Published online Jun 28, 2013. doi: 10.3748/wjg.v19.i24.3831
Revised: February 10, 2013
Accepted: April 28, 2013
Published online: June 28, 2013
Processing time: 175 Days and 4.9 Hours
AIM: To determine whether an active intervention is beneficial for the survival of elderly patients with hepatocellular carcinoma (HCC).
METHODS: The survival of 740 patients who received various treatments for HCC between 1983 and 2011 was compared among different age groups using Cox regression analysis. Therapeutic options were principally selected according to the clinical practice guidelines for HCC from the Japanese Society of Hepatology. The treatment most likely to achieve regional control capability was chosen, as far as possible, in the following order: resection, radiofrequency ablation, percutaneous ethanol injection, transcatheter arterial chemoembolization, transarterial oily chemoembolization, hepatic arterial infusion chemotherapy, systemic chemotherapy including molecular targeting, or best supportive care. Each treatment was used alone, or in combination, with a clinical goal of striking the best balance between functional hepatic reserve and the volume of the targeted area, irrespective of their age. The percent survival to life expectancy was calculated based on a Japanese national population survey.
RESULTS: The median ages of the subjects during each 5-year period from 1986 were 61, 64, 67, 68 and 71 years and increased significantly with time (P < 0.0001). The Child-Pugh score was comparable among younger (59 years of age or younger), middle-aged (60-79 years of age), and older (80 years of age or older) groups (P = 0.34), whereas the tumor-node-metastasis stage tended to be more advanced in the younger group (P = 0.060). Advanced disease was significantly more frequent in the younger group compared with the middle-aged group (P = 0.010), whereas there was no difference between the middle-aged and elderly groups (P = 0.75). The median survival times were 2593, 2011, 1643, 1278 and 1195 d for 49 years of age or younger, 50-59 years of age, 60-69 years of age, 70-79 years of age, or 80 years of age or older age groups, respectively, whereas the median percent survival to life expectancy were 13.9%, 21.9%, 24.7%, 25.7% and 37.6% for each group, respectively. The impact of age on actual survival time was significant (P = 0.020) with a hazard ratio of 1.021, suggesting that a 10-year-older patient has a 1.23-fold higher risk for death, and the overall survival was the worst in the oldest group. On the other hand, when the survival benefit was evaluated on the basis of percent survival to life expectancy, age was again found to be a significant explanatory factor (P = 0.022); however, the oldest group showed the best survival among the five different age groups. The youngest group revealed the worst outcomes in this analysis, and the hazard ratio of the oldest against the youngest was 0.35 for death. The survival trends did not differ substantially between the survival time and percent survival to life expectancy, when survival was compared overall or among various therapeutic interventions.
CONCLUSION: These results suggest that a therapeutic approach for HCC should not be restricted due to patient age.
Core tip: Progressive population aging worldwide demands consensus development for decision making to treat elderly patients. A simple comparison of survival days is confounded by aging; therefore, age compensation is mandatory to evaluate survival benefits among different age groups. In this study, age difference was compensated by life expectancy in a hepatocellular carcinoma cohort. The authors suggested that age itself might not be a critical determinant for the selection of a therapeutic option. This study emphasizes the importance of clarifying risk determinants specific for elderly patients with respect to individual aspects and medical economy.
- Citation: Suda T, Nagashima A, Takahashi S, Kanefuji T, Kamimura K, Tamura Y, Takamura M, Igarashi M, Kawai H, Yamagiwa S, Nomoto M, Aoyagi Y. Active treatments are a rational approach for hepatocellular carcinoma in elderly patients. World J Gastroenterol 2013; 19(24): 3831-3840
- URL: https://www.wjgnet.com/1007-9327/full/v19/i24/3831.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i24.3831
The Japanese population is aging more rapidly than that of any other nation in the world. In 1990, approximately one in eight people in Japan were aged 65 years or older[1]. This level was already the highest in Asia, although somewhat lower than in most developed European countries. Since then, the population of Japan has aged because of an increasing life expectancy and a falling birth rate[2]. As a result, as many as one in four of the Japanese population will be at least 65 years old by the year 2025[3], which is a much higher ratio than that predicted for any other country.
There is much controversy concerning medical interventions for elderly patients. Zhang et al[4] reported that an interventional scheme should not be changed on the basis of the age of patients facing the treatment of myocardial infarction, whereas Teo et al[5] reported that the addition of percutaneous coronary intervention to optimal medical therapy did not improve the clinical outcomes in patients 65 years of age or older. An active treatment is recommended for elderly patients suffering from subarachnoid hemorrhage[6,7]. However, a conservative treatment was reported to be superior for elderly patients in the management of traumatic dental axis fracture[8]. Although some benefit of active treatment for hepatocellular carcinoma (HCC) in elderly patients has been suggested, the debate still continues as to how fast patients suffering from HCC are actually getting older and whether the survival benefit offered by active interventions is comparable between the young and the elderly.
In this report, aging trends among patients actively treated for HCC were evaluated over 25 years from 1986 to 2011 at a single institution in Japan. To compare the survival benefits between the relatively young and the elderly, survival was compared after adjusting the absolute survival time or life expectancy. Finally, a case presentation of an elderly patient who was successfully managed is used to illustrate the risks and benefits of active interventions for HCC in elderly patients.
Clinicopathological data were retrospectively analyzed for 918 patients who were admitted between 1983 and 2011 for the first time for management of HCC in our hospital. The subjects’ basic characteristics are shown in Table 1. To compare the ages of patients during each 5-year interval of the overall study period, 840 patients were selected who were admitted between 1986 and 2010. Only 740 patients who had already died or who had been followed for longer than a year in our hospital were included in survival analyses using actual survival time. Among these 740 cases, 504 could be allocated a life expectancy because these data are available for each age and gender since 1996 in Japan. To compare survival among different age groups, patients were classified into five groups according to their ages; 49 years of age or younger (-49), 50-59 years of age (50s), 60-69 years of age (60s), 70-79 years of age (70 s) and 80 years of age or older (80+).
Hepatic nodules were radiographically diagnosed as HCC when they fulfilled at least one of the following criteria based on dynamic computed tomography (CT)/magnetic resonance imaging and/or CT during hepatic arteriography/CT during arterial portography: (1) the typical hemodynamics of classical HCC, with a substantial arterial phase enhancement followed by a washout with a corona-like peripheral enhancement in an equilibrium phase; or (2) with similar characteristics as coexisting nodules that had already been diagnosed as HCC.Otherwise, a histological diagnosis was made.
Therapeutic options were principally selected according to the clinical practice guidelines for HCC from the Japanese Society of Hepatology, 2009[9]. The treatment most likely to achieve regional control capability was chosen, as far as possible, in the following order: resection, radiofrequency ablation (RFA), microwave coagulation (MWC), percutaneous ethanol injection (PEI), transcatheter arterial chemoembolization (TACE), transarterial oily chemoembolization (TOCE), hepatic arterial infusion chemotherapy (HAIC), systemic chemotherapy including molecular targeting or best supportive care. Each treatment was used alone or in combination, such as RFA after TACE or TACE following HAIC, with a clinical goal of striking the best balance between functional hepatic reserve and the volume of the targeted area. Stereotactic radiotherapy was considered when loco-regional treatments were indicated but not applicable, whereas liver transplantation was selected by an exclusive decision process. Treatments were classified into four groups: (1) loco-regional including resection, RFA, MWC and PEI; (2) interventional radiology (IVR) including TACE and TOCE; (3) chemotherapy (Cx) including HAIC and systemic chemotherapy; and (4) other, including stereotactic radiotherapy, proton beam and liver transplantation, which were applied to only 11 patients in total. If pleural treatments were added as an adjunct, the case was classified into a group according to the applied treatment with the highest regional control capability.
Measuring hepatitis B surface antigen (HBsAg), anti-hepatitis C virus (HCV), anti-mitochondrial, anti-M2 and anti-nuclear antibodies serologically defined background liver diseases. A habitual daily alcohol intake of more than 60 g was considered alcohol abuse. Nonalcoholic steatohepatitis was diagnosed on the basis of histological findings, whereas Budd-Chiari syndrome was diagnosed angiographically. Patients who were negative for all of the above criteria were considered not definitive for a background liver disease. The institutional review board of our institution, which did not require informed consent for a retrospective study using medical records or imaging examinations, approved the present study, which conformed to the ethical guidelines of the 2008 Declaration of Helsinki.
HBsAg and anti-HCV antibodies were detected by a chemiluminescence immunoassay using the ARCHITECT HBsAg QT and ARCHITECT HCV kits (Abbott Japan Co. Ltd., Chiba, Japan), respectively. Serum anti-mitochondrial and anti-M2 antibodies were quantified using the commercial kits AMA FluoroAID-1 and Mesacup mitochondria M2 (MBL Co. Ltd., Nagoya, Japan), respectively. Total and Lens culinaris agglutinin A-reactive α-fetoprotein (AFP) serum concentrations were quantified with a liquid-phase binding assay system (LiBASys; Wako Pure Chemical Indus-tries Ltd., Osaka, Japan). L3 was calculated as a percentage of Lens culinaris agglutinin A-reactive species against total AFP. Serum des-γ-carboxy prothrombin (DCP) was measured using an electro-chemiluminescence immunoassay (Wako Pure Chemical Industries Ltd, Osaka, Japan). Other blood biochemistries were routinely measured in the clinical laboratories of our hospital.
Two expert histologists independently rendered histological diagnoses based on microscopic observations of tissues stained with hematoxylin and eosin, silver, iron, periodic acid-Schiff, periodic acid-Schiff with diastase digestion, and azan. When there was any discordance between the two histologists, the specimen was reviewed to reach a consensus diagnosis.
The Japanese life expectancy per year for each gender at a specific age is available for 1996 onwards and was downloaded from the Ministry of Health, Labour and Welfare[1]. The life expectancy for our cohort was plotted in three dimensions using O-Chart Standard software (ONO SOKKI Co., Ltd., Yokohama, Japan). The survival timefor each case was divided by the life expectancy to obtain the percent life expectancy (%LE).
Patient ages were compared using the Kruskal-Wallis test, and Dunn’s multiple comparison tests were used to compare the different periods in 5-year intervals. The influence of multiple factors on survival was evaluated using Cox regression analysis. The comparisons of categorical data were performed with the Fisher’s exact test or the χ2 test among three or two different age groups, respectively. Overall survival was demonstrated by calculating Kaplan-Meier survival fractions. All analyses were performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, United States), except for the multivariate analysis, which was performed using PASW statics 17.0 (SPSS Inc., Chicago, United States). A two-tailed P value less than 0.05 was considered statistically significant after Bonferroni correction.
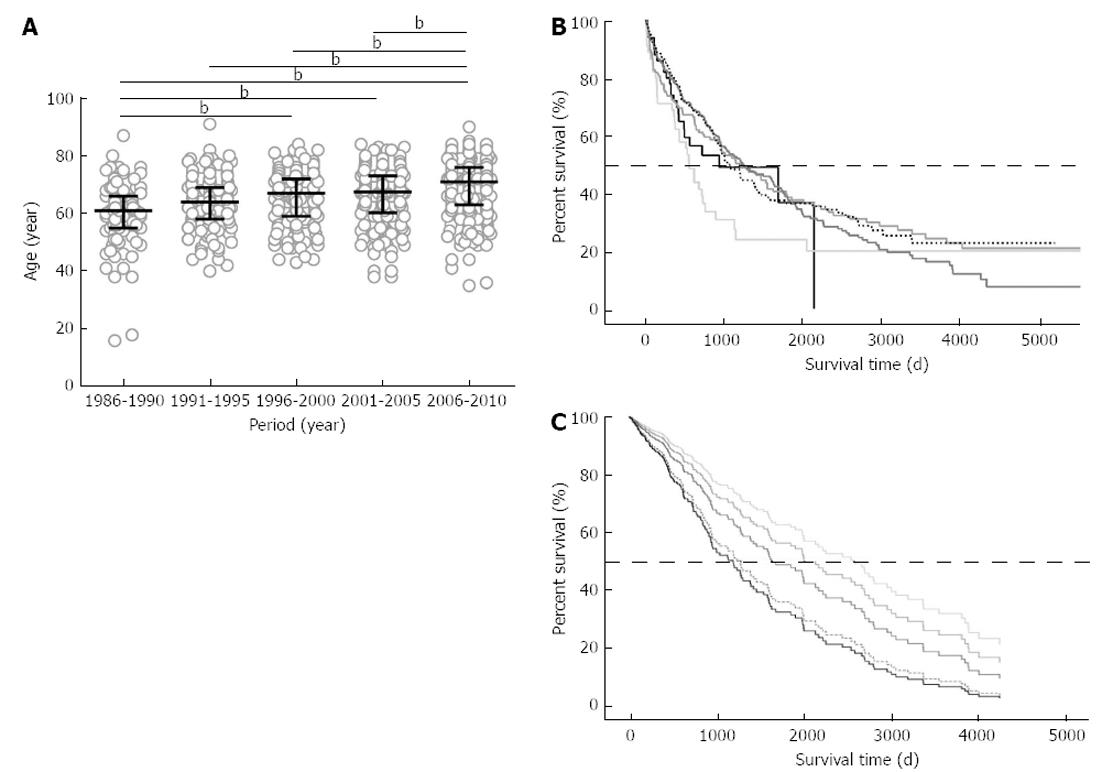
The median ages for each 5-year interval steadily increased from 61 (interquartile range: 55-66) years of age (from 1986 to 1990) to 71 (63-76) years of age (from 2006 to 2010), as shown in Figure 1A. The median age was significantly different among the periods (P < 0.0001), and the median age of these patients increased by 10 years in the last 20 years. The patients admitted from 2006 to 2010 were significantly older than the patients who were hospitalized during any other periods (P < 0.001 vs 1986-1990, 1991-1995, 1996-2000; and P < 0.01 vs 2001-2005).
The median survival time for 740 patients who were deceased or were followed longer than 1 year was calculated from the Kaplan-Meier survival fractions as 1094 d. When the patients’ ages were categorized into the five groups to test the dependence of survival on age, there was no significant survival difference among the groups (P = 0.41), and the least median survival time was 553 d in the -49 group, as shown in Figure 1B. For the Cox regression analysis, 379 cases were included because they were not missing values for the 10 explanatory variables: (1) age (years); (2) gender (male/female): (3) HBsAg (-/+); (4) anti-HCV (-/+);(5) AFP (log10); (6) L3 (%); (7) DCP (log10); (8) Child-Pugh class (A/B/C); (9) HCC stage (I/II/III/IV); and (10) therapy (loco-regional/IVR/Cx/other). Among these 10 variables, age, AFP, DCP, HBsAg, anti-HCV, Child-Pugh class, HCC stage and therapy were determined to be significant factors that influenced survival time (Table 2). The impact of age on survival time was found to be significant (P = 0.020), with a hazard ratio of 1.021, suggesting that a 10-year-older patient has a 1.23-fold higher risk of death. When the survival differences among the five age groups were estimated with the Cox proportional hazards model on the basis of the above 10 explanatory factors, overall survival was poorer with age and was the worst in the 80+ group, as shown in Figure 1C. The risk of death in the 80+ group was 2.41-times higher when compared with that of the -49 group.
| Variable | Significance | HR | 95%CI for HR | |
| Lower | Upper | |||
| Age | 0.020 | 1.021 | 1.003 | 1.040 |
| Gender | 0.652 | 1.079 | 0.775 | 1.503 |
| HBsAg | 0.029 | 1.598 | 1.051 | 2.432 |
| anti-HCV | 0.036 | 1.503 | 1.027 | 2.198 |
| AFP | 0.000 | 1.314 | 1.135 | 1.521 |
| L3 | 0.288 | 1.004 | 0.997 | 1.012 |
| DCP | 0.005 | 1.216 | 1.061 | 1.395 |
| Child-Pugh class | ||||
| A | 0.000 | |||
| B | 0.000 | 2.307 | 1.562 | 3.408 |
| C | 0.001 | 3.373 | 1.617 | 7.035 |
| Tumor stage1 | ||||
| I | 0.000 | |||
| II | 0.148 | 1.504 | 0.865 | 2.616 |
| III | 0.051 | 1.751 | 0.997 | 3.074 |
| IV | 0.000 | 5.715 | 2.985 | 10.939 |
| Therapy category | ||||
| Loco-regional | 0.000 | |||
| IVR | 0.000 | 2.567 | 1.800 | 3.661 |
| Chemotherapy | 0.000 | 2.861 | 1.675 | 4.889 |
| Others | 0.000 | 6.151 | 2.505 | 15.107 |
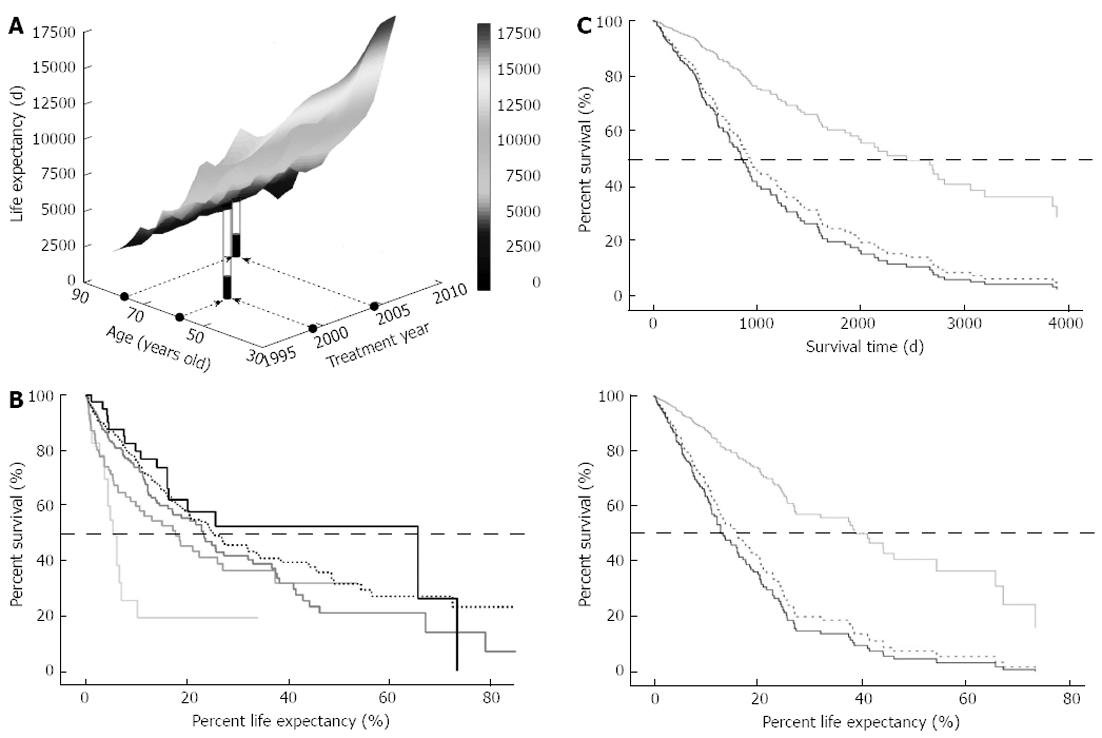
It may be reasonable to assume that older patients will have shorter survival times irrespective of effective treatments, preserved functional hepatic reserve, or other factors, simply because of their shorter residual length of life. To compare the survival from the point of aging, survival was normalized by life expectancy. A ratio of survival days to the expected residual life length is defined as the %LE. Life expectancy data for each age and gender are available from 1996 onward in Japan; therefore, life expectancy was plotted for the 504 cases in our cohort (Figure 2A). Overall survival based on %LE revealed a median survival percentage of 22.9%. When the survival based on %LE was compared among the five different age groups, the median survival was significantly different among the groups (Figure 2B, P < 0.0001), ranging from 5.4% in the -49 group to the best rate of 65.7% in the 80+ group.
Among 504 patients, 174 cases were excluded from further analyses because a therapeutic intervention was never performed or because one or more of the 10 explanatory candidate factors was not measured. Among the remaining 330 cases, only two patients received therapies that were categorized in “other”. Finally, 328 cases were subjected to Cox regression analysis to investigate the survival differences associated with different therapeutic modalities. As shown in Figure 2C, the survival curves were very similar to the evaluations based on survival time (upper) and %LE (lower). Both analyses showed that loco-regional therapies far surpassed IVR and Cx in terms of survival benefit.
When the relationship between survival time and %LE was evaluated in each case, however, it became clear that the two survival indicators were not consistent. For example, the same survivals of 1779 d for males at 59 and 77 years of age in 1999 and 2004 gave rise to very different %LE values of 22.4% and 48.6%, respectively, as indicated in Figure 2A. In another example, a 69-year-old female in 2002 and a male of the same age in 1999 survived 2693 and 1980 d, respectively. Because their life expectancies were 7125 and 5168 d, respectively, the shorter absolute survival value for the male surpassed the female’s longer survival in terms of %LE at 37.8% and 38.3%, respectively. Taken together, %LE is a potential alternative for evaluating survival benefit in HCC patients among different age groups.
Although the overall survival trends and survival benefits of different therapies were consistent between the analyses using dependent variables of survival time and %LE, the two analyses indicated substantially different survival benefits when the survival was compared among the different age groups. A comparison based on survival time in 330 cases revealed that the 80+ group had the worst survival (Figure 3A upper panel), consistent with a similar finding in the initial cohort of 760 patients (Figure 1C). However, when the survival benefit was evaluated on the basis of %LE, age was again found to be a significant explanatory factor (P = 0.022), but the 80+ group showed the best survival among the five different age groups, as shown in the lower panel of Figure 3A. Intriguingly, the -49 group revealed the worst outcomes in this analysis. The hazard ratio of the 80+ group against the -49 group was 0.35 for death (Table 3). The other significant explanatory factors for %LE were HBsAg and anti-HCV, AFP, DCP, Child-Pugh score, tumor-node-metastasis (TNM) stage, and therapeutic options, and the maximal hazard ratios for each variable were 1.71, 1.60, 1.35, 1.24, 2.14, 3.88 and 3.37, respectively, suggesting that age is one of the most powerful determinants of %LE.
| Variable | Significance | HR | 95%CI for HR | |
| Lower | Upper | |||
| Gender | 0.072 | 0.717 | 0.499 | 1.030 |
| HBsAg | 0.043 | 1.709 | 1.018 | 2.869 |
| anti-HCV | 0.043 | 1.597 | 1.015 | 2.511 |
| AFP | 0.000 | 1.348 | 1.145 | 1.587 |
| L3 | 0.421 | 1.004 | 0.995 | 1.012 |
| DCP | 0.015 | 1.243 | 1.043 | 1.482 |
| Child-Pugh class | ||||
| A | 0.001 | |||
| B | 0.001 | 2.144 | 1.393 | 3.300 |
| C | 0.084 | 2.375 | 0.891 | 6.332 |
| Tumor stage1 | ||||
| I | 0.000 | |||
| II | 0.554 | 1.216 | 0.637 | 2.320 |
| III | 0.180 | 1.554 | 0.816 | 2.960 |
| IV | 0.000 | 3.879 | 1.871 | 8.045 |
| Therapy category | ||||
| Loco-regional | 0.000 | |||
| IVR | 0.000 | 2.755 | 1.816 | 4.179 |
| Chemotherapy | 0.000 | 3.365 | 1.884 | 6.010 |
| Others | 0.246 | 3.359 | 0.435 | 25.971 |
| Age group | ||||
| 49 years/younger | 0.240 | |||
| 50s | 0.242 | 0.598 | 0.253 | 1.415 |
| 60s | 0.107 | 0.496 | 0.211 | 1.164 |
| 70s | 0.047 | 0.436 | 0.192 | 0.990 |
| 80 years/older | 0.041 | 0.348 | 0.126 | 0.958 |
Functional hepatic reserve and anatomical tumor extent were compared among three groups: a younger group of -49 and 50s, a middle-aged group consisting of 60s and 70s, and an elderly group of 80+. As shown in the upper panel of Figure 3B, the functional hepatic reserve, as assessed by the Child-Pugh class, did not differ among the three groups (P = 0.34), while the TNM stage tended to be more advanced in the younger group (Figure 3B middle panel, P = 0.060). Advanced disease was significantly more frequent in the younger group as compared to the middle-aged group (P = 0.010), whereas there was no difference between the middle-aged and elderly groups (P = 0.75). A similar trend was observed for HBsAg positivity (P < 0.0001). In the younger group, HBsAg was positive in 45.7% of patients, while it was positive in only 14.8% and 6.7% of patients in the middle-aged and elderly groups, respectively, which led to a significant difference between the younger and middle-aged groups (P < 0.0001), but no significant difference between the middle-aged and elderly groups (P = 0.23).
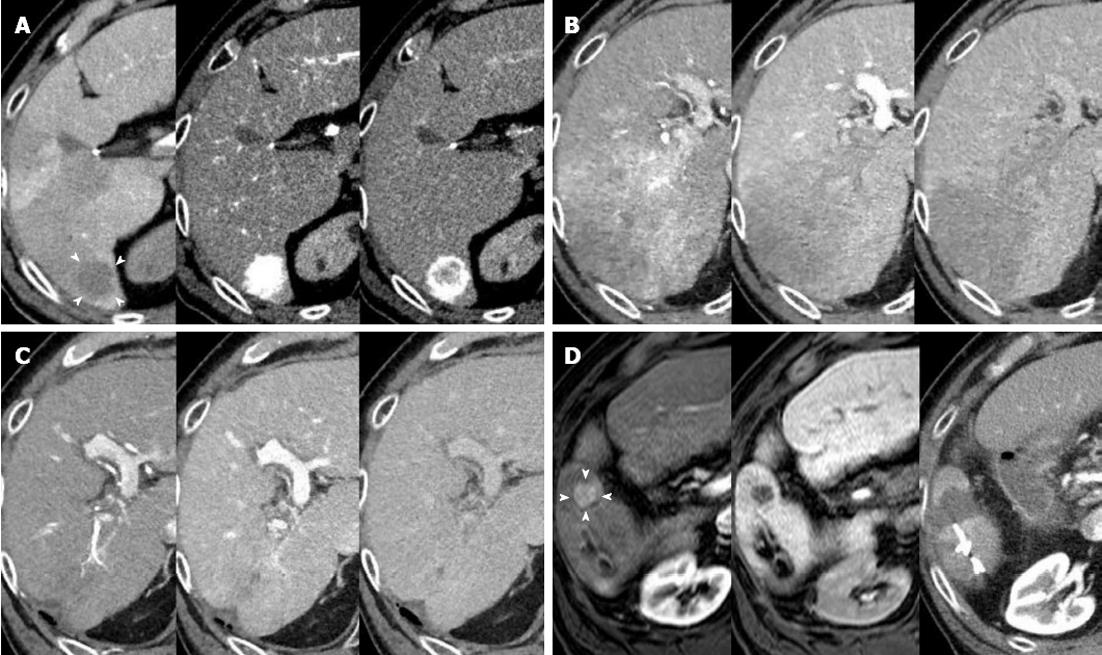
This case is an example of an 85-year-old Japanese male who was admitted to our hospital on April 2009 for the treatment of an HCC that was approximately 20 mm in diameter and located in segment 6 (Figure 4A). He suffered from HCV infection and diabetes mellitus, for which he had used insulin by injection for more than a decade, and he had one prior hepatic resection for HCC. His life expectancy upon admission was 6.27 years. RFA was completed after TACE through the right intercostal space under ultrasound guidance. Twenty-five months after the ablation, however, a follow-up CT revealed that the HCC had spread to wide areas of segments 6 and 7 with portal vein tumor thrombus that extended up to the right main trunk (Figure 4B). Relying on the Child A-class preserved hepatic functional reserve, HAIC was our recommendation at this stage, delivered through a catheter that was implemented and connected to a port under the skin. After receiving written informed consent from the patient, 125 mg of 5-fluorouracil and 5 mg of cis-diamminedichloroplatinum were infused over 23 h and 60 min, respectively, through the common hepatic artery, and repeated for 5 consecutive days. After 2 d of no drug administration, the same schedule was performed for the following 2 wk. The 5-d HAIC was then repeated every 2-3 mo. The tumor and portal vein tumor thrombi gradually disappeared to reveal an enormous tumor reduction by July 2012 (Figure 4C). A new lesion appeared in segment 5 and gradually enlarged to 15 mm in diameter; therefore, RFA was performed again on August 2012 (Figure 4D). In September 2012, the patient turned 89 years old and has survived for 42 mo (55.8% of LE) since the initial RFA. He is in good shape with a PS of 0 and without severe complaints.
In this study, we introduced a new indicator, the %LE, to evaluate whether active treatments for HCC are beneficial for patients over 80 years of age. Considering that morbidity, mortality and other health-related outcomes are generally compared in populations after adjusting for age structures, the age should also be normalized when survival is compared among different age groups. Based on the assumption of a community downscaling to an individual, the life expectancy adjustment among individuals should correspond to age adjustment among communities. As shown in Figure 1B and C, there is a large difference in survival curves between the Kaplan-Meier fractions and the Cox hazards after compensation using 10 explanatory factors. The worst survival of the -49 group in the Kaplan-Meier analysis was the best survival in the Cox regression. In contrast, when %LE was used, the order of survival was consistent between the Kaplan-Meier and Cox regression analyses (Figures 2B and 3A lower panel). The explanatory factors for survival time and %LE in the Cox regression analyses were consistent, and the analyses using survival time or %LE revealed similar survival curves among the different therapeutic approaches (Figure 2C); therefore, it is suggested that the factor that explains the large difference between the Kaplan-Meier and Cox regression analyses using survival time is age. Therefore, it is assumed that the impact of age on survival irrespective of liver pathophysiology can be normalized using %LE instead of survival time.The worst survival of the -49 group in the %LE analysis concurs with the common clinical experience in Japan of higher HBsAg positivity in the younger generation of HCC, leading to the onset of HCC at advanced stages[10]. Taken together, these data suggest that the %LE can be a useful factor to compare survival benefit, especially between cohorts of patients with large differences in age.
It is controversial whether active intervention is beneficial to elderly patients, and inconsistent recommendations have been reported for various diseases, including HCC. Studies that demonstrate an adverse influence of aging on survival in HCC were all reported more than 20 years ago[11-14], whereas recent reports emphasized the benefit of active treatments in the elderly[15-18], suggesting improvements of the medical and social environments over the past decades. Unfortunately, however, all recent reports discussed survival benefits in the elderly principally based on Kaplan-Meier survival fractions. As shown in Figure 1B, a simple comparison of survival time does not reveal survival differences among various age groups. However, one should not conclude that age is not a significant factor for survival on the basis of Kaplan-Meier analysis, because survival varies widely among the different age groups in the Cox regression analysis (Figure 1C). Although our conclusion is consistent with previous reports that a therapeutic scheme should not be changed because of a patient’s age, this study explains the rationale of active intervention for HCC in the elderly more theoretically. However, our conclusions are based on the study from a single institution and a limited number of patients. To establish the best approach for the elderly patients, a multicenter study should be conducted using a larger cohort.
Generally, our institution applies the same process to decide a treatment strategy irrespective of the patient’s age, and this approach led to the best survival in the elderly in terms of %LE, as shown in this study. We also presented the case of a patient over 80 years of age, for whom active interventions such as RFA, TACE or HAIC were safely applied and provided survival benefit. Although it is important to thoroughly assess the risks and to obtain written informed consent, because aging is associated with a progressive deterioration of organ function that reduces the functional reserve to recover from stress/complications[19], our data suggested that age itself should not be a reason to change a treatment strategy. The number of elderly patients in one institution is generally limited, as is the case in this study; therefore, it is difficult to clarify risk determinants that are specific for elderly patients. Future trials should include efforts to enroll the elderly and to clarify factors specific for them that determine outcomes, not only for physical function, but also for quality of life and independence. Facing the world’s highest proportion of elderly people, Japanese society should play a primary role in the application of guidelines for the elderly subset by achieving maximal benefits while minimizing risks.
Increasing life expectancy and a falling birth rate have lead to population aging worldwide, especially in developed countries. As a result, the proportion of elderly patients is steadily rising in many diseases, including hepatocellular carcinoma (HCC). There is much controversy concerning medical interventions for elderly patients; therefore, it is important to clarify a treatment strategy specific for the elderly in terms of both survival benefit and medical resources.
Aging itself has a significant impact on survival days; therefore, a simple comparison of survival days among different age groups may not be suitable for evaluating survival benefit with regard to aging. Another approach is required to compensate for the effect of aging on survival.
To avoid the confounding effect between survival days and aging, the research team led by Takeshi Suda from Division of Gastroenterology and Hepatology, Niigata University introduced percent life expectancy instead of survival days as a novel indicator for the evaluation of survival benefit. Using percent life expectancy, the authors finally concluded that a therapeutic approach for HCC should not be restricted because of patient age.
Facing progressive population aging, medical society should play a primary role in the application of guidelines for the elderly subset by achieving maximal benefits while minimizing risks. In future trials with larger cohorts, percent life expectancy should play an important role in evaluating survival benefits associated with aging.
Computed tomography (CT) during hepatic arteriography and CT during arterial portography are one of the best ways to evaluate perfusion characteristics of a nodular lesion in the liver, and provide the diagnostic rationale for HCC. Radiofrequency ablation therapy and percutaneous ethanol injection are puncture-based locoregional approaches for HCC. Both are usually applied percutaneously under ultrasound guidance, and utilize radiofrequency waves and anhydrous ethanol, respectively, to degenerate cancer cells. Transcatheter arterial chemoembolization and transarterial oily chemoembolization are interventional radiology including the embolization of hepatic arteries feeding HCC. These techniques employ gelatin and oil for embolization, respectively, and gelatin achieves a prolonged obstruction. The tumor markers of α-fetoprotein, L3, and des-γ-carboxy prothrombin are commonly measured as useful independent indicators for the biological malignant potential of HCC.
Facing the world highest elderly ratio in Japan, it is necessary to clarify whether a medical decision process that is applied for the general population provides similar benefits for elderly patients in terms of survival. For this purpose, the authors introduced a new indicator, percent life expectancy, to normalize aging effects, and evaluated the survival benefit among different age groups. Many developed countries, such as United States and China, are also facing population aging; therefore, it is important to clarify risk determinants specific for the elderly in terms of individual aspects and medical economy. This manuscript will have a significant impact and the hepatology community will recognize the importance of the gerontological aspect.
P- Reviewer Hwang SG S- Editor Wen LL L- Editor Stewart GJ E- Editor Li JY
| 1. | Available from: http: //www.stat.go.jp/english/index.htm. |
| 2. | Available from: http: //www.mhlw.go.jp/english/database/db-hw/index.html. |
| 3. | Available from: http: //www.stat.go.jp/data/sekai/02.htm. |
| 4. | Zhang Q, Zhang RY, Zhang JS, Hu J, Yang ZK, Zheng AF, Zhang X, Shen WF. Outcomes of primary percutaneous coronary intervention for acute ST-elevation myocardial infarction in patients aged over 75 years. Zhonghua Yixve Zazhi. 2006;119:1151-1156. [PubMed] |
| 5. | Teo KK, Sedlis SP, Boden WE, O’Rourke RA, Maron DJ, Hartigan PM, Dada M, Gupta V, Spertus JA, Kostuk WJ. Optimal medical therapy with or without percutaneous coronary intervention in older patients with stable coronary disease: a pre-specified subset analysis of the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive druG Evaluation) trial. J Am Coll Cardiol. 2009;54:1303-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Asano S, Hara T, Haisa T, Okamoto K, Kato T, Ohno H, Hasuo K, Kondo T. Outcomes of 24 patients with subarachnoid hemorrhage aged 80 years or older in a single center. Clin Neurol Neurosurg. 2007;109:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Johansson M, Cesarini KG, Contant CF, Persson L, Enblad P. Changes in intervention and outcome in elderly patients with subarachnoid hemorrhage. Stroke. 2001;32:2845-2949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Fagin AM, Cipolle MD, Barraco RD, Eid S, Reed JF, Li PM, Pasquale MD. Odontoid fractures in the elderly: should we operate? J Trauma. 2010;68:583-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Clinical Practice Guidelines for Hepatocellular Carcinoma - The Japan Society of Hepatology 2009 update. Hepatol Res. 2010;40 Suppl 1:2-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Dohmen K, Shigematsu H, Irie K, Ishibashi H. Comparison of the clinical characteristics among hepatocellular carcinoma of hepatitis B, hepatitis C and non-B non-C patients. Hepatogastroenterology. 2003;50:2022-2027. [PubMed] |
| 11. | Chlebowski RT, Tong M, Weissman J, Block JB, Ramming KP, Weiner JM, Bateman JR, Chlebowski JS. Hepatocellular carcinoma. Diagnostic and prognostic features in North American patients. Cancer. 1984;53:2701-2706. [PubMed] |
| 12. | Falkson G, Cnaan A, Schutt AJ, Ryan LM, Falkson HC. Prognostic factors for survival in hepatocellular carcinoma. Cancer Res. 1988;48:7314-7318. [PubMed] |
| 13. | Calvet X, Bruix J, Ginés P, Bru C, Solé M, Vilana R, Rodés J. Prognostic factors of hepatocellular carcinoma in the west: a multivariate analysis in 206 patients. Hepatology. 1990;12:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 169] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Okada S, Okazaki N, Nose H, Yoshimori M, Aoki K. Prognostic factors in patients with hepatocellular carcinoma receiving systemic chemotherapy. Hepatology. 1992;16:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Hazama H, Omagari K, Matsuo I, Masuda J, Ohba K, Sakimura K, Kinoshita H, Isomoto H, Murase K, Kohno S. Clinical features and treatment of hepatocellular carcinoma in eight patients older than eighty years of age. Hepatogastroenterology. 2001;48:1692-1696. [PubMed] |
| 16. | Thornton RH, Covey A, Petre EN, Riedel ER, Maluccio MA, Sofocleous CT, Brody LA, Getrajdman GI, D’Angelica M, Fong Y. A comparison of outcomes from treating hepatocellular carcinoma by hepatic artery embolization in patients younger or older than 70 years. Cancer. 2009;115:5000-5006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Dohmen K, Shirahama M, Shigematsu H, Irie K, Ishibashi H. Optimal treatment strategy for elderly patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Huang J, Li BK, Chen GH, Li JQ, Zhang YQ, Li GH, Yuan YF. Long-term outcomes and prognostic factors of elderly patients with hepatocellular carcinoma undergoing hepatectomy. J Gastrointest Surg. 2009;13:1627-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Colloca G, Santoro M, Gambassi G. Age-related physiologic changes and perioperative management of elderly patients. Surg Oncol. 2010;19:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |