Published online Jun 7, 2013. doi: 10.3748/wjg.v19.i21.3332
Revised: February 18, 2013
Accepted: March 6, 2013
Published online: June 7, 2013
Processing time: 172 Days and 15.2 Hours
AIM: To describe protease serine 1 (PRSS1) gene mutations in patients with autoimmune pancreatitis (AIP) and the clinical features of AIP.
METHODS: Fourteen patients with AIP, 56 with other chronic pancreatitis, 254 with pancreatic cancer and 120 normal controls were studied. The mutations and polymorphisms of four genes involved with pancreatitis or pancreatic cancer, PRSS1, SPINK1, CFTR and MEN1, were sequenced. The pathogenic mechanism of AIP was investigated by comparing the wild-type expression system with the p.81Leu→Met mutant expression system.
RESULTS: Two novel mutations (p.81Leu→Met and p.91Ala→Ala) were found in PRSS1 gene from four patients with AIP. PRSS1_p.81Leu→Met mutation led to a trypsin display reduction (76.2%) combined with phenyl agarose (Ca2+ induced failure). Moreover, the ratio of trypsin/amylase in patients with AIP was higher than in the patients with pancreatic cancer and other pancreatitis. A large number of lymphocytes and plasma cells were found in the bile ducts accompanied by hyperplasia of myofibroblasts.
CONCLUSION: Autoimmune pancreatitis may be related to PRSS1 gene mutations.
Core tip: Novel mutations (p.81Leu→Met and p.91Ala→Ala) were found in protease serine 1 gene from the patients with autoimmune pancreatitis. Trypsinogen abnormal activation resulted in multiple organ injuries. And this offers direct evidence in support of the trypsinogen gene mutation and abnormal immune system.
-
Citation: Gao F, Li YM, Hong GL, Xu ZF, Liu QC, He QL, Lin LQ, Weng SH.
PRSS1 _p.Leu81Met mutation results in autoimmune pancreatitis. World J Gastroenterol 2013; 19(21): 3332-3338 - URL: https://www.wjgnet.com/1007-9327/full/v19/i21/3332.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i21.3332
Most of the earlier literature about autoimmune pancreatitis (AIP) came from Japan[1-3]. AIP has been referred to a variety of names including sclerosing pancreatitis, tumefactive pancreatitis, and nonalcoholic destructive pancreatitis, depending in part on the specific pathologic findings and the presence of extrapancreatic manifestations. However, it is generally believed that the pathologic heterogeneity may reflect different stages or manifestations of the same disease. Immunoglobulin G4 (IgG4) positive plasma cells infiltration is considered a marker for the disease and can be detected in the pancreas and a variety of other tissues[3-7]. Unfortunately, serum IgG4 increase was not found in all patients with AIP and more than half of the patients with AIP had normal serum IgG4[8-10]. It is urgent to find some more specific diagnosis technology (including the molecular markers). Genetic mutation is often involved in immune system disorders, and protease serine 1 (PRSS1), cystic fibrosis conductance regulator (CFTR), serine protease inhibitor Kazal type 1 (SPINK1) and multiple endocrine neoplasia 1 (MEN1) mutation is followed by pancreatitis or pancreatic cancer[11-15]. We are keen on identifing these genes targeted by the inflammatory process in AIP. Although typsin was historically believed to be immunologically active, it is now continued to be verified that abnormal activation of typsin can be recognized by the immune system. This study aimed to determine whether PRSS1 gene p.T81M mutation contributes to the functions of calcium-induced trypsinogen activation and to explore its role in autoimmune pancreatitis.
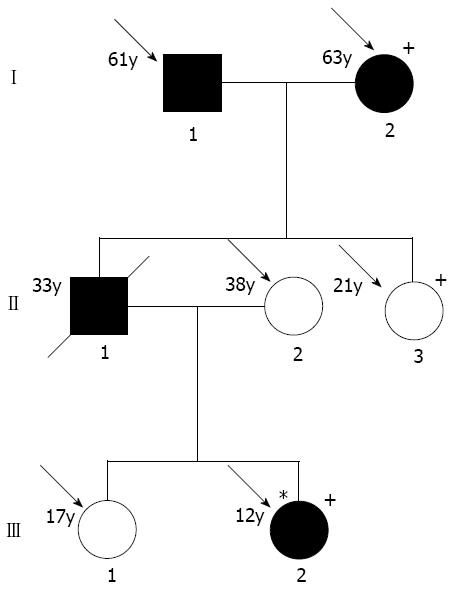
This study was approved by the Fujian Medical University Ethics Committee and all study participants gave informed consent for DNA analyses. Clinical information for the survey was obtained by personal interviews using a structured questionnaire and/or clinical trials. AIP diagnostic criteria were as follows: (I) pancreatic imaging studies show diffuse narrowing of the main pancreatic duct with irregular wall (more than 1/3 of length of the entire pancreas); (II) laboratory data demonstrate abnormally elevated levels of serum gamma globulin and/or IgG, or the presence of autoantibodies; and (III) histopathologic examination of the pancreas shows fibrotic changes with lymphocyte and plasma cell infiltration. For diagnosis, criterion I (pancreatic imaging) must be presented with criterion II (laboratory data) and/or III (histopathologic findings)[16-19]. Total one pedigree (Figure 1) and 12 unrelated patients with AIP, 56 with chronic pancreatitis, 254 with pancreatic cancer and 120 normal controls seen in the past six years were studied.
Genomic DNA was extracted from peripheral blood and other tissue specimens using a QIAamp DNA mini kit (Qiagen, Hilden, Germany). Primer pairs and experimental condition were used to generate specific fragments according to the references[12,15]. The polymerase chain reaction (PCR) products were purified for sequencing after electrophoresis on an agarose gel. For sequencing, a Perkin Elmer Big Dye Sequencing kit (Perkin-Elmer, Shelton, CT, United States) and an ABI PRISM7700 sequencer (Perkin-Elmer ABI, Foster, CA, United States) were used.
Four genes involved in pancreatitis/pancreatic cancer, PRSS1, SPINK1, CFTR and MEN1, were sequenced according to references[12-15]. A 20 μL mixture was prepared for each reaction and contained 1 × HotStarTaq buffer, 2.0 mmol/L Mg2+, 0.2 mmol/L dNTP, 0.2 μmol/L of each primer, 1 U HotStarTaq polymerase (Qiagen Inc., Valencia, CA, United States) and 1 μL template DNA. The cycling program was 95 °C for 15 min; 11 cycles of 94 °C for 15 s, 62 ± 0.5 °C per cycle for 40 s, 72 °C for 1 min; 24 cycles of 94 °C for 15 s, 57 °C for 30 s, 72 °C for 1 min, and 72 °C for 2 min. PCR purification was completed using SAP and Exo I 1 U SAP, and 6 U Exo I was added into 8 μL PCR products. The mixture was incubated at 37 °C for 60 min, followed by incubation at 70 °C for 10 min.
Pancreatic tissues were stained with haematoxylin and eosin, modified gomori trichrome, periodic acid-Schiff stain and IgG4 special staining.
The serum trypsin was tested with ELISA kits (R and D Systems, Minneapolis, MN, United States) and amylase with latex-enhanced nephelometric immunoassay (Dade Behring Marburg GmbH, Germany).
The complete mutated (p.T81M) and wild-type PRSS1 cDNA were introduced into plasmid pMD18-T (TaKaRa, China) and transformed into Escherichia coli DH5 competent cells. Primers were designed for PCR amplification. The forward primer was 5’-TGCAATTGTATGGCACCATTCGACGATGATGACAAGAT-3’ and the reverse primer was 5’-GAGTCGACTCAGCTAATTAAGCTTAGTG-3’. In addition, MunI and SalI digestion sites were designed in the forward and reverse primers, respectively. The expression products underwent isolation, purification and renaturation. Benzoyl L-arginine ethyl ester served as a substrate, and absorbance (A253) was measured at 253 nm within 30 min. The specific enzyme activity was calculated as follows: specific activity = enzyme activity/mg of protein = ΔA253/t × 1000/(ε × t × 0.001), where t refers to time (min) and ε refers to the amount of proteinase (μg) during the detection.
Binding of 45Ca2+ to wild-type recoverin and the L81M mutant was investigated as described previously[20]. In brief, 100 mol/L protein was dissolved in 20 mmol/L HEPES-KOH, pH 7.5, 100 mmol/L NaCl and 1 mmol/L DTT, and then it was transferred to centricon 10 devices. 45Ca2+ was added, the samples were centrifuged for 1 min, and radioactivity of the filtrate was counted. Next, non-radioactive Ca2+ was added and the centrifugation procedure was repeated. Protein-bound Ca2+ versus free Ca2+ was determined from the excess Ca2+ in the protein sample in the ultrafiltration.
Informed consent was obtained before treatment. Glucocorticoids were administered empirically [oral prednisone (40 mg) once daily with a 5-mg taper every 2 wk]. At the same time, oral acid-suppressing agents and calcium were also given.
The auxiliary test results of the patients with AIP (male/female = 7:7) are shown in Table 1. There was significant weight loss (2-9 kg/12 mo) and abnormally increased serum IgG4 level: 5/14 (+).
| Number | 1 (I1) | 2 (II2) | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Type | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 |
| Sex | M | F | M | M | F | F | M | F | M | F | F | F | M | |
| Age/onset | 61/59 | 63/52 | 70/58 | 59/52 | 68/62 | 70/62 | 53/46 | 46/46 | 60/48 | 59/52 | 62/60 | 62/55 | 48/42 | 33/32 |
| Weight loss (kg/12 mo) | 5 | 3 | 3 | 4 | 6 | 2 | 8 | 3 | 5 | 8 | 6 | 3 | 6 | 9 |
| Nausea/vomit | +/- | -/- | +/+ | -/- | +/+ | -/- | +/+ | -/- | +/- | -/- | +/- | -/- | +/- | -/- |
| IgG (0-16) | 12.5 | 6.9 | 11 | 9.6 | 8.5 | 6.3 | 23.5 | 26.9 | 21 | 15.2 | 11.3 | 26.3 | 18.5 | 6.9 |
| IgG4 (0.08-1.4 g/L) | 2.53 | 0.82 | 0.89 | 1.12 | 2.69 | 0.77 | 1.75 | 0.25 | 0.96 | 4.25 | 2.01 | 0.75 | 0.63 | 0.56 |
| Glucose (mmoL/L) | 14.32 | 4.56 | 5.14 | 18.69 | 4.33 | 6.55 | 4.25 | 6.35 | 7.15 | 8.66 | 4.12 | 5.6 | 4.23 | 5.02 |
| Trypsin (ng/mL) | 28.65 | 63.55 | 52.45 | 33.65 | 56.55 | 32.12 | 23.15 | 56.99 | 87.02 | 74.52 | 63.05 | 56.23 | 78.06 | 12.66 |
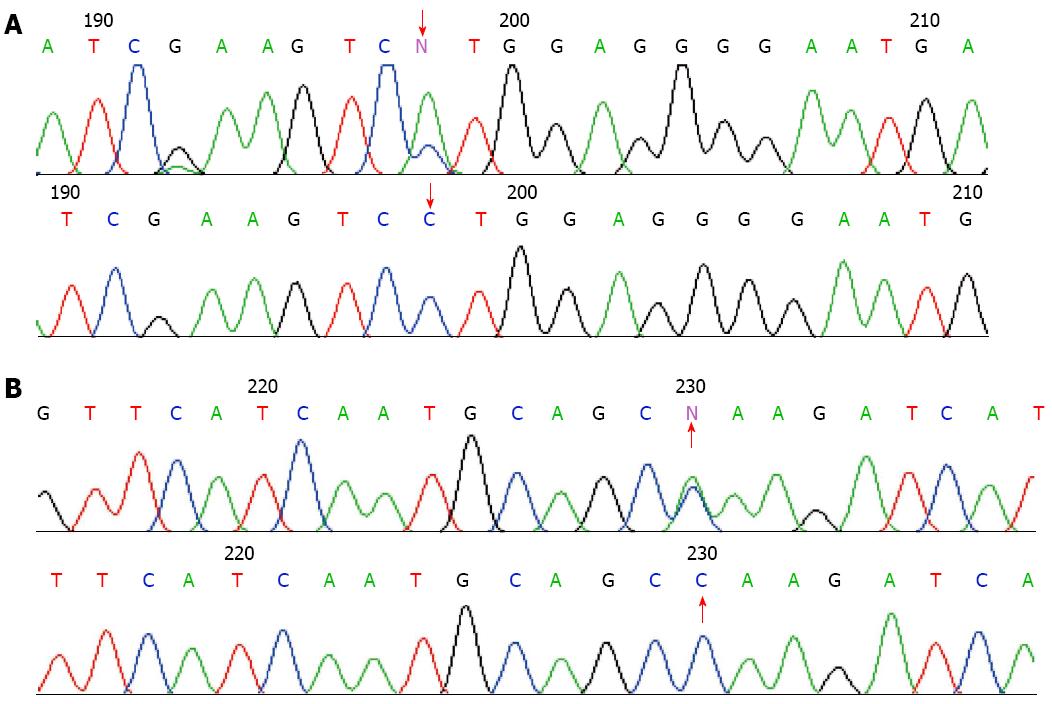
In the affected patients, novel mutations were found in the genes coding for PRSS1 (Figure 2). They were PRSS1_c.247 C > A (p.81Leu→Met) (No. 1, 2, 6 and 7) and PRSS1_c.279 C > A (p.91Ala→Ala) (No. 7). None of these mutations were found in the normal controls and other patients with chronic pancreatitis and pancreatic cancer.
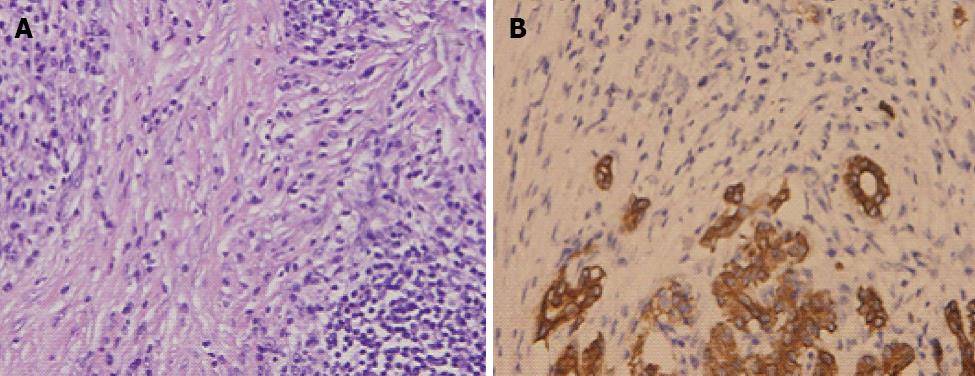
Histopathologic examination of the pancreas revealed a characteristic lymphoplasmacytic infiltrate of lymphocytes and IgG4 positive plasma cells, and interstitial fibrosis and acinar cell atrophy in later stages. However, localization and the degree of duct wall infiltration were variable. It has been proposed that a cytologic smear is rich in inflammatory cells. The sensitivity and the specificity of these criteria for differentiating AIP from neoplasia are unknown. A large number of lymphocytes and plasma cells were found in the bile ducts accompanied by hyperplasia of myofibroblasts (Figure 3A). The number of pancreatic acini was markedly reduced (Figure 3B) (immunohistochemistry by CK, CD3, CD20, CD38, CD68 and vimentin).
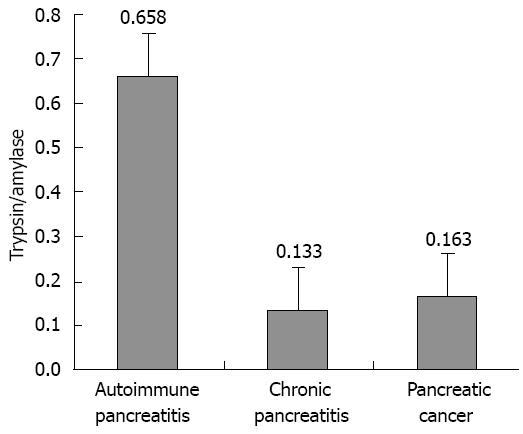
Patients were treated with glucocorticoids for 3-6 mo, and the jaundice improved. Throughout the course of the disease, the trypsin (ng/mL)/amylase (U/L) ratio was higher in patients with AIP (0.658 ± 0.309) than in patients with pancreatic cancer (0.163 ± 0.087) or other types of chronic pancreatitis (0.133 ± 0.095) (Figure 4).
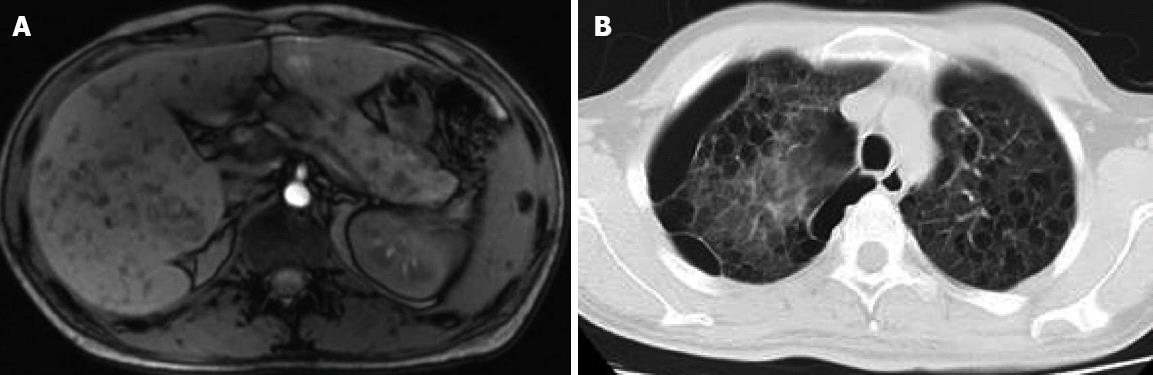
Computed tomography found a diffusely enlarged hypodense pancreas or a focal mass and retroperitoneal lymph node enlargement that may be mistaken for a pancreatic cancer. Magnetic resonance imaging revealed diffusely decreased signal intensity and delayed enhancement on dynamic scanning. The characteristic endoscopic retrograde cholangiopancreatographic finding was segmental or diffuse irregular narrowing of the main pancreatic duct. The characteristic magnetic resonance cholangiopancreatographic finding was partial intrahepatic bile duct dilatation or narrowness and multiple focal high signal intensity in the liver. Multiple cysts occurred in the liver, pancreas, spleen and other organs (Figure 5).
A UV spectrophotometer was used to measure the activity of trypsinogen before and after enterokinase activation at 253 nm (ΔOD253). The activity of products of the mutated gene remained unchanged after enterokinase activation. Using the aforementioned formula, the calculated specific activity of renatured recombinant trypsin was 126-183 BAEE U/mg, and the calculated specific activity of wild-type trypsin was 123-165 BAEE U/mg after enterokinase activation, showing that p.T81M mutation did not affect the activity of trypsin.
According to the literature[20], the binding of recoverin to phenyl-agarose is thought to depend on the Ca2+-induced exposure of hydrophobic residues and not on the presence of the myristoyl group. Could the non-myristoylated L81M variant bind to phenyl-agarose in a fashion similar to the wild-type? In fact, the binding capacity of the mutant protein was lower (76.2% of that of the wild-type protein).
Six patients (No. 2, 3, 5, 6, 7 and 10) were treated with glucocorticoids for 3-6 mo, and the jaundice improved. The serum levels of total and direct bilirubin were reduced significantly. Wheezing was markedly improved and the body weight increased (2-5 kg/mo). The symptoms of the other 8 patients were improved to different degrees.
AIP shares many presenting symptoms with pancreatic carcinoma. Fortunately, AIP can be effectively treated and cured. Serum IgG4 levels are usually abnormally increased[6,9,21-24], but increased serum IgG4 levels are also found in patients with pancreatic cancer and some patients without AIP. Indeed, genetic analyses identified a specific gene, PRSS1, for hereditary pancreatitis and other types of chronic pancreatitis in 1996. Some PRSS1 mutations enhance trypsinogen autoactivation, explaining the young age of patients at onset of AIP. Other mutations may render some patients more susceptible to pancreatitis in the presence of other insults to the pancreas[10-12]. In Japan, a strong association with the HLA-DRB1*0405/DQB1*0401 haplotype has been identified[25]. However, the relationship has not been reported in other ethnic groups , which prompted us to search for AIP-related genes[25-30].
Although p.T81A mutant protein is not associated with functional activity, it binds to the sites that are quite dissimilar from 56Q-57W-58V-59V (i.e., classical Ca2+-binding sites)[20]. On the basis of this observation, a refined model of the role of the myristoyl group as an intrinsic allosteric modulator is proposed. Ca2+ stabilizes the hydrophobic pore structure of the trypsin molecule and increases folding to permit formation of two ionic bonds with β trypsin. Asp102, His57, Ser195 catalytic triad and Asp189, Gly216, Gly226 displayed by the substrate binding pocket of the trypsin are more stable, thereby improving the efficiency of the enzyme catalysis[20,31-33].
There is no controversy that trypsinogen activation plays a very important role in early pancreatitis. However, what activates trypsinogen is not entirely clear. Recent researches have focused on the relationship between the original concentration of intracellular calcium and trypsin activation within acinar cells. Trypsinogen activation starts in the apical part of the acinar cells after supramaximal cholecystokinin stimulation. It is highly dependent on the release of calcium ions within subcellular structures[32-35] and on repetitive calcium transients. Normally, trypsin cannot be activated by calcium. Mutation at the trypsin calcium binding point blocks such a conventional activation pathway[20]. The gene mutation significantly increases the catalytic activity of trypsin but has no effect on the expression level of the mutant. Increased trypsin synthesis and secretion results in ectopic activation, leading to the occurrence of pancreatitis.
The abnormal increase in serum IgG4 is considered to be an indication of AIP. Unfortunately, serum IgG4 level is normal in more than half of the patients with AIP. In this study, most of our patients with AIP had normal serum IgG4 levels. Our findings suggest that patients with AIP can present with a variety of clinical phenotypes, and that genetic heterogeneity and clinical heterogeneity are features of AIP. In addition, studies of mutations in the PRSS1 gene have helped elucidate the molecular mechanism underlying the pathogenesis of AIP. Furthermore, high trypsin/amylase ratio contributes to the diagnosis of AIP.
Autoimmune pancreatitis (AIP) is an autoimmune chronic pancreatitis which is characterized by infiltration of lymphocytes and plasma cells, and pancreatic fibrosis and dysfunction. AIP and pancreatic cancer have similar clinical manifestations and findings on imaging, thus AIP is often misdiagnosed as pancreatic cancer, resulting in unnecessary surgical intervention. AIP not only poses a diagnostic challenge for clinicians, but can lead to severe and irreversible injury to patients.
The etiology of AIP is poorly understood. It has been reported that genetic factors play an important role in the pathogenesis of AIP. Parkdo et al reported that substitution of aspartic acid at the 57th position of haploid DQβ1 of the histocompatibility leukocyte antigen is closely related to the recurrence of AIP. The trypsinogen genes are inserted in the TCR vβ site; thus, the trypsinogen gene and TCR vβ gene share the same loci, and this arrangement is conserved in humans, mice, and chickens.
PRSS1_p.81Leu→Met mutation leads to a trypsin display reduction (76.2%) combined with phenyl agarose (Ca2+ induced failure) which induced premature activation of trypsinogen and caused AIP-related polycystic lesions in the lungs, liver, gallbladder, pancreas, and spleen. Although typsin was historically believed to be immunologically active, it is now continue to be verified that abnormal activation of typsin can be recognized by the immune system.
The authors’ findings suggest that AIP can present with polycystic lesions in multiple organs, which can be found in patients with hereditary AIP, as well as those with sporadic AIP. In addition, functional studies have elucidated the molecular mechanisms underlying the pathogenesis of AIP due to the presence of mutations of the PRSS1 gene. Additional clinical studies are required to investigate the clinical heterogeneity and molecular pathogenesis of AIP.
This paper reports the novel mutations (p.81Leu→Met and p.91Ala→Ala) found in PRSS1 gene from patients with AIP. This finding is intriguing, but some of the cases described in the paper may not be AIP for the reasons written in major comments.
P- Reviewer Goto N S- Editor Zhai HH L- Editor Ma JY E- Editor Ma S
| 1. | Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694-2699. [PubMed] |
| 2. | Brauner E, Lachter J, Ben-Ishay O, Vlodavsky E, Kluger Y. Autoimmune pancreatitis misdiagnosed as a tumor of the head of the pancreas. World J Gastrointest Surg. 2012;4:185-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, Ochi Y, Nakayama K, Tokutake Y, Katsuyama Y, Saito S. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology. 2002;122:1264-1269. [PubMed] |
| 4. | van Heerde MJ, Biermann K, Zondervan PE, Kazemier G, van Eijck CH, Pek C, Kuipers EJ, van Buuren HR. Prevalence of autoimmune pancreatitis and other benign disorders in pancreatoduodenectomy for presumed malignancy of the pancreatic head. Dig Dis Sci. 2012;57:2458-2465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Kim JH, Chang JH, Nam SM, Lee MJ, Maeng IH, Park JY, Im YS, Kim TH, Kim CW, Han SW. Newly developed autoimmune cholangitis without relapse of autoimmune pancreatitis after discontinuing prednisolone. World J Gastroenterol. 2012;18:5990-5993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Hart PA, Moyer AM, Yi ES, Hogan MC, Pearson RK, Chari ST. IgG4-related paratesticular pseudotumor in a patient with autoimmune pancreatitis and retroperitoneal fibrosis: an extrapancreatic manifestation of IgG4-related disease. Hum Pathol. 2012;43:2084-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Shimosegawa T. The amendment of the Clinical Diagnostic Criteria in Japan (JPS2011) in response to the proposal of the International Consensus of Diagnostic Criteria (ICDC) for autoimmune pancreatitis. Pancreas. 2012;41:1341-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Löhr JM, Faissner R, Koczan D, Bewerunge P, Bassi C, Brors B, Eils R, Frulloni L, Funk A, Halangk W. Autoantibodies against the exocrine pancreas in autoimmune pancreatitis: gene and protein expression profiling and immunoassays identify pancreatic enzymes as a major target of the inflammatory process. Am J Gastroenterol. 2010;105:2060-2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Hirano K, Matsubara S, Tada M, Isayama H, Sasahira N, Koike K. Autoimmune pancreatitis with low serum immunoglobulin G4 level associated with annular pancreas. Pancreas. 2012;41:811-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Sugino K, Gocho K, Ishida F, Kikuchi N, Hirota N, Sato K, Sano G, Isobe K, Sakamoto S, Takai Y. Acquired hemophilia A associated with IgG4-related lung disease in a patient with autoimmune pancreatitis. Intern Med. 2012;51:3151-3154. [PubMed] |
| 11. | Patel H, Levine J, Weinstein T. Combination of CFTR gene mutation and autoimmune pancreatitis presenting as necrotizing pancreatitis. Pancreas. 2012;41:970-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Liu Q, Lin X, Liu J, Liu A, Gao F. The -409 C/T genotype of PRSS1 protects against pancreatic cancer in the Han Chinese population. Dig Dis Sci. 2012;57:573-579. [PubMed] |
| 13. | Freitag TL, Cham C, Sung HH, Beilhack GF, Durinovic-Belló I, Patel SD, Bronson RT, Schuppan D, Sønderstrup G. Human risk allele HLA-DRB1*0405 predisposes class II transgenic Ab0 NOD mice to autoimmune pancreatitis. Gastroenterology. 2010;139:281-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Kamisawa T, Tsuruta K, Okamoto A, Horiguchi S, Hayashi Y, Yun X, Yamaguchi T, Sasaki T. Frequent and significant K-ras mutation in the pancreas, the bile duct, and the gallbladder in autoimmune pancreatitis. Pancreas. 2009;38:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Liu QC, Zhuang ZH, Zeng K, Cheng ZJ, Gao F, Wang ZQ. Prevalence of pancreatic diabetes in patients carrying mutations or polymorphisms of the PRSS1 gene in the Han population. Diabetes Technol Ther. 2009;11:799-804. [PubMed] |
| 16. | Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the honolulu consensus document. Pancreatology. 2010;10:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Ebbo M, Grados A, Daniel L, Vély F, Harlé JR, Pavic M, Schleinitz N. IgG4-related systemic disease: emergence of a new systemic disease? Literature review. Rev Med Interne. 2012;33:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Okazaki K, Uchida K, Fukui T. Recent advances in autoimmune pancreatitis: concept, diagnosis, and pathogenesis. J Gastroenterol. 2008;43:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Kamisawa T, Tabata T, Hara S, Kuruma S, Chiba K, Kanno A, Masamune A, Shimosegawa T. Recent advances in autoimmune pancreatitis. Front Physiol. 2012;3:374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Weiergräber OH, Senin II, Philippov PP, Granzin J, Koch KW. Impact of N-terminal myristoylation on the Ca2+-dependent conformational transition in recoverin. J Biol Chem. 2003;278:22972-22979. [PubMed] |
| 21. | Takita M, Itoh T, Matsumoto S, Shimoda M, Chujo D, Iwahashi S, Tamura Y, Onaca N, Naziruddin B, Bartlett BL. Autoimmune chronic pancreatitis with IgG4-related pancreatic pseudocyst in a patient undergoing total pancreatectomy followed by autologous islet transplantation: a case report. Pancreas. 2013;42:175-177. [PubMed] |
| 22. | Kojima M, Sipos B, Klapper W, Frahm O, Knuth HC, Yanagisawa A, Zamboni G, Morohoshi T, Klöppel G. Autoimmune pancreatitis: frequency, IgG4 expression, and clonality of T and B cells. Am J Surg Pathol. 2007;31:521-528. [PubMed] |
| 23. | Algül H, Chari ST. Lymphotoxin in the pathogenesis of autoimmune pancreatitis: a new player in the field. Gastroenterology. 2012;143:1147-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Fusaroli P, Kypraios D, Caletti G, Eloubeidi MA. Pancreatico-biliary endoscopic ultrasound: a systematic review of the levels of evidence, performance and outcomes. World J Gastroenterol. 2012;18:4243-4256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Ota M, Ito T, Umemura T, Katsuyama Y, Yoshizawa K, Hamano H, Kawa S. Polymorphism in the KCNA3 gene is associated with susceptibility to autoimmune pancreatitis in the Japanese population. Dis Markers. 2011;31:223-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 26. | Chang MC, Chang YT, Tien YW, Liang PC, Jan IS, Wei SC, Wong JM. T-cell regulatory gene CTLA-4 polymorphism/haplotype association with autoimmune pancreatitis. Clin Chem. 2007;53:1700-1705. [PubMed] |
| 27. | Ota M, Katsuyama Y, Hamano H, Umemura T, Kimura A, Yoshizawa K, Kiyosawa K, Fukushima H, Bahram S, Inoko H. Two critical genes (HLA-DRB1 and ABCF1)in the HLA region are associated with the susceptibility to autoimmune pancreatitis. Immunogenetics. 2007;59:45-52. [PubMed] |
| 28. | Umemura T, Ota M, Hamano H, Katsuyama Y, Muraki T, Arakura N, Kawa S, Kiyosawa K. Association of autoimmune pancreatitis with cytotoxic T-lymphocyte antigen 4 gene polymorphisms in Japanese patients. Am J Gastroenterol. 2008;103:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Okumura F, Sakuma H, Nakazawa T, Hayashi K, Naitoh I, Miyabe K, Yoshida M, Yamashita H, Ohara H, Inagaki H. Analysis of VH gene rearrangement and somatic hypermutation in type 1 autoimmune pancreatitis. Pathol Int. 2012;62:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Sultan M, Werlin S, Venkatasubramani N. Genetic prevalence and characteristics in children with recurrent pancreatitis. J Pediatr Gastroenterol Nutr. 2012;54:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137:1509-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Guvench O, Price DJ, Brooks CL. Receptor rigidity and ligand mobility in trypsin-ligand complexes. Proteins. 2005;58:407-417. [PubMed] |
| 33. | Buch I, Giorgino T, De Fabritiis G. Complete reconstruction of an enzyme-inhibitor binding process by molecular dynamics simulations. Proc Natl Acad Sci USA. 2011;108:10184-10189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 514] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 34. | Sherwood MW, Prior IA, Voronina SG, Barrow SL, Woodsmith JD, Gerasimenko OV, Petersen OH, Tepikin AV. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci USA. 2007;104:5674-5679. [PubMed] |
| 35. | Krajewski E, Krajewski J, Spodnik JH, Figarski A, Kubasik-Juraniec J. Changes in the morphology of the acinar cells of the rat pancreas in the oedematous and necrotic types of experimental acute pancreatitis. Folia Morphol (Warsz). 2005;64:292-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |