661-20665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 449] [Article Influence: 23.6] [Reference Citation Analysis (0)] |