Published online Jun 21, 2013. doi: 10.3748/wjg.v19.i23.3642
Revised: March 20, 2013
Accepted: April 27, 2013
Published online: June 21, 2013
Processing time: 176 Days and 18.5 Hours
AIM: To assess the impact of fast-track surgery (FTS) on hospital stay, cost of hospitalization and complications after radical total gastrectomy.
METHODS: A randomized, controlled clinical trial was conducted from November 2011 to August 2012 in the Department of Digestive Surgery, Xijing Hospital of Digestive Diseases, the Fourth Military Medical University. A total of 122 gastric cancer patients who met the selection criteria were randomized into FTS and conventional care groups on the first day of hospitalization. All patients received elective standard D2 total gastrectomy. Clinical outcomes, including duration of flatus and defecation, white blood cell count, postoperative pain, duration of postoperative stay, cost of hospitalization and complications were recorded and evaluated. Two specially trained doctors who were blinded to the treatment were in charge of evaluating postoperative outcomes, discharge and follow-up.
RESULTS: A total of 119 patients finished the study, including 60 patients in the conventional care group and 59 patients in the FTS group. Two patients were excluded from the FTS group due to withdrawal of consent. One patient was excluded from the conventional care group because of a non-resectable tumor. Compared with the conventional group, FTS shortened the duration of flatus (79.03 ± 20.26 h vs 60.97 ± 24.40 h, P = 0.000) and duration of defecation (93.03 ± 27.95 h vs 68.00 ± 25.42 h, P = 0.000), accelerated the decrease in white blood cell count [P < 0.05 on postoperative day (POD) 3 and 4], alleviated pain in patients after surgery (P < 0.05 on POD 1, 2 and 3), reduced complications (P < 0.05), shortened the duration of postoperative stay (7.10 ± 2.13 d vs 5.68 ± 1.22 d, P = 0.000), reduced the cost of hospitalization (43783.25 ± 8102.36 RMB vs 39597.62 ± 7529.98 RMB, P = 0.005), and promoted recovery of patients.
CONCLUSION: FTS could be safely applied in radical total gastrectomy to accelerate clinical recovery of gastric cancer patients.
Core tip: Fast-track surgery (FTS) is a promising program for surgical patients, and has been applied in several surgical diseases. The value of FTS in radical distal gastrectomy has been demonstrated recently, but the safety and efficacy of FTS for radical total gastrectomy requires further evaluation. The present study showed that FTS was feasible for perioperative care in radical total gastrectomy. Compared with conventional care, FTS could shorten the duration of flatus and defecation, accelerate the decrease in white blood cell count, decrease postoperative complications, shorten the postoperative stay, reduce the cost of hospitalization, and promote postoperative recovery of patients.
- Citation: Feng F, Ji G, Li JP, Li XH, Shi H, Zhao ZW, Wu GS, Liu XN, Zhao QC. Fast-track surgery could improve postoperative recovery in radical total gastrectomy patients. World J Gastroenterol 2013; 19(23): 3642-3648
- URL: https://www.wjgnet.com/1007-9327/full/v19/i23/3642.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i23.3642
Fast-track surgery (FTS) was initiated by the Danish surgeon H Kehlet in the field of elective colorectal surgery in the 1990s[1,2], and has rapidly gained popularity around the world because of its significant benefits and safety[3]. The core elements of FTS include: epidural or regional anesthesia, perioperative fluid management, minimally invasive techniques, optimal pain control, early initiation of oral feeding and early mobilization[4]. The combination of these approaches has led to a significant reduction in complication rates, morbidity and mortality rates, duration of hospital stay and costs of hospitalization, and finally, greatly improved postoperative recovery[5-7]. In recent years, FTS has been applied in several surgical diseases, include radical prostatectomy[8], cardiac surgery[9], total knee replacement[10], cesarean section[11], coronary artery bypass grafting[12], it has also been used for specific procedures in children[13] and the elderly[14].
Gastric cancer is the fourth most common cancer worldwide but the second leading cause of cancer mortality[15], and it is more common in men and in developing countries. Up to now, surgery has been the most common treatment. For radical gastrectomy, conventional elective gastric resection and perioperative care are associated with a morbidity of 20%-46%, a mortality of 0.8%-10%[16] and a postoperative hospital stay of 8-13 d[17]. The high rate of complications leads to prolonged duration of hospital stay and increased costs of hospitalization.
The value of FTS in radical distal gastrectomy has been demonstrated recently[18,19], but the safety and efficacy of FTS in radical total gastrectomy still requires further evaluation. Therefore, we performed a slightly modified fast-track protocol in gastric cancer patients in our department. We evaluated the feasibility and safety of FTS in gastric cancer patients through a prospective, randomized comparative study.
This study was performed in Xijing Hospital of Digestive Diseases affiliated to the Fourth Military Medical University from November 2011 to August 2012. Selection criteria were: (1) diagnosis of gastric cancer based on clinical symptoms, imaging and pathology; (2) age between 18 and 75 years; (3) no preoperative radiotherapy or chemotherapy; (4) no distant metastasis; (5) no history of primary diabetes mellitus, bowel obstruction, severe cardiopulmonary diseases, and immune related diseases; (6) no pregnancy or breast feeding; (7) an American Society of Anesthesiologists (ASA) score of I or II; (8) undergoing elective standard D2 total gastrectomy; and (9) written informed consent was obtained from the patient and the family. Gastric cancer patients meeting the selection criteria were randomly divided into a FTS group and a conventional care group immediately after admission. The sample size of 122 patients (61 cases in each group) was calculated with an alpha level of 0.05 and 90% power for primary endpoints.
This study was approved by the Ethics Committee of Xijing Hospital. This study was registered under chictr.org, identifier number ChiCTR-TRC-11001440.
All the patients were clearly informed about the aims and details of the present study and signed consent forms. Random numbers were generated by computer. Eligible patients were randomly assigned in a 1:1 ratio. The specially trained investigator prepared allocation envelopes for the doctors of the enrolled patients. The investigator did not contact the patients throughout the clinical trial. The doctors and nurses administering the interventions and collecting the data had no role in the randomization process. Two specially trained doctors who were blinded to the treatment were in charge of evaluating postoperative outcomes, discharge and follow-up.
The patients were admitted to the hospital 1-2 d before surgery. A slightly modified fast-track protocol proposed by Kehlet et al[20] was used in the present FTS group. Patients in the conventional surgery group received conventional perioperative care. Details of the interventions are listed in Table 1. Both groups were protocol-driven, with appropriate protocol details for patients, surgeons and nurses to ensure compliance.
| Perioperative intervention | Conventional | Fast-track surgery |
| Diet before surgery | No intake of food and drink after supper the day before surgery | Intake of 1000 mL 14% carbohydrate drink 12 h before and 350 mL 14% carbohydrate drink 3 h before surgery. |
| Anesthesia | Tracheal intubation and general anesthesia | Tracheal intubation and general anesthesia |
| Thermal insulation during operation | No thermal insulation, room temperature was maintained at 22 °C | Thermal insulation of the body and extremities, body temperature was maintained at 36 °C |
| Operation procedure | Standard laparotomy approach | Standard laparotomy approach |
| Placement of abdominal drainage | Use of abdominal drainage tube | No routine use of abdominal drainage tube |
| Analgesia after operation | Standard use of patient-controlled analgesic pump | Infiltration of surgical wounds with ropivacaine at the end of surgery and 24 h after surgery. Oral intake of 200 mg celecoxib twice daily |
| Mobilization after operation | Mobilize out of bed on patients’ own request | Encourage patients to mobilize out of bed |
| Diet after operation | Oral intake initiated after flatus (following a stepwise plan from water to other liquids to semi-fluids to normal food) | Oral intake of 500-1000 mL glucose saline on the day of surgery. Intake of 2000-3000 mL liquid food containing 1000 kcal to 1200 kcal per day from the 1st day after surgery |
| Intravenous nutrition after operation | Infusion of glucose saline and amino acid injection iv on the day of surgery. Infusion of parenteral nutrition (25 kcal/kg of body weight) iv before oral intake. Appropriate level of iv fluid intake based on the volume of liquid intake and output, and physiological need | Infusion of parenteral nutrition iv if oral intake is not adequate. Appropriate level of iv fluid intake based on the volume of liquid intake and output, and physiological need |
| Removal of nasogastric tube | Removal of nasogastric tube after flatus | Removal of nasogastric tube within 24 h after surgery |
| Removal of urine catheter | Removal of urine catheter on the 3rd or 4th day after surgery | Removal of urine catheter within 24 h after surgery |
| Antibiotics | Standard use of antibiotics for 3 d after surgery | Standard use of antibiotics before and once after surgery |
Patients were considered dischargeable postoperatively if they met the following criteria: normal body temperature, pain controlled with oral analgesics, normal mobilization, no discomfort, normal oral diet, no parenteral nutrition, normal gastrointestinal function (normal flatus and defecation), Karnofsky Performance Status Scale score exceeding 80, and willing to go home.
After discharge, the patients were followed up by our specially trained surgeons through telephone within the first 24 h and once per week for 4 wk, and the patients could also contact us if they had any discomfort. The patients were readmitted if any of the following occurred: hyperpyrexia, abdominal pain, bowel obstruction, gastrointestinal hemorrhage, malnutrition, infection and poor healing of the wound.
The primary clinical endpoints were the duration of hospital stay and the cost of hospitalization. The second clinical endpoints were incidence of complications such as pneumonia, surgical site infection, abdominal infection, anastomotic leak, and bowel obstruction. We recorded preoperative data on age, sex, body mass index (BMI), nutritional risk screening (NRS) 2002 score, ASA score, differentiation status, TNM classification, white blood cell (WBC) count, hemoglobin, albumin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Surgical-related data such as operation time and blood loss were also recorded. Postoperative data such as timing of first flatus and defecation, duration of hospital stay, the cost of hospitalization and complications were recorded. WBC was measured from postoperative day (POD) 1 to POD 5. Pain intensity was evaluated from POD 1 to POD 5 using a visual analog scale (VAS).
Data were processed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, United States). Numerical variables were expressed as the mean ± SD unless otherwise stated. Differences between the two groups were tested using a two-tailed Student t test. Discrete variables were analyzed using the χ2 test or Fisher’s exact test. A P value < 0.05 was considered statistically significant.
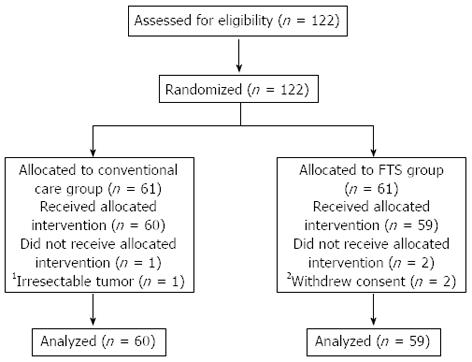
A total of 119 patients finished the study, including 60 patients in the conventional care group and 59 patients in the FTS group. Two patients were excluded from the FTS group after withdrawing consent. One patient was excluded from the conventional care group because of an irresectable tumor (Figure 1). The preoperative baseline characteristics of the two groups are compared in Table 2. There were no significant differences between the two groups in age, sex, BMI, NRS 2002 score, ASA score, differentiation status, TNM classification, WBC count, hemoglobin, albumin, ALT, AST, operation time and blood loss (all P > 0.05).
| Characteristics | Conventional | Fast-track surgery | P value |
| Age, yr | 55.79 ± 10.06 | 54.98 ± 11.35 | 0.682 |
| Sex | 0.689 | ||
| Male/female | 44/16 | 41/18 | |
| BMI | 21.01 ± 1.78 | 22.44 ± 3.51 | 0.061 |
| NRS 2002 score | 0.81 ± 1.10 | 1.08 ± 1.41 | 0.424 |
| ASA score | 0.364 | ||
| I/II | 1/59 | 3/56 | |
| Differentiation status | 0.857 | ||
| Well differentiated | 6 | 4 | |
| Moderately differentiated | 20 | 17 | |
| Poorly differentiated | 34 | 38 | |
| TNM classification | 0.324 | ||
| I/II/III | 8/31/2021 | 14/12/33 | |
| White blood cell | 6.20 ± 1.74 | 6.05 ± 2.08 | 0.671 |
| Hemoglobin, g/L | 133.36 ± 22.03 | 130.65 ± 22.41 | 0.52 |
| Albumin, g/L | 44.42 ± 4.89 | 42.83 ± 4.65 | 0.082 |
| ALT | 17.91 ± 11.35 | 21.29 ± 15.55 | 0.195 |
| AST | 21.84 ± 11.46 | 25.83 ± 17.00 | 0.151 |
| Operation time, min | 242.38 ± 72.89 | 226.11 ± 65.87 | 0.214 |
| Blood loss, mL | 221.17 ± 122.55 | 230.55 ± 171.82 | 0.735 |
Pain intensity was evaluated from POD 1 to POD 5 in the two groups (Table 3). VAS analysis showed that pain intensity of patients in the FTS group was significantly lower than that of patients in the conventional care group on POD 1-3 (P < 0.05).
| Time | Conventional | Fast-track surgery | P value |
| Postoperative pain intensity | |||
| POD 1 | 5.41 ± 1.45 | 4.32 ± 1.65 | 0.000 |
| POD 2 | 4.43 ± 1.54 | 3.39 ± 1.65 | 0.001 |
| POD 3 | 3.63 ± 1.48 | 2.76 ± 1.36 | 0.002 |
| POD 4 | 3.02 ± 1.45 | 2.51 ± 1.87 | 0.119 |
| POD 5 | 2.21 ± 1.39 | 2.30 ± 1.56 | 0.789 |
| White blood cell count | |||
| POD 1 | 14.81 ± 5.34 | 14.55 ± 5.04 | 0.793 |
| POD 2 | 15.36 ± 5.36 | 12.26 ± 4.78 | 0.002 |
| POD 3 | 11.80 ± 4.80 | 9.35 ± 3.83 | 0.005 |
| POD 4 | 8.56 ± 3.70 | 7.52 ± 3.57 | 0.223 |
| POD 5 | 6.37 ± 2.34 | 6.91 ± 3.34 | 0.684 |
The WBC counts of patients in the two groups were measured in the morning of POD 1 to POD 5 (Table 3). The WBC count in the conventional care group and FTS group were both elevated on POD 1. Although the WBC count in the conventional care group continued to rise on POD 2, the WBC count in the FTS group began to drop (P < 0.05). The WBC count in the conventional care group began to drop on POD 3, but was significantly higher than in the FTS group (P < 0.05).
The outcomes were summarized in Table 4. Compared with the conventional care group, the patients in the FTS group showed significantly accelerated recovery of gastrointestinal function in terms of time to first flatus and first defecation (P < 0.05). The duration of postoperative stay of the FTS group was significantly shorter than that of the conventional care group (P < 0.05) and the cost of hospitalization was also significantly lower (P < 0.05).
| Conventional | Fast-track surgery | P value | |
| Clinical outcomes | |||
| First flatus, h | 79.03 ± 20.26 | 60.97 ± 24.40 | 0.000 |
| First defecation, h | 93.03 ± 27.95 | 68.00 ± 25.42 | 0.000 |
| Postoperative stay, d | 7.10 ± 2.13 | 5.68 ± 1.22 | 0.000 |
| Cost of hospitalization, RMB | 43783.25 ± 8102.36 | 39597.62 ± 7529.98 | 0.005 |
| Postoperative complications | |||
| Total cases | 17 | 6 | 0.019 |
| Pneumonia | 10 | 5 | 0.269 |
| Incision infection | 3 | 1 | 0.619 |
| Urinary infection | 1 | 0 | 1.000 |
| Abdominal infection | 1 | 0 | 1.000 |
| Gastric retention | 0 | 0 | |
| Anastomotic leak | 0 | 0 | |
| Deep-vein thrombosis | 0 | 0 | |
| Ileus | 1 | 0 | 1.000 |
| Reoperation | 1 | 0 | 1.000 |
| Readmission | 0 | 0 | |
| Mortality | 0 | 0 | |
Table 4 summarizes the complications and readmissions in each group. The overall complication rate in the FTS group (10.17%) was significantly lower than in the conventional group (28.33%, P = 0.019). In the conventional care group, 10 patients suffered from pneumonia, 3 patients suffered from incision infection, 1 patient experienced urinary infection, 1 patient experienced abdominal infection, and 1 patient underwent reoperation because of ileus. In the FTS group, 5 patients suffered from pneumonia and 1 experienced incision infection. All the patients were cured by surgery or conservative treatment.
The aim of the present study was to evaluate the safety, efficacy and outcome of FTS protocol employed in the perioperative treatment of gastric cancer in comparison with conventional perioperative treatment. The data of the present study showed that the FTS protocol was feasible for perioperative care of gastric cancer patients who underwent radical total gastrectomy. Compared with conventional care, FTS could shorten the duration of flatus and defecation, accelerate the decrease in WBC, decrease postoperative complications, shorten the duration of postoperative stay, reduce the cost of hospitalization, and eventually promote postoperative recovery of the patients.
Optimal pain control is very important. Pain can not only result in stress[21], but also affects the mobilization of patients after surgery. Early mobility or activity is recognized as a critical step in fast-track care. Bed rest not only increases muscle loss and insulin resistance, but also decreases pulmonary function and supply of oxygen to tissues[22]. It has been reported that opioids may result in nausea, vomiting and fatigue that counteract the benefits of FTS[23]. Therefore, routine use of opioids was avoided in the FTS group. In our present study, the infiltration of surgical wounds with ropivacaine and oral intake of celecoxib were applied instead of a patient-controlled analgesia pump. Pain intensity was evaluated from POD 1 to POD 5 after surgery using the VAS. The results showed that VAS in the FTS group was significantly lower than that of conventional care group. This indicated that ropivacaine combined with celecoxib had a better analgesic effect than an analgesic pump, and the better analgesic effect in the FTS group ensured a longer duration of mobilization out of bed.
Conventionally, the duration of antibiotic use is 2-3 d after gastrectomy. In the present study, the antibiotics were only applied before and after surgery in the FTS group (Table 1). We noticed that even with shorter use of antibiotics in the FTS group, the WBC decreased earlier and faster than in the conventional postoperative care group.
Nasogastric tubes have been used traditionally for decompression after gastric surgery and remain a routine part of postoperative care in many centers. Nasogastric tubes are often left for several days until the first flatus after gastric resection. This is based on the rationale that this can prevent aspiration, and reduce the risk of intestinal obstruction and anastomotic leak in clinical practice. Previous studies have shown that the small intestine might return to normal enterocinesia 6 h after abdominal surgery[24]. Recent studies comparing nasogastric decompression vs no decompression demonstrated that a gastric tube may induce pulmonary complications after gastric cancer surgery[25,26] and prolong the time to first flatus with no difference in anastomotic leak rate[27]. Therefore, placement of a nasogastric tube is unnecessary. In our present study, a nasogastric tube was not routinely used in FTS group and was removed within th 24 h after surgery.
Multiple studies have demonstrated that drains are unnecessary after gastrointestinal surgery[28]. The placement of abdominal drainage is prone to increased feelings of pain, intra-abdominal fluid collection, infection, internal organ injuries and risk of fistulas, resulting in delayed recovery[17]. Alvarez Uslar et al[29] reported that operative morbidity and hospital stay were significantly higher in patients who underwent total gastrectomy with abdominal drains than that in patients without drains. However, we refrain from abolishing use of abdominal drains for total gastrectomy in China. Since all the patients received D2 total gastrectomy, the degree of lymph node dissection could lead to a higher risk of chyle leakage. Therefore, the use of drains after total gastrectomy continues to be an issue for debate in the development of FTS.
An early postoperative oral diet can hasten the return of gut function, protect gut mucosal barrier function, and enhance portal circulation[30]. Early enteral nutrition with dietary fiber can alleviate intestinal barrier dysfunction and decrease the incidence of bacterial translocation[31]. Although early enteral nutrition increases the incidence of vomiting and flatulence, a series of reports showed that it can reduce the risk of postoperative complications and mortality[32], facilitate postoperative restoration without increasing the incidence of fistulas[33], and be safety applied in gastrectomy[34]. In the present study, the majority patients in the FTS group well tolerated an early oral diet or enteral nutrition by jejunal feeding tube. We noticed that nausea and vomiting was rare, but abdominal distension did occur in some patients, the symptoms only lasted for a short time based on adequate mobilization out of bed and did not result in severe complications.
It is reported that the postoperative hospital stay of gastric patients could be deceased to 3.8 d in FTS group[35]. In the present study, the mean postoperative stay of patients in FTS group was 5 d, which was longer than that reported in the literature. We found that the traditional Chinese concepts of patients are the main obstacles. They believe that surgery could cause great damage to their bodies, and they could not recover in a short time. Thus, they worried about their safety after discharge from hospital. Therefore, preoperative patient instruction and education is crucial to the outcome of FTS[36]. It will let the patients fully understand the safety, efficacy and benefits of FTS, and guarantee the compliance of patients with medical and FTS protocols.
From the view of the doctors, concern about anastomotic leakage was the main reason which affected early discharge. A series of studies showed that the FTS protocol did not increase the incidence of anastomotic leakage[37], and revealed that education of FTS concepts was also very critical for doctors. Compliance with the FTS protocol is the main factor influencing the outcome of FTS[38]. Thus, we established a study group made up of a researcher, surgeons, anesthesiologists and nurses. We periodically conducted meetings with all staff about the details of FTS, in order to ensure the quality of the study.
The limitation of our present study was the inadequate adherence to the FTS protocol. Epidural analgesia was critical for FTS. Intraoperative application and postoperative use of epidural analgesia could block sympathetic activation to outside stimulation, inhibit hormone secretions of the hypothalamic-pituitary-adrenal axis, and finally attenuate responses to stress[39]. In our present study, tracheal intubation and general anesthesia were applied in both groups, which may partially decrease the efficacy of FTS.
The present study indicates that FTS could promote postoperative recovery, decrease the rate of complications, shorten the duration of hospital stay, and reduce the cost of hospitalization. Our data indicate that FTS is a safe and efficient perioperative management strategy in patients undergoing radical total gastrectomy. Along with the further understanding of stress and development of FTS perioperative care, FTS could probably be safely applied in critically ill patients and emergency surgery, and major operations such as tumor resection may become day procedures in the near future.
Fast-track surgery (FTS) is a promising comprehensive program for surgical patients in elective surgery. In recent years, FTS has been applied in several surgical diseases, include radical prostatectomy, cardiac surgery, total knee replacement, cesarean section, and coronary artery bypass grafting. It has also been used for specific procedures in children and elderly. The value of FTS in radical distal gastrectomy has been demonstrated recently, but the safety and efficacy of FTS in radical total gastrectomy still requires further evaluation.
The value of FTS in radical distal gastrectomy has been demonstrated recently. Chen et al evaluate the safety and effectiveness of fast-track surgery combined with laparoscopy-assisted radical distal gastrectomy for gastric cancer. They found that a combination of FTS and laparoscopy-assisted radical distal gastrectomy in gastric cancer is safe, feasible, and efficient and can improve nutritional status, lessen postoperative stress, and accelerate postoperative rehabilitation.
The present study showed that the FTS protocol was feasible for perioperative care of gastric cancer patients. Compared with conventional care, FTS could shorten the duration of flatus and defecation, accelerate the decrease in white blood cell count, decrease postoperative complications, shorten the duration of postoperative stay, reduce the cost of hospitalization, and eventually promote postoperative recovery of patients.
The data indicate that FTS is a safe and efficient perioperative management strategy in patients undergoing radical total gastrectomy. Along with further understanding of stress, and development of FTS perioperative care, FTS could probably be safely applied in critically ill patients and emergency surgery, and major operations such as tumor resection may become day procedures in the near future.
FTS: Fast-track surgery, initiated by the Danish surgeon H Kehlet in the field of elective colorectal surgery in the 1990s, is a promising comprehensive program for surgical patients in elective surgery; the visual analogue scale is a psychometric response scale which can be used in questionnaires. It is a measurement instrument for subjective characteristics that cannot be directly measured.
This was a good study in which the authors indicates that FTS could promote postoperative recovery, decrease rate of complications, shorten duration of hospital stay, and reduce the cost of hospitalization. However, the author should think about the reason of more pneumonia in conventional care group although it is not significant.
P- Reviewers Gokhan K, Mario K S- Editor Zhai HH L- Editor Cant MR E- Editor Zhang DN
| 1. | Bardram L, Funch-Jensen P, Jensen P, Crawford ME, Kehlet H. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet. 1995;345:763-764. [PubMed] |
| 2. | Kehlet H, Slim K. The future of fast-track surgery. Br J Surg. 2012;99:1025-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Slim K. Fast-track surgery: the next revolution in surgical care following laparoscopy. Colorectal Dis. 2011;13:478-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ. 2001;322:473-476. [PubMed] |
| 5. | Wang G, Jiang Z, Zhao K, Li G, Liu F, Pan H, Li J. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg. 2012;16:1379-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Ionescu D, Iancu C, Ion D, Al-Hajjar N, Margarit S, Mocan L, Mocan T, Deac D, Bodea R, Vasian H. Implementing fast-track protocol for colorectal surgery: a prospective randomized clinical trial. World J Surg. 2009;33:2433-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 825] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 8. | Gralla O, Haas F, Knoll N, Hadzidiakos D, Tullmann M, Romer A, Deger S, Ebeling V, Lein M, Wille A. Fast-track surgery in laparoscopic radical prostatectomy: basic principles. World J Urol. 2007;25:185-191. [PubMed] |
| 9. | Jawahar K, Scarisbrick AA. Parental perceptions in pediatric cardiac fast-track surgery. AORN J. 2009;89:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Husted H, Troelsen A, Otte KS, Kristensen BB, Holm G, Kehlet H. Fast-track surgery for bilateral total knee replacement. J Bone Joint Surg Br. 2011;93:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Antipin EE, Uvarov DN, Svirskiĭ DA, Antipina NP, Nedashkovskiĭ EV, Sovershaeva SL. [Realization of Fast track surgery principles during cesarean section]. Anesteziol Reanimatol. 2011;33-36. [PubMed] |
| 12. | Liang YX, Zhou YB, Shen Y, Gu MN. Whether awake coronary artery bypass grafting is contrary to fast-track surgery? Eur J Cardiothorac Surg. 2012;41:719; author reply 720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Mattioli G, Palomba L, Avanzini S, Rapuzzi G, Guida E, Costanzo S, Rossi V, Basile A, Tamburini S, Callegari M. Fast-track surgery of the colon in children. J Laparoendosc Adv Surg Tech A. 2009;19 Suppl 1:S7-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Day A, Fawcett WJ, Scott MJ, Rockall TA. Fast-track surgery and the elderly. Br J Anaesth. 2012;109:124; author reply 124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Price TJ, Shapiro JD, Segelov E, Karapetis CS, Pavlakis N, Van Cutsem E, Shah MA, Kang YK, Tebbutt NC. Management of advanced gastric cancer. Expert Rev Gastroenterol Hepatol. 2012;6:199-208; quiz 209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 753] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 17. | Wang D, Kong Y, Zhong B, Zhou X, Zhou Y. Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg. 2010;14:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Chen Hu J, Xin Jiang L, Cai L, Tao Zheng H, Yuan Hu S, Bing Chen H, Chang Wu G, Fei Zhang Y, Chuan Lv Z. Preliminary experience of fast-track surgery combined with laparoscopy-assisted radical distal gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1830-1839. [PubMed] |
| 19. | Kim JW, Kim WS, Cheong JH, Hyung WJ, Choi SH, Noh SH. Safety and efficacy of fast-track surgery in laparoscopic distal gastrectomy for gastric cancer: a randomized clinical trial. World J Surg. 2012;36:2879-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641. [PubMed] |
| 21. | Panerai AE. Pain stress and headache. Neurol Sci. 2012;33 Suppl 1:S1-S3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Soop M, Carlson GL, Hopkinson J, Clarke S, Thorell A, Nygren J, Ljungqvist O. Randomized clinical trial of the effects of immediate enteral nutrition on metabolic responses to major colorectal surgery in an enhanced recovery protocol. Br J Surg. 2004;91:1138-1145. [PubMed] |
| 23. | Rugyte D, Edberg KE. [Patient-controlled analgesia in the treatment of postoperative pain in children and adolescents]. Medicina (Kaunas). 2002;38:1078-1082. [PubMed] |
| 24. | Nelson R, Tse B, Edwards S. Systematic review of prophylactic nasogastric decompression after abdominal operations. Br J Surg. 2005;92:673-680. [PubMed] |
| 25. | Carrère N, Seulin P, Julio CH, Bloom E, Gouzi JL, Pradère B. Is nasogastric or nasojejunal decompression necessary after gastrectomy? A prospective randomized trial. World J Surg. 2007;31:122-127. [PubMed] |
| 26. | Yoo CH, Son BH, Han WK, Pae WK. Nasogastric decompression is not necessary in operations for gastric cancer: prospective randomised trial. Eur J Surg. 2002;168:379-383. [PubMed] |
| 27. | Yang Z, Zheng Q, Wang Z. Meta-analysis of the need for nasogastric or nasojejunal decompression after gastrectomy for gastric cancer. Br J Surg. 2008;95:809-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1147] [Cited by in RCA: 1191] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 29. | Alvarez Uslar R, Molina H, Torres O, Cancino A. Total gastrectomy with or without abdominal drains. A prospective randomized trial. Rev Esp Enferm Dig. 2005;97:562-569. [PubMed] |
| 30. | Henriksen MG, Jensen MB, Hansen HV, Jespersen TW, Hessov I. Enforced mobilization, early oral feeding, and balanced analgesia improve convalescence after colorectal surgery. Nutrition. 2002;18:147-152. [PubMed] |
| 31. | Hou H, Ping X, Zhu Y, Zhao Z, Li Y, Li J. Dietary fiber alleviates intestinal barrier dysfunction in post-trauma rats. Clin Invest Med. 2010;33:E117. [PubMed] |
| 32. | Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg. 2009;13:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 33. | Berberat PO, Ingold H, Gulbinas A, Kleeff J, Müller MW, Gutt C, Weigand M, Friess H, Büchler MW. Fast track--different implications in pancreatic surgery. J Gastrointest Surg. 2007;11:880-887. [PubMed] |
| 34. | Suehiro T, Matsumata T, Shikada Y, Sugimachi K. Accelerated rehabilitation with early postoperative oral feeding following gastrectomy. Hepatogastroenterology. 2004;51:1852-1855. [PubMed] |
| 35. | Grantcharov TP, Kehlet H. Laparoscopic gastric surgery in an enhanced recovery programme. Br J Surg. 2010;97:1547-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Carter J, Szabo R, Sim WW, Pather S, Philp S, Nattress K, Cotterell S, Patel P, Dalrymple C. Fast track surgery: a clinical audit. Aust N Z J Obstet Gynaecol. 2010;50:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Chopra SS, Schmidt SC, Fotopoulou C, Sehouli J, Schumacher G. Evidence-based perioperative management: strategic shifts in times of fast track surgery. Anticancer Res. 2009;29:2799-2802. [PubMed] |
| 38. | Maessen J, Dejong CH, Hausel J, Nygren J, Lassen K, Andersen J, Kessels AG, Revhaug A, Kehlet H, Ljungqvist O. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg. 2007;94:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 370] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 39. | Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, Sage D, Futter M, Saville G, Clark T. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1551] [Cited by in RCA: 1265] [Article Influence: 50.6] [Reference Citation Analysis (0)] |