Published online Jun 7, 2013. doi: 10.3748/wjg.v19.i21.3309
Revised: April 16, 2013
Accepted: May 7, 2013
Published online: June 7, 2013
Processing time: 125 Days and 9.4 Hours
AIM: To compare the efficacy of capecitabine and oxaliplatin (XELOX) with 5-fluorouracil, folinic acid and oxaliplatin (FOLFOX6) in gastric cancer patients after D2 dissection.
METHODS: Between May 2004 and June 2010, patients in our gastric cancer database who underwent D2 dissection for gastric cancer at the First Affiliated Hospital of Sun Yat-Sen University were retrospectively analyzed. A total of 896 patients were enrolled into this study according to the established inclusion and exclusion criteria. Of these patients, 214 received the XELOX regimen, 48 received FOLFOX6 therapy and 634 patients underwent surgery only without chemotherapy. Overall survival was compared among the three groups using Cox regression and propensity score matched-pair analyses.
RESULTS: Patients in the XELOX and FOLFOX6 groups were younger at the time of treatment (median age 55.2 years; 51.2 years vs 58.9 years), had more undifferentiated tumors (70.1%; 70.8% vs 61.4%), and more lymph node metastases (80.8%; 83.3% vs 57.7%), respectively. Overall 5-year survival was 57.3% in the XELOX group which was higher than that (47.5%) in the surgery only group (P = 0.062) and that (34.5%) in the FOLFOX6 group (P = 0.022). Multivariate analysis showed that XELOX therapy was an independent prognostic factor (hazard ratio = 0.564, P < 0.001). After propensity score adjustment, XELOX significantly increased overall 5-year survival compared to surgery only (58.2% vs 44.2%, P = 0.025) but not compared to FOLFOX6 therapy (48.5% vs 42.7%, P = 0.685). The incidence of grade 3/4 adverse reactions was similar between the XELOX and FOLFOX6 groups, and more patients suffered from hand-foot syndrome in the XELOX group (P = 0.018).
CONCLUSION: Adjuvant XELOX therapy is associated with better survival in patients after D2 dissection, but does not result in a greater survival benefit compared with FOLFOX6 therapy.
Core tip: This original study retrospectively analyzed the efficacy of adjuvant chemotherapy and compared the effects of adjuvant capecitabine and oxaliplatin (XELOX) therapy with 5-fluorouracil, folinic acid and oxaliplatin (FOLFOX6) therapy in gastric cancer patients undergoing D2 dissection. Propensity score matched-pair analysis was performed to account for biases associated with retrospective data. Adjuvant XELOX was significantly associated with improved survival after D2 dissection compared to surgery alone following multivariate Cox regression and propensity score matched analyses, however, the XELOX regimen did not result in a greater survival benefit compared with the FOLFOX6 regimen. Our findings suggest that adjuvant XELOX therapy should be considered in curable gastric cancer patients.
- Citation: Wu Y, Wei ZW, He YL, Schwarz RE, Smith DD, Xia GK, Zhang CH. Efficacy of adjuvant XELOX and FOLFOX6 chemotherapy after D2 dissection for gastric cancer. World J Gastroenterol 2013; 19(21): 3309-3315
- URL: https://www.wjgnet.com/1007-9327/full/v19/i21/3309.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i21.3309
Gastric cancer (GC) is the fourth prevalent cancer and second most common cause of cancer-related deaths worldwide, with half of all patients in East Asia[1-4]. Radical resection remains the only curative treatment for GC[1,5,6]. Radical gastrectomy with D2 lymphadenectomy is the standard treatment for patients with resectable GC in Eastern countries[1,6,7] and is now recommended in Western countries[3,8-10]. However, even if radical resection is performed, about 50% patients have recurrence within 5 years of surgery[5,11,12] and 50%-90% of patients die due to disease relapse[13]. Therefore, novel approaches such as multimodality therapies are being explored to improve treatment outcome. Adjuvant chemotherapy has been intensively investigated for several decades, but definitive evidence is limited especially in patients after D2 dissection[14-16].
The chemotherapeutic regimen after GC resection is heterogeneous throughout the world and the optimal regimen has not yet been determined[13,17]. S-1 plus cisplatin is considered the standard regimen for advanced GC in Japan[18,19], while its application outside Japan remains uncertain. In the United States, the standard of care is adjuvant bolus 5-fluorouracil (5-FU)-based chemoradiotherapy for resected GC based primarily on the results of the Intergroup 116 trial[20]. In European countries, the epirubicin, cisplatin and 5-FU (ECF) regimen was associated with survival benefits in the MAGIC trial[21], but not in the Intergroup CALGB 80101 trial[22]. Recently, the CLASSIC trial[23] carried out in 37 centers in South Korea, China and Taiwan showed that 6 mo of adjuvant capecitabine and oxaliplatin (XELOX) chemotherapy improved 3-year disease-free survival compared with surgery alone. However, this was a pre-specified interim efficacy analysis and not yet formally validated. Nevertheless, based on published data, adjuvant chemotherapy might improve survival, however, further definite evidence is needed, and a clearly superior strategy has not yet emerged. Adjuvant XELOX and 5-FU, folinic acid and oxaliplatin (FOLFOX6) chemotherapy has been widely used in GC patients. However, few studies have directly compared their efficacy.
In the present study, we used our GC database prospectively established since 1994 and retrospectively analyzed the efficacy of adjuvant XELOX and FOLFOX6 therapy in GC patients undergoing D2 dissection. This study aimed to: (1) examine the benefit of adjuvant chemotherapy; (2) compare the prognosis of patients undergoing D2 dissection plus XELOX or FOLFOX6 with that of patients undergoing surgery only; and (3) account for biases associated with treatment selection of adjuvant chemotherapy in retrospective data through propensity score adjusted analysis.
From May 2004 to June 2010, 1083 patients in our database underwent gastrectomy with D2 nodal dissection for GC at the First Affiliated Hospital of Sun Yat-Sen University. This study was approved by the Ethical Review Committee of the First Affiliated Hospital of Sun Yat-Sen University. All patients signed an informed consent. The stage of gastric carcinoma was determined according to the TNM Classification of Malignant Tumors established by the International Union against Cancer 7th edition[24].
The inclusion criteria for this study were as follows: (1) histologically proven advanced GC, radical gastrectomy with D2 lymph-node dissection and R0 surgery; (2) patients aged between 20 and 75 years; (3) no preoperative chemotherapy, immunotherapy or radiotherapy; (4) patients receiving chemotherapy 4 wk after surgery; and (5) no synchronous or metachronous cancers.
Five exclusion criteria were employed: (1) age > 75 or < 20 years; (2) hepatic, renal, pulmonary or cardiac dysfunction; (3) severe postoperative complications, such as anastomotic fistula and pancreatic fistula; (4) less than 15 lymph nodes retrieved; and (5) loss to follow-up.
According to the inclusion and exclusion criteria, a total of 896 patients were included in the final analysis. Of these patients, 214 (23.9%) received XELOX chemotherapy (the XELOX group), 48 (5.4%) received FOLFOX6 chemotherapy (the FOLFOX6 group), and 634 (70.7%) underwent surgery only without chemotherapy (the surgery only group).
The XELOX regimen consisted of 3-wk cycles of oral capecitabine (1000 mg/m2 twice daily on days 1-14 of each cycle plus intravenous oxaliplatin (130 mg/m2 on day 1 of each cycle). The FOLFOX6 regimen consisted of 2-wk cycles of intravenous oxaliplatin 85-100 mg/m2 and leucovorin 200 mg/m2 over 2 h on day 1 of each cycle plus 5-FU 400 mg/m2 bolus with infusional 5-FU 2400 mg/m2 in 48 h of each cycle. The median chemotherapy duration was 8 cycles in the XELOX group and 7 in the FOLFOX6 group. All patients underwent weekly clinical evaluation and routine blood examinations during chemotherapeutic treatment. Chemotherapeutic adverse reactions were graded on a 0-4 scale for acute and sub-acute toxicity in accordance with the WHO guidelines for anti-cancer drugs.
Follow-up assessments were performed every 3 mo for the first 2 years after surgery and then every 6 mo until the patient’s death. The survival status of patients was ascertained in December 2011. Median follow-up was 39.7 mo (range 6-87.6 mo).
Analyses were performed using the SPSS 18.0 software (SPSS, Inc., Chicago, IL, United States). The overall survival was recorded from the date of surgery to the date of death from any cause or last follow-up. Survival curves and overall 5-year survival rates were established according to the Kaplan-Meier and Log-Rank methods. Hazard ratios for death were calculated by Cox regression analysis with backward model selection. The potential prognostic factors entered into the Cox regression model were as follows: chemotherapy (surgery only vs XELOX vs FOLFOX6); age (continuous variable); gender; tumor location (whole stomach vs upper vs middle vs lower vs remnant stomach); pathological T category (T1 and 2 vs T3 vs T4a vs T4b); pathological N category (N0 vs N1 vs N2 vs N3a vs N3b); macroscopic Borrmann type (I and II vs III vs IV and V); and tumor differentiation (undifferentiated vs differentiated). Two-sided P values were calculated for all tests. P values less than 0.05 were considered statistically significant.
To reduce selection biases associated with retrospective data, propensity score matched-pair analysis was performed with 1 to 1 matching (XELOX and surgery only; XELOX and FOLFOX6). The propensity score method was used to determine the probability of an individual patient having received a certain treatment as a function of several confounding covariates that were collapsed into a single predictor[25-27]. Individuals were matched for age, gender, tumor location, Borrmann type, number of retrieved nodes, tumor differentiation, pathological T category and pathological N category. In total, there were 370 patients in the XELOX vs surgery only analysis (n = 185 for adjuvant XELOX and n = 185 for surgery only) and 74 patients in the XELOX vs FOLFOX6 analysis (n = 37 for XELOX and n = 37 for FOLFOX6). Kaplan-Meier methods and Log-Rank analyses were then performed.
The clinicopathological characteristics of the 896 patients are shown in Table 1. Compared to the surgery only group, patients in the XELOX and FOLFOX6 groups were younger at the time of treatment (median age 55.2 years; 51.2 years vs 58.9 years), had more undifferentiated tumors (70.1%; 70.8% vs 61.4%), and more lymph node metastases (80.8%; 83.3% vs 57.7%), respectively. There were no significant differences in baseline features between the XELOX and FOLFOX6 groups.
| Surgery only | FOLFOX6 | XELOX | P value1 | |||
| (n = 634) | (n = 48) | P value | (n = 214) | P value | ||
| Age (yr) | ||||||
| Mean ± SD | 58.9 ± 12.5 | 51.2 ± 13.2 | < 0.001 | 55.2 ± 11.4 | < 0.001 | 0.051 |
| Median | 60 | 52 | 57 | |||
| Tumor location, whole stomach | 23 (3.6) | 5 (10.4) | 7 (3.3) | 0.124 | ||
| Upper | 192 (30.3) | 11 (22.9) | 55 (25.7) | |||
| Middle | 148 (23.3) | 16 (33.3) | 61 (28.5) | |||
| Lower | 235 (37.1) | 14 (29.2) | 87 (40.7) | |||
| Remnant stomach | 36 (5.7) | 2 (4.2) | 4 (1.9) | |||
| Macroscopic Borrmann type | 0.035 | 0.014 | 0.064 | |||
| I | 39 (6.2) | 0 (0.0) | 13 (6.1) | |||
| II | 171 (27) | 7 (14.6) | 39 (18.2) | |||
| III | 333 (52.5) | 30 (62.5) | 138 (64.5) | |||
| IV and V | 91 (14.4) | 11 (22.9) | 24 (11.2) | |||
| Histological type | 0.219 | 0.013 | 0.919 | |||
| Differentiated | 245 (38.6) | 14 (29.2) | 64 (29.9) | |||
| Undifferentiated | 389 (61.4) | 34 (70.8) | 150 (70.1) | |||
| Pathological T category | < 0.001 | < 0.001 | 0.087 | |||
| T1 and 2 | 117 (18.5) | 0 (0.0) | 9 (4.2) | |||
| T3 | 73 (11.5) | 1 (2.1) | 23 (10.7) | |||
| T4a | 342 (53.9) | 41 (85.4) | 152 (71) | |||
| T4b | 102 (16.1) | 6 (12.5) | 30 (14) | |||
| Retrieved node | ||||||
| Mean ± SD | 26.4 ± 15.9 | 32.1 ± 19.2 | 0.019 | 29.7 ± 14.5 | 0.004 | 0.339 |
| Median (range) | 24 (0-102) | 31 (0-89) | 27 (1-91) | |||
| Pathological N category | 0.005 | < 0.001 | 0.085 | |||
| N0 | 268 (42.3) | 8 (16.7) | 41 (19.2) | |||
| N1 | 91 (14.4) | 7 (14.6) | 43 (20.1) | |||
| N2 | 108 (17) | 12 (25) | 52 (24.3) | |||
| N3a | 89 (14) | 9 (18.8) | 56 (26.2) | |||
| N3b | 78 (12.3) | 12 (25) | 22 (10.3) | |||
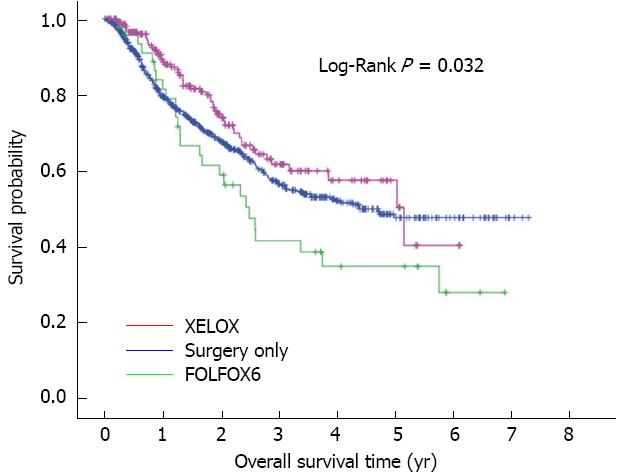
In the 896 patients, the overall mean and median survival were 57.8 and 52.7 mo, respectively. Overall 3-year and 5-year survival rates were 56.4% and 47.5% in the surgery only group, 61.5% and 57.3% in the XELOX group, and 41.3% and 34.5% in the FOLFOX6 group, respectively. Overall survival rate in the XELOX group was higher than that in the surgery only group (P = 0.069) and the FOLFOX6 group (P = 0.022) (Figure 1). Following multivariate analysis, adjuvant chemotherapy was found to have a prognostic influence on the hazard ratio (HR) for death (P = 0.001), and the HR for death was 0.564 (95%CI: 0.416-0.765; P < 0.001) for XELOX therapy (Table 2). In addition, pathological T category, pathological N category, tumor location, and macroscopic Borrmann type were also prognostic factors for overall survival. Recurrence rate was 24.3% (52/214) in the XELOX group, 31.3% (15/48) in the FOLFOX6 group and 32.8% (208/634) in the surgery only group. Recurrence rate in the XELOX group was lower than that in the surgery only (P = 0.021) and the FOLFOX6 (P = 0.36) groups.
| HR and 95%CI | Overall | ||||
| HR | Lower | Upper | P value | P value | |
| Adjuvant chemotherapy | 0.001 | ||||
| Surgery only | Reference | ||||
| FOLFOX6 | 0.762 | 0.503 | 1.156 | 0.201 | |
| XELOX | 0.564 | 0.416 | 0.765 | < 0.001 | |
| Pathological N category | < 0.001 | ||||
| N0 | Reference | ||||
| N1 | 1.613 | 1.049 | 2.483 | 0.030 | |
| N2 | 2.952 | 2.011 | 4.334 | < 0.001 | |
| N3a | 3.938 | 2.702 | 5.739 | < 0.001 | |
| N3b | 5.025 | 3.382 | 7.465 | < 0.001 | |
| Tumor location | 0.003 | ||||
| Whole stomach | Reference | ||||
| Upper | 0.671 | 0.421 | 1.071 | 0.094 | |
| Middle | 0.663 | 0.414 | 1.064 | 0.088 | |
| Lower | 0.681 | 0.422 | 1.099 | 0.115 | |
| Remnant stomach | 1.454 | 0.791 | 2.675 | 0.228 | |
| Macroscopic Borrmann type | < 0.001 | ||||
| I and II | Reference | ||||
| III | 1.199 | 0.875 | 1.642 | 0.258 | |
| IV and V | 2.142 | 1.469 | 3.124 | < 0.001 | |
| Pathological T category | < 0.001 | ||||
| T1 and 2 | |||||
| T3 | 1.112 | 0.477 | 2.590 | 0.806 | |
| T4a | 3.010 | 1.574 | 5.757 | 0.001 | |
| T4b | 4.944 | 2.477 | 9.870 | < 0.001 | |
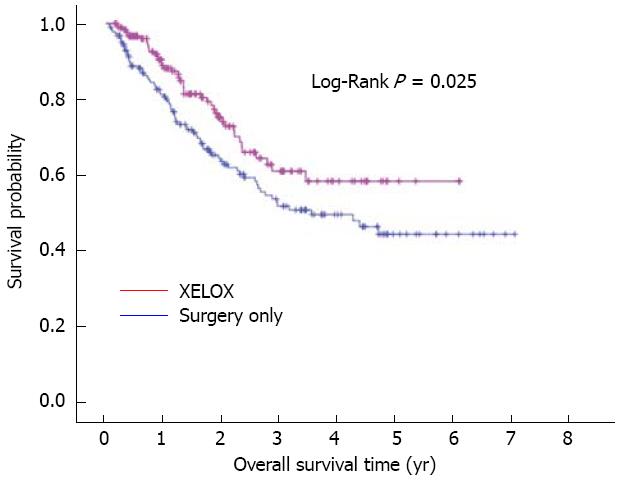
In an attempt to eliminate treatment selection bias associated with retrospective data, a propensity score matched-pair analysis was performed using the following variables: age, gender, tumor location, Borrmann type, number of retrieved nodes, tumor differentiation, pathological T category and pathological N category. This resulted in a total of 370 patients for the XELOX vs surgery only analysis with 185 cases per treatment arm, and a total of 74 patients for the XELOX vs FOLFOX6 analysis with 37 cases per treatment arm. There were no significant differences (P > 0.05) among the matched variables by treatment group (results not shown). On matched analysis, adjuvant XELOX therapy significantly improved overall survival compared to surgery only with longer mean overall survival (51.2 mo vs 48.9 mo) and better 3-year (60.9% vs 51.7%) and 5-year survival (58.2% vs 44.2%, P = 0.025) (Figure 2). However, there was no significant difference in overall survival between the XELOX and FOLFOX6 groups (P = 0.685). Overall 3-year survival was 42.7% in the FOLFOX6 group and 48.5% in the XELOX group with a median survival of 37.5 and 44.7 mo, respectively.
Grade 3/4 adverse reactions in the XELOX and FOLFOX6 groups are shown in Table 3. A total of 75 patients in the XELOX group (35%) experienced grade 3/4 adverse events similar to the rate in the FOLFOX6 group (19 patients, 39.6%, P = 0.618). More patients in the XELOX group experienced hand-foot syndrome than in the FOLFOX6 group (9.8% vs 0%, P = 0.018).
| Adverse reactions | FOLFOX6 | XELOX | P value |
| (n = 48) | (n = 214) | ||
| At least one adverse event | 19 (39.6) | 75 (35) | 0.618 |
| Vomitting | 5 (10.4) | 16 (7.5) | 0.555 |
| Anorexia | 5 (10.4) | 20 (9.3) | 0.788 |
| Oral mucositis | 1 (2.0) | 3 (1.4) | 0.557 |
| Diarrhea | 4 (8.3) | 14 (6.5) | 0.751 |
| Hand-foot syndrome | 0 (0.0) | 21 (9.8) | 0.018 |
| Peripheral neurotoxicity | 9 (18.8) | 25 (11.7) | 0.232 |
| Leukocyte/neutropenia | 7 (14.5) | 16 (7.5) | 0.153 |
| Thrombocytopenia | 4 (8.3) | 14 (6.5) | 0.751 |
| ALT/AST increase | 1 (2.0) | 5 (2.3) | 0.999 |
This study showed that adjuvant XELOX was significantly associated with improved survival after D2 dissection for GC compared to D2 dissection alone, regardless of age, tumor differentiation, and nodal status. After adjustment for confounders in the propensity score analysis, adjuvant XELOX therapy improved 5-year overall survival by approximately 14% (P = 0.025) compared to surgery only, but was not associated with a greater survival benefit than FOLFOX6 therapy (48.5% vs 42.7%, P = 0.685). These results demonstrated that adjuvant XELOX therapy should be considered in curable GC patients.
D2 gastrectomy has been the standard surgical procedure for GC in Eastern countries for several decades. For accurate pathological N category, at least 15 nodes should be retrieved for pathological examination according to the International Union against Cancer 7th edition[24]. Accurate tumor stage supports correct prognostic evaluation. This is why patients with less than 15 nodes were excluded in the current study. However, few studies have compared D2 surgery only with D2 surgery plus adjuvant chemotherapy in patients with GC. The JCOG 9206-1 clinical trial[28] failed to demonstrate the survival benefits of adjuvant chemotherapy with intravenous mitomycin, fluorouracil, and cytarabine followed by oral fluorouracil after D2 or greater dissection. The JCOG 9206-2 phase III trial also showed no survival benefit with adjuvant intraperitoneal and intravenous cisplatin followed by oral fluorouracil in serosa-positive GC after D2 dissection compared to D2 dissection alone[29]. However, the phase III CLASSIC study[23] reported a significant improvement in 3-year disease-free survival with the XELOX regimen compared to surgery alone. Our study demonstrated that adjuvant XELOX significantly improved 5-year survival compared to D2 dissection only. As one center involved in the CLASSIC trial, results in the current study were consistent with the preliminary 3-year report of the CLASSIC trial and further confirmed the efficacy of adjuvant XELOX chemotherapy after D2 dissection in the Chinese population based on overall 5-year survival data. Compared to the CLASSIC trial, the 3-year overall survival rate was relatively low in our study (61.5% vs 83% in the XELOX group and 57.3% vs 78% in the control group). The main reasons for this may be related to the different population and advanced disease in our study. To eliminate selection biases associated with retrospective data, propensity score adjusted and matched-pair analyses were performed, and adjuvant XELOX therapy showed improved survival after D2 dissection in GC patients compared to surgery only. Our findings demonstrated that adjuvant XELOX was an effective therapy for patients with resectable GC.
In this study, adjuvant XELOX therapy was associated with a significantly higher overall survival than FOLFOX6 before propensity score adjusted analysis. However, this significant difference disappeared after propensity score matched analysis. A similar phenomenon occurred during a comparison between XELOX therapy and surgery only with or without propensity score analysis. These discrepant results showed that unbalanced features associated with retrospective data existed between these two groups, even if no significant unbalanced baseline factors were found before propensity score adjustment. The relatively small sample size in the FOLFOX6 group limited the efficacy of propensity score analysis and the reliability of our conclusion. Different chemotherapy regimens lead to different effects, and some chemotherapy regimens may not result in survival improvement[30]. The main differences between the XELOX and FOLFOX6 regimens were oral capecitabine and intravenous 5-FU. Although 5-FU is the most established single-agent drug in palliative chemotherapeutic GC care, the tumor response to 5-FU was reported to be inadequately predicted in SCID mouse models with sometimes no antitumor effects observed[31]. A retrospective study compared the effects of the XELOX regimen with the FOLFOX6 regimen in GC patients after D2 dissection and found no survival difference (5-year survival rate: 34% vs 29%) between these two regimens[32]. A meta-analysis showed that the HR for death for oral capecitabine-containing regimens was 0.94 (95%CI: 0.89-1.00; P = 0.0489) in patients with advanced gastrointestinal cancers compared to 5-FU-containing regimens[33]. Our study showed no survival benefit for XELOX therapy compared to FOLFOX6 therapy and similar severe side effects were observed in these two groups. However, oral capecitabine was found to be more convenient and less dangerous than intravenous 5-FU. Few patients had nausea and vomiting during XELOX therapy, although more patients suffered from hand-foot syndrome. Most patients undergoing FOLFOX6 therapy did not complete all the required treatment courses due to frequent hospital visits for biweekly treatment and adverse effects related to eating, drinking and sleeping. This may be why more patients and doctors preferred the XELOX regimen.
In conclusion, this study demonstrated that adjuvant XELOX therapy was associated with survival benefits in GC patients after D2 dissection and provides a superior option for curable GC patients.
The authors would like to acknowledge the assistance of Dong-Lian Chen in maintaining our database.
The clinical treatment of gastric cancer (GC) is challenging and overall survival is poor. Adjuvant chemotherapy has been intensively investigated for several decades but definitive evidence is limited especially for patients after D2 dissection.
Adjuvant capecitabine and oxaliplatin (XELOX) and 5-fluorouracil, folinic acid and oxaliplatin (FOLFOX6) chemotherapy have been widely used in GC patients. However, few studies have directly compared their efficacy and the optimal regimen of adjuvant chemotherapy has not yet been determined.
The authors found that adjuvant XELOX was significantly associated with improved survival after D2 dissection compared to surgery alone following multivariate Cox regression and propensity score matched analyses. However, the XELOX regimen did not result in a greater survival benefit than the FOLFOX6 regimen.
The findings in this study demonstrated that adjuvant XELOX therapy should be considered in curable GC patients.
The propensity score method is used to determine the probability of an individual patient having received a certain treatment as a function of several confounding covariates that are collapsed into a single predictor.
The optimal chemotherapeutic regimen after resected advanced GC is still one of the clinical challenges. Based on previous studies, whether adjuvant chemotherapy can improve GC survival is still uncertain. Although XELOX (capecitabine and oxaliplatin) and FOLFOX6 (5-fluorouracil, folinic acid and oxaliplatin) regimens have been widely used in clinics, few studies have shown direct evidence to indicate their efficacy. This study compared the efficacy of XELOX and FOLFOX6 for GC patients after D2 dissection, and the findings have some scientific and clinical significance.
P- Reviewers Han X, Shen J S- Editor Song XX L- Editor A E- Editor Li JY
| 1. | Sasako M, Inoue M, Lin JT, Khor C, Yang HK, Ohtsu A. Gastric Cancer Working Group report. Jpn J Clin Oncol. 2010;40 Suppl 1:i28-i37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Modern surgery for gastric cancer--Japanese perspective. Scand J Surg. 2006;95:232-235. [PubMed] |
| 3. | Kodera Y, Schwarz RE, Nakao A. Extended lymph node dissection in gastric carcinoma: where do we stand after the Dutch and British randomized trials? J Am Coll Surg. 2002;195:855-864. [PubMed] |
| 4. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 5. | Schwarz RE, Zagala-Nevarez K. Recurrence patterns after radical gastrectomy for gastric cancer: prognostic factors and implications for postoperative adjuvant therapy. Ann Surg Oncol. 2002;9:394-400. [PubMed] |
| 6. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [PubMed] |
| 7. | Zhang CH, Zhan WH, He YL, Chen CQ, Huang MJ, Cai SR. Spleen preservation in radical surgery for gastric cardia cancer. Ann Surg Oncol. 2007;14:1312-1319. [PubMed] |
| 8. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1308] [Article Influence: 87.2] [Reference Citation Analysis (1)] |
| 9. | Degiuli M, Sasako M, Calgaro M, Garino M, Rebecchi F, Mineccia M, Scaglione D, Andreone D, Ponti A, Calvo F. Morbidity and mortality after D1 and D2 gastrectomy for cancer: interim analysis of the Italian Gastric Cancer Study Group (IGCSG) randomised surgical trial. Eur J Surg Oncol. 2004;30:303-308. [PubMed] |
| 10. | Okines A, Verheij M, Allum W, Cunningham D, Cervantes A. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v50-v54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 11. | Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87:353-357. [PubMed] |
| 12. | Xu J, Zhang C, He Y, Wu H, Wang Z, Song W, Li W, He W, Cai S, Zhan W. Lymphatic endothelial cell-secreted CXCL1 stimulates lymphangiogenesis and metastasis of gastric cancer. Int J Cancer. 2012;130:787-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 14. | Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362:858-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Zhang C, Awasthi N, Schwarz MA, Schwarz RE. Establishing a peritoneal dissemination xenograft mouse model for survival outcome assessment of experimental gastric cancer. J Surg Res. 2013;182:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Oyama K, Fushida S, Kinoshita J, Makino I, Nakamura K, Hayashi H, Nakagawara H, Tajima H, Fujita H, Takamura H. Efficacy of pre-operative chemotherapy with docetaxel, cisplatin, and S-1 (DCS therapy) and curative resection for gastric cancer with pathologically positive para-aortic lymph nodes. J Surg Oncol. 2012;105:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Osti MF, Agolli L, Bracci S, Monaco F, Tubin S, Minniti G, De Sanctis V, Enrici RM. Adjuvant chemoradiation with 5-fluorouracil or capecitabine in patients with gastric cancer after D2 nodal dissection. Anticancer Res. 2012;32:1397-1402. [PubMed] |
| 18. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [PubMed] |
| 19. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1422] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 20. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [PubMed] |
| 21. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [PubMed] |
| 22. | Ku GY, Ilson DH. Adjuvant therapy in esophagogastric adenocarcinoma: controversies and consensus. Gastrointest Cancer Res. 2012;5:85-92. [PubMed] |
| 23. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1291] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 24. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6461] [Article Influence: 430.7] [Reference Citation Analysis (0)] |
| 25. | D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265-2281. [PubMed] |
| 26. | Newgard CD, Hedges JR, Arthur M, Mullins RJ. Advanced statistics: the propensity score--a method for estimating treatment effect in observational research. Acad Emerg Med. 2004;11:953-961. [PubMed] |
| 27. | Schwarz RE, Smith DD, Keny H, Iklé DN, Shibata SI, Chu DZ, Pezner RD. Impact of intraoperative radiation on postoperative and disease-specific outcome after pancreatoduodenectomy for adenocarcinoma: a propensity score analysis. Am J Clin Oncol. 2003;26:16-21. [PubMed] |
| 28. | Nashimoto A, Nakajima T, Furukawa H, Kitamura M, Kinoshita T, Yamamura Y, Sasako M, Kunii Y, Motohashi H, Yamamoto S. Randomized trial of adjuvant chemotherapy with mitomycin, Fluorouracil, and Cytosine arabinoside followed by oral Fluorouracil in serosa-negative gastric cancer: Japan Clinical Oncology Group 9206-1. J Clin Oncol. 2003;21:2282-2287. [PubMed] |
| 29. | Miyashiro I, Furukawa H, Sasako M, Yamamoto S, Nashimoto A, Nakajima T, Kinoshita T, Kobayashi O, Arai K. Randomized clinical trial of adjuvant chemotherapy with intraperitoneal and intravenous cisplatin followed by oral fluorouracil (UFT) in serosa-positive gastric cancer versus curative resection alone: final results of the Japan Clinical Oncology Group trial JCOG9206-2. Gastric Cancer. 2011;14:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, Price T, Anderson H, Iveson T, Hickish T. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002;20:1996-2004. [PubMed] |
| 31. | Wacheck V, Heere-Ress E, Halaschek-Wiener J, Lucas T, Meyer H, Eichler HG, Jansen B. Bcl-2 antisense oligonucleotides chemosensitize human gastric cancer in a SCID mouse xenotransplantation model. J Mol Med (Berl). 2001;79:587-593. [PubMed] |
| 32. | Chen S, Feng X, Li Y, Yuan X, Zhou Z, Chen Y. Efficacy and safety of XELOX and FOLFOX6 adjuvant chemotherapy following radical total gastrectomy. Oncol Lett. 2012;3:781-786. [PubMed] |
| 33. | Cassidy J, Saltz L, Twelves C, Van Cutsem E, Hoff P, Kang Y, Saini JP, Gilberg F, Cunningham D. Efficacy of capecitabine versus 5-fluorouracil in colorectal and gastric cancers: a meta-analysis of individual data from 6171 patients. Ann Oncol. 2011;22:2604-2609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |