Published online Jun 7, 2013. doi: 10.3748/wjg.v19.i21.3281
Revised: March 28, 2013
Accepted: April 27, 2013
Published online: June 7, 2013
Processing time: 202 Days and 9.9 Hours
AIM: To examine the possible ameliorative effect of breastfeeding and the uptake of human colostrum against coeliac disease in autistic rats.
METHODS: Female rats were fed a standard diet and received a single intraperitoneal injection of 600 mg/kg sodium valproate on day 12.5 after conception. In study 1, neonatal rats were randomly subjected to blood tests to investigate autism. In study 2, the 1st group was fed by the mother after an injection of interferon-γ (IFN-γ) and administration of gliadin. The pups in the 2nd group were prevented from accessing maternal milk, injected IFN-γ, administered gliadin, and hand-fed human colostrum. The normal littermates fed by the table mothers were injected with physiological saline and served as normal controls in this study.
RESULTS: The protein concentration was higher in group 2 than in group 1 in the duodenum (161.6 ± 9 and 135.4 ± 7 mg/g of tissue, respectively, P < 0.01). A significant increase (P < 0.001) in body weight was detected in human colostrum-treated pups on post natal day (PND) 7 and 21 vs suckling pups in group 1. A delay in eye opening was noticed in the treated rats in group 1 on PND 13 compared with the control group and group 2. Administration of a single intraperitoneal injection of 600 mg/kg sodium valproate on day 12.5 after conception resulted in significantly reduced calcium and vitamin D levels in study 1 compared with the control groups (P < 0.001). However, human colostrum uptake inhibited increases in the level of transglutaminase antibody in autistic pups with coeliac disease.
CONCLUSION: The effects of early-life nutrition and human colostrum on the functional maturation of the duodenal villi in autistic rats with coeliac disease that might limit or prevent the coeliac risk with autism.
Core tip: Research examining the potential benefits of using breastfeeding and/or human colostrum for a wide range of gastroenterologic conditions of coeliac disease in autistic rats which is never studied before. Early results are encouraging and we envisage the standard use of human colostrum in the clinical management of gastrointestinal diseases within the next decade.
- Citation: Selim ME, Al-Ayadhi LY. Possible ameliorative effect of breastfeeding and the uptake of human colostrum against coeliac disease in autistic rats. World J Gastroenterol 2013; 19(21): 3281-3290
- URL: https://www.wjgnet.com/1007-9327/full/v19/i21/3281.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i21.3281
Two retrospective studies, which analysed representative populations of children with autism, have reported gastrointestinal tract (GI) symptoms in 20% of young children previously diagnosed with autism[1]. In contrast, prospective reports from paediatric gastroenterology and general autism clinics have described GI symptoms in 46%-84% of patients with autism spectrum disorder (ASD)[2]. However, there are few prevalence estimates from population-based epidemiologic studies. Reported GI abnormalities include low activities of disaccharidase enzymes, defective sulphation of ingested phenolic amines (Tylenol), bacterial overgrowth with a greater diversity and number of clostridial species, more numerous Paneth cells, increased intestinal permeability, and positive effects on behavioural cognition following dietary intervention[3]. Coeliac disease (CD) exhibits a multifactorial aetiology, and both genetic and environmental factors contribute to disease development. CD is a human leukocyte antigen (HLA)-associated disorder, and the majority of CD patients express HLA-DQ2 and HLA-DQ8 to a lesser extent[4]. Disease development is a consequence of the ingestion of immunogenic fragments in gluten by genetically predisposed subjects[5]. The treatment of CD involves a lifelong diet, which may cause difficulties, because avoiding gluten completely is almost impossible. However, over the last few years, new studies have suggested that prevention of CD may be possible[6]. The statement “prevention is better than a cure” is desirable for every disease, but is especially true for CD. Primary prevention avoids the development of a disease. In CD, primary prevention aims to avoid CD by intervening before the onset of the initial disease processes. In this context, we will outline the rationale for primary prevention in CD, which is based on factors contributing to CD and the possibility to improve tolerance to (food) allergens. Breastfeeding and colostrum provide immunological integration between the mother and neonate. Breastfeeding has immunological advantages, which allow it to prevent infections. Moreover, there is evidence that breastfeeding protects against cardiovascular disorders, obesity, Crohn’s disease, colitis ulcerosa, allergies, Diabetes mellitus type I and other (autoimmune) disorders such as CD[7]. The risk of these disorders could increase if the duration of breastfeeding is less than 3-6 mo[8]. During the lactation period, breast milk undergoes three different phases with different milk compositions: colostrum, first milk and mature milk[9]. Colostrum is the first milk produced after birth and is particularly rich in immunoglobulins, antimicrobial peptides (e.g., lactoferrin and lactoperoxidase), and other bioactive molecules, including growth factors. Colostrum is important for the nutrition, growth, and development of new-born infants and contributes to the immunologic defence of neonates. The composition of mammary secretions changes continuously throughout the suckling period; however, for the purposes of this research, we define colostrum as the milk produced in the first 48 h after birth. Breast milk contains all of the immunoglobulins (IgA, IgE, IgG, IgD and IgM)[10]. However, for new-borns, the most important immunoglobulins are IgA and IgG. IgA, specifically secretory IgA, provides mucosal defence, and IgG antibodies support tissue defence. Several studies describe the detection of wheat gliadins and other gluten peptides in breast milk along with specific IgA-antibodies against gliadin[11].
The low levels of gluten in breast milk could potentially be involved in the induction of oral tolerance to gluten in breastfed infants. The concentration of IgA antigliadin is highest in colostrum and decreases after one month[11]. Many studies have been published regarding the preventive effect of breastfeeding in CD. An important systematic review and meta-analysis of observational studies on breastfeeding and CD by Akobeng et al[12] concludes that breastfeeding offers protection against the development of CD. However, it is unclear whether breastfeeding permanently protects against the development of CD or whether it only delays the onset of symptoms[11]. The mechanism of protection against CD by breast milk is not well understood. Hanson et al[9] suggested that breastfeeding modulates the early exposure of the neonate’s intestinal mucosa to microbes and limits bacterial translocation through the gut mucosa. In addition, by preventing inflammation in the gut, breastfeeding also diminishes the passage of gluten peptides into the lamina propria and prevents the development of CD[10]. Another possible preventive mechanism is that human colostrum may decrease tissue transglutaminase expression in the gut and diminish the generation of deaminated gluten peptides[13]. The immune-modulating properties of human colostrum may also be exerted through a T-cell-specific suppressive effect, which has been shown in experiments on peripheral lymphocytes stimulated with phytohaemagglutinin, OKT3 and allo-antigens[11]. Thus, human colostrum may be an ideal antimicrobial for selective targeting in the gastrointestinal tract in autistic rats with coeliac disease.
Female Wistar rats with controlled fertility cycles were mated overnight. The morning when spermatozoa were found was designated as the first day of gestation. The females were fed a standard diet and received a single intraperitoneal injection of 600 mg/kg of sodium valproate on day 12.5 after conception. The control females were fed a normal standard diet and injected with physiological saline. Sodium valproate (Sigma) was dissolved in saline at a concentration of 250 mg/mL. Administration of this dose of sodium valproate to rats during embryogenesis has been shown to result in a maximum level of total VPA (900 μg/mL) in maternal plasma in less than 1 h with a mean plasma elimination half-life of 2.3 h[14]. The dams were housed individually and were allowed to raise their own litters. To induce coeliac disease, autistic male neonatal rats from valproate-treated females were divided into 2 experimental groups (twenty pups/group) and treated with interferon-γ (IFN-γ) (1000 U per animal administered intraperitoneally) after birth. Gliadin (0.5 and 3 mg) was intragastrically administered to the pups of both groups on day 0 and 3, and a 30 mg challenge dose was administered on day 20 (24 h before the termination of the experiment)[15]. The control pups from control females received physiological saline until the experiment was terminated.
In study 1, male Wistar neonatal rats were randomly selected from the above experimental groups before the treatment and were subjected to blood tests to investigate autism. Study 2 consisted of two experimental groups of new-born male autistic rats. The pups in group 1 were randomly assigned to be mother-fed after injection of IFN-γ and administration of gliadin. The pups in group 2 were collected from the mothers immediately after birth to prevent suckling of maternal milk. No foster nursing took place because the major objective was to study pup viability. The animals were injected with IFN-γ, administered gliadin, placed in an infant incubator to control body temperature and hand-fed human colostrum every 3-4 h using a silicone rubber tube. The method was both time and labour intensive; however, the method was essential because gastrostomy of new-born rats is associated with a very high surgery-related death rate. Normal littermates fed by control mothers were injected with physiological saline and served as normal non-treated controls in this study.
The total DNA content in tissue was assayed using the diphenylamine method of Burton[16] and determined by spectrophotometry. Assays for total protein content[17] were performed on homogenates and supernatants; the concentrations were determined using spectrophotometry (SpectraMAX Plus, Molecular Devices, Sunnyvale, CA, United States).
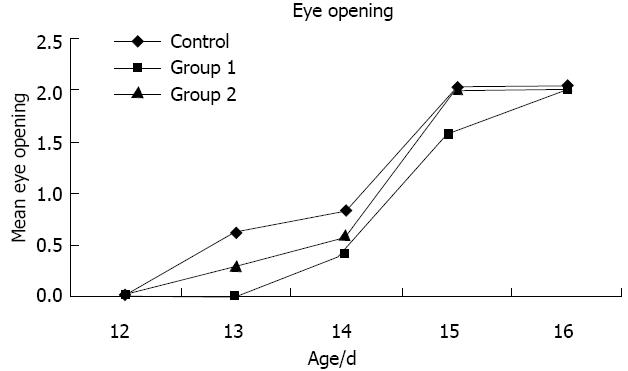
On post natal day (PND) 0, the pup weights were determined, and the pups were examined for malformations. The measurement of the pup weights was repeated on PND 7, 14 and 21 when the offspring were weaned from their mothers in all of the experimental groups. Eye opening was observed once daily from day 12 to 16 and rated as follows: on day 0, both eyes were closed; on day 1, one eye opened; and on day 2, both eyes opened.
Study 1 (to investigate autism): In study 1, blood samples from each experimental group (ten pups) from groups 1 and 2 and the control group were pooled for analyses before the administration of IFN-γ and gliadin in groups 1 and 2 or physiological saline in the control group. The samples were obtained from the eye using capillary tubes and were collected in ethylene diamine tetraacetic acid tubes containing aprotinin at 0 °C and centrifuged at 1600 g for 15 min. The plasma was analysed for 1-epinephrine, norepinephrine, catecholamine, and 2-serotonin using high performance liquid chromatography as previously described[18,19]. The serum was analysed for 3-calcium and 25-OH vitamin D after collection in microfuge tubes using a chemiluminescent microparticle immunoassay[20].
IFN-γ: Recombinant rat IFN-γ (PRP24; Serotec, Oxford, United Kingdom) was used in this study. IFN-γ was lyophilised (0.1 mg), reconstituted in 0.3 mL of distilled water, and stored until use in aliquots of 50 μL (10000 U) at -70 °C. A dose of 1000 U was used for each new-born rat. After application of IFN-γ, the pups were fed either milk from their mother or human colostrum.
Gliadin administration: Gliadin (from wheat gluten, G-3375; Sigma, St. Louis, MO, United States) was administered intragastrically using a silicon tube. Young rats were repeatedly administered gliadin in the following doses: day 0, 0.5 mg in one intragastric dose and day 3, 3 mg in one intragastric dose. The pups in each group were euthanised at 21 d of age after receiving a provocative dose of 30 mg of gliadin per animal 24 h before euthanisation (on postnatal day PND 20).
Blood test study 2 (to investigate coeliac disease): Study 2 aimed to investigate coeliac disease in autistic pups on PND 21. After the application of interferon-γ in group 1 (suckling pups receiving gliadin) and group 2 (human colostrum and gliadin), serum tissue transglutaminase tTG antibody titres were measured quantitatively by an enzyme-linked immunosorbent assay (QuantaLite tTG, Inova Diagnostics, CA, United States). Additionally, all the chemical analyses for study 1 were repeated on PND 21 for the two experimental groups and the control group.
Small samples from the duodenum of each experimental group on PND 21 were immediately fixed in 3% phosphate-buffered glutaraldehyde (pH = 7.4; 4 °C) for 2 h. The tissues were postfixed in 1% aqueous osmium tetraoxide in an appropriate buffer for 1 h and embedded in Epon. Ultrathin sections (80-100 nm) were prepared and stained with uranyl acetate and lead citrate. To examine the duodenum using scanning electron microscopy, the tissues were fixed as described previously, dehydrated, mounted on aluminium stubs with conductive carbon glue, and sputter coated with a 100 Å layer of gold. The control and treated samples were examined using a Joel EX 1200 transmission electron microscope at the central lab of King Saud University.
SPSS 13.0 was used for the statistical analysis. The data were expressed as the mean ± SD and were analysed using one-way analysis of variance followed with a post-hoc test for multiple comparisons. The differences were considered significant at the P < 0.05 level.
As shown in Table 1, the duodenal DNA and protein concentrations were significantly different between group 1 and group 2 and significantly higher in group 2 compared with the control animals. The protein concentration in the duodenum was significantly higher in group 2 compared with group 1 (151.6 ± 9 mg/g vs 122.4 ± 7 mg/g of tissue, respectively). However, no significant difference in the duodenal DNA concentration was observed between group 1 and the control group P > 0.05 (6.1 ± 0.06 vs 6.3 ± 0.03, respectively).
As shown in Table 2, the pup weight decreased significantly (P < 0.001) in the autistic neonatal pups on PND 0 (study 1) compared with the normal control pups. The animals from group 1 (suckling pups administered gliadin) exhibited a statistically significantly (P < 0.001) reduced increase in body weight until weaning compared with the control group. (21.40 ± 2.36 g vs 33.4 ± 2.7 g, respectively). A significant increase (P < 0.001) in body weight was detected in human colostrum-treated pups on PND 7 and 21 compared with suckling pups in group 1. However, no significant difference was noticed between the two groups on PND 0 (P > 0.05). The stools were sticky, and the animals showed long bone malformations in autistic rats (results not shown).
As shown in Figure 1, there was a delay in eye opening in the treated rats in group 1 on PND 13 compared with the control group and group 2. No difference was observed on PND 12 and 16 between group 1 and group 2 (P > 0.05) vs the control group.
Table 3 shows the changes in biochemical parameters of control and autistic neonatal pups on PND 0. The mean concentrations of epinephrine, norepinephrine and serotonin were greatly increased in study 1 compared with the control group (P < 0.001). Administration of a single intraperitoneal injection of 600 mg/kg sodium valproate on day 12.5 after conception resulted in significantly reduced calcium and vitamin D levels in study 1 vs the control group (P < 0.001).
Mean vitamin D: As shown in Table 4, a significant difference in the vitamin D level was observed between group 2 and the control group (33.62 ± 0.581 ng/mL vs 44.36 ± 0.302 ng/mL; P < 0.05). Furthermore, on PND 21, the pups in group 1 exhibited a significantly lower mean vitamin D level vs the control group and group 2 (9.4 ± 0.225 ng/mL vs 44.36 ± 0.302 ng/mL and 33.62 ± 0.581 ng/mL; P < 0.01). Treatment with human colostrum inhibited decreases in the level of vitamin D in autistic pups with coeliac disease (P < 0.01).
| Parameters | Vitamin D (ng/mL) | Serotonin (μg/L) | Epinephrine (ng/L) | Norepinephrine (ng/L) | Transglutaminase (g/L) | Calcium (mg/dL) |
| Method | CMIA | HPLC | HPLC | HPLC | ELISA | Calorimetric |
| Control | 44.36 ± 0.302 | 9.12 ± 0.126 | 21.73 ± 0.29 | 110.46 ± 0.99 | 0.9 ± 0.066 | 10.91 ± 0.241 |
| Group 1 | 9.4 ± 0.225b | 30.5 ± 0.565b | 76.22 ± 1.15b | 204.87 ± 1.83b | 3.1 ± 0.149b | 7.31 ± 0.411a |
| Group 2 | 33.62 ± 0.581bd | 15.32 ± 0.367bd | 56.15 ± 0.07bc | 155.42 ± 0.08bc | 1.6 ± 1.021ad | 10.12 ± 0.819c |
Mean serotonin: As shown in Table 4, a significant increase in the mean serotonin levels was detected in group 1 vs the control pups (30.5 ± 0.565 ng/L vs 9.12 ± 0.126 ng/L; P < 0.001). A significant decrease in the serotonin level was detected in group 2, which was treated with human colostrum, vs group 1 (15.32 ± 0.367 ng/L vs 30.5 ± 0.565 ng/L; P < 0.001).
Mean epinephrine: As shown in Table 4, the mean epinephrine concentration was significantly increased (P < 0.001) in all of the treated groups including group 1 and group 2 on PND 21 vs the control group (76.22 ± 1.15 ng/L and 56.15 ± 0.071 ng/L vs 21.73 ± 290 ng/L, respectively). However, the epinephrine level was significantly (P < 0.01) decreased in group 2 pups vs group 1 pups (56.15 ± 0.071 ng/L vs 76.22 ± 1.15 ng/L, respectively).
Mean norepinephrine: As shown in Table 4, the mean norepinephrine concentration was significantly increased (P < 0.01) in all of the treated groups including group 1 and group 2 on PND 21 vs the control group (204.87 ± 1.83 ng/L and 155.42 ± 0.086 ng/L vs 110.46 ± 0.990 ng/L, respectively). Moreover, a significant change was detected in group 2 vs group 1 pups (P < 0.05).
Mean transglutaminase: As shown in Table 4, the mean transglutaminase concentrations were significantly increased (P < 0.01) in all of the treated groups including group 1 (suckling pups administered gliadin) and group 2 (human colostrum and gliadin) on PND 21 vs the normal controls (3.1 ± 0.149 g/L and 1.6 ± 1.021 g/L vs 0.9 ± 0.066 g/L, respectively). Furthermore, the mean transglutaminase level was significantly (P < 0.01) increased in the group 1 pups vs the group 2 pups. Moreover, a significant change was detected in the group 2 pups compared with the control pups. Treatment with human colostrum inhibited the increased level of transglutaminase in autistic pups with coeliac disease vs group 1 suckling pups.
Mean calcium: As shown in Table 4, the calcium level was decreased in group 1 vs the control group and group 2 (7.31 ± 0.411 vs 10.91 ± 0.241 and 10.12 ± 0.819; P < 0.05). No significant difference in the calcium level was observed between group 2 and the control group (P > 0.05). Therefore, the human colostrum treatment was more effective than mother’s milk in increasing the calcium level.
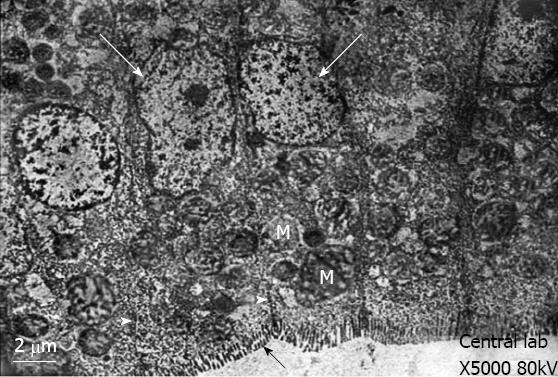
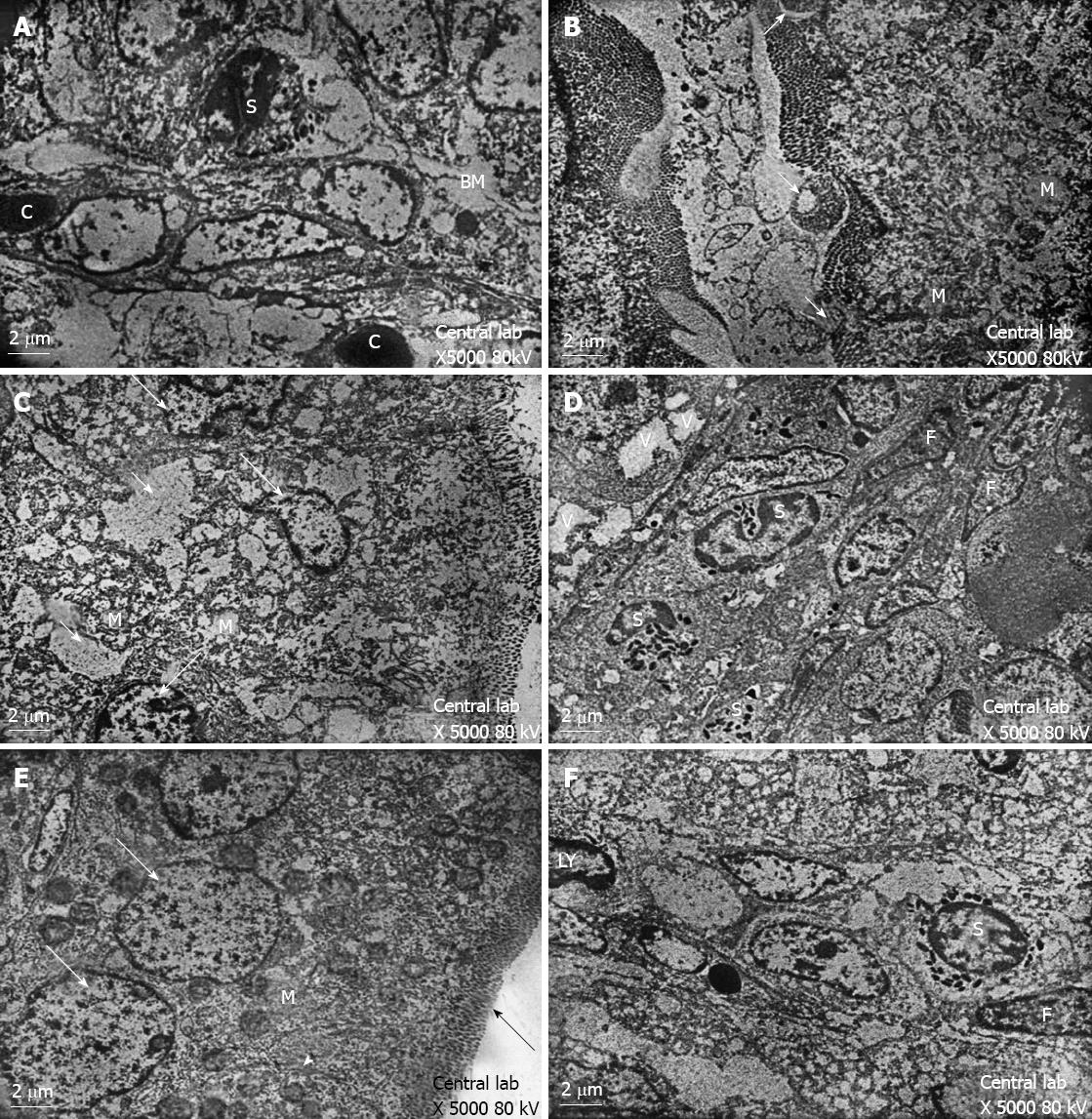
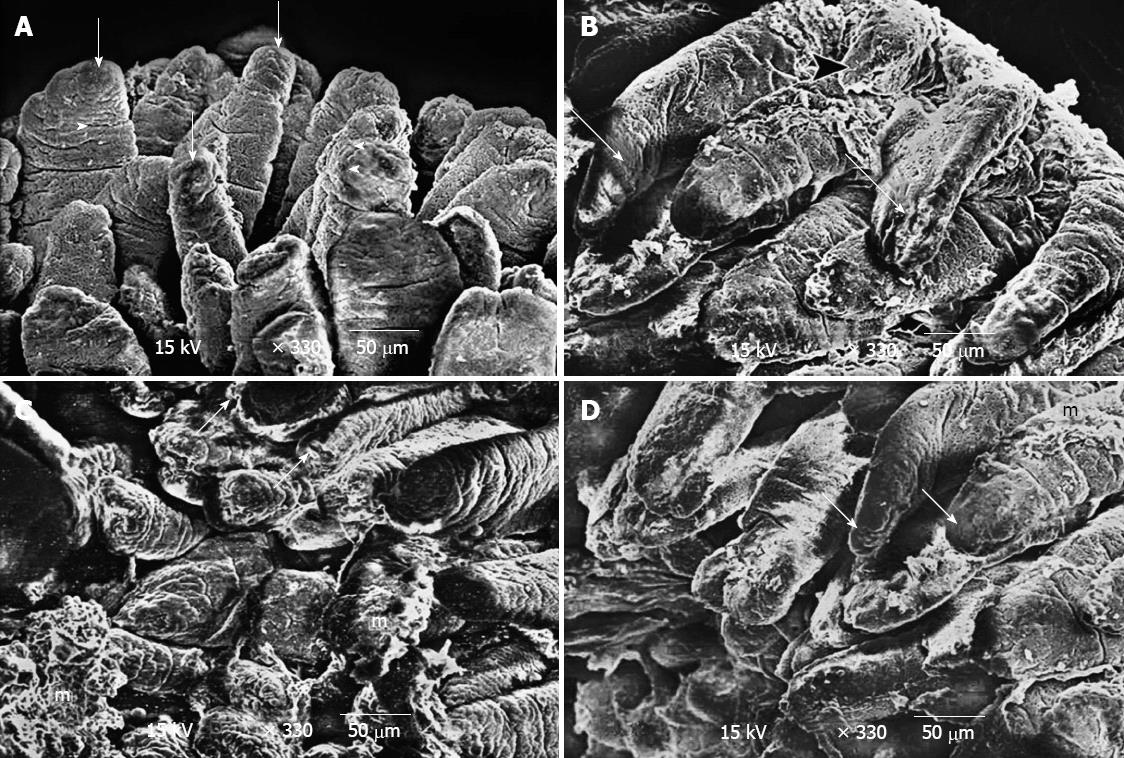
Control group: Transmission electron microscopy showed that the enterocytes exhibited a regular microvillus border, and cell junctions were typically observed between the enterocytes (Figure 2) along with scattered abundant mitochondria (Figure 2). The cores of the villi possessed normal appearances with normal capillaries and connective tissue cells (Figure 3A). The surface of the duodenal villi was examined using scanning electron microscopy and revealed some villi with intact tips and borders. The openings of goblet cells were observed on the surface of the villi (Figure 4A).
Group 1: The electron microscopic study showed localised areas of loss of the microvillus borders in the enterocytes with swollen mitochondria and vacuolation in the cytoplasm (Figure 3B). Irregular appearance of the nuclei of the enterocytes was also observed (Figure 3C), and the core of the villi showed cellular infiltration (Figure 3D). Scanning electron microscopy showed deformed villi (Figure 4B), villi with distorted appearances and the accumulation of mucous (Figure 4C).
Group 2: The ultrastructure of the enterocytes of the duodenal villi was similar to that of the control group. The microvillus border was regular and continuous. The enterocytes showed vesicular nuclei and abundant mitochondria (Figure 3E). The core of the villi appeared to be normal with normal capillaries (Figure 3F). Scanning electron microscopy showed that the villi of the duodenum of group 2 appeared to be normal and were similar to the control group (Figure 4D).
In this investigation, the repeated administration of human colostrum until weaning in group 2 increased the mucosal restitution of duodenal tissue as shown in Figures 3E, 3F and 4D, which was observed by electron microscopy. However, the breast feeding in group 1 could not overcome the atrophy of the gut caused by coeliac disease in autistic rats as shown in Figures 3B-D, 4B and 4C. This suggested that epidermal growth factor (EGF) in colostrum (200 μg/L) and milk (30-50 μg/L) in humans and many other species acts as a luminal surveillance peptide that prevents gut damage[21] and stimulates the repair process at sites of injury[22]. Transforming growth factor-α (TGF-α) is a 50-amino acid molecule present in human colostrum and milk at much lower concentrations (2.2-7.2 μg/L)[23] than EGF and occurs in the gastrointestinal mucosa at sites of injury, which supports its role in mucosal growth and repair. TGF-β-like molecules are present in high concentrations in both bovine milk (1-2 mg/L) and colostrum (20-40 mg/L)[24]. The concentrations are sufficient enough to mediate the ability to maintain gastrointestinal integrity in suckling neonates. The previous results are consistent with our previous report, which shows that treatment with bovine colostrum has a positive effect on duodenum injury in autistic rats with coeliac disease[25]. Insulin-like growth factor (somatomedins) (IGF-I) concentrations are much higher in bovine colostrum compared with human colostrum (500 μg/L vs 18 μg/L)[26,27]. And even lower concentrations in mature bovine milk (10 μg/L) are observed[28]. IGF-I is known to promote protein accretion (i.e., it is an anabolic agent[29]) and is at least partially responsible for mediating the growth-promoting activity of growth hormone. In rodents, two growth factors play an important role in the process of regeneration and proliferation of intestinal enterocytes: EGF and transforming TGF-α. However, TGF-α has not been found in rat maternal milk[30]. This may explain the decreased bone malformation in group 2, which was treated with human colostrum (results not shown), compared with group 1 rats. Growth factors are so named because they stimulate the growth of various cell lines in vitro; however, the functions of these peptide-based molecules are diverse. There are also marked species differences in the nature and concentration of growth factor constituents. For example, human colostrum contains much higher concentrations of EGF than bovine colostrum, while the reverse is true for insulin-like IGFs-I and II. In our investigation, the uptake of human colostrum in group 2 showed significant improvements compared with suckling pups in group 1 when assessed by body weight. The mean body weight measured in group 2 was highest at the end of weaning (29.6 ± 1.75) and significantly differed from the mean body weight observed on other days in group 1 and compared with the control. An additional consequence of different routes of immunoglobulin transmission relates to the changes in the relative contents of immunoglobulins, which occurs during the transition from colostrum to milk in certain species. For example, the profile of immunoglobulins in human colostrum is similar to milk; the IgA level is high in both colostrum and milk (88%-90% of total immunoglobulin). IgA protects the gastrointestinal tract (GIT) from various diseases in new-borns, which is reflected in large changes in the profile of immunoglobulins during the transition from colostrum to mature milk[31]. IgA-deficient patients predominantly suffer from respiratory and gastrointestinal infections because secretory IgA has important functions in protecting mucosal surfaces[31]. Human colostrum has a low IgG content (2%), and the IgG required to provide systemic immunity is transferred across the placenta before birth[32]. In contrast, the colostral IgG content in many other species is typically greater than 75% of the total immunoglobulin content.
Based on the aforementioned information, in our study, we used this unique model to induce gluten enteropathy. We found that the concurrent administration of IFN-γ intraperitoneally and gliadin intragastrically in group 1 followed by a second intragastric dose of gliadin resulted in a failure to detoxify antigens from the gut, which may lead to cognitive impairments and coeliac crises on day 14. The impairments included loose stools with short and deformed duodenal villi in some pups, while other pups exhibited sloughing of the tips as observed by scanning electron microscopy. Local loss of the brush border, irregular nuclei and vacuolation of some enterocytes were observed using transmission electron microscopy on day 21. The previous result may be due to the presence of antigens in the diet that can cross into the mucosa more easily via a disrupted intestinal barrier. The antigens may cause local inflammatory reactions and generate proinflammatory cytokine signals that affect the GIT. The previously described damage was not observed in group 2, which was administered human colostrum until weaning, using scanning and transmission electron microscopy as shown in Figures 3E, 3F and 4D. This could be explained by the report from another group[33], who revealed that the concentration of IgA antigliadin is the highest in colostrum and decreases after a month. This may diminish the effect of gluten peptides on the lamina propria and prevent the initiation of damage to intestinal villi that was observed in group 1.
Leptin levels, which supports duodenal protection in colostrum and milk, was correlated with the whole fat and choline phospholipid content of colostrum. The association between leptin and fat globule membrane content results from its protective properties during gut transit in the new-born animal[34]. Leptin receptor expression has been found in the small intestine, which suggests that the intestinal epithelium is a direct target of leptin action[35]. The concentration of leptin was 56% lower in mature milk (day 10) vs colostrum (13.90 g/L vs 6.14 g/L, respectively; P < 0.001). The colostrum and milk leptin levels correlated with fat (0.90; P < 0.001) and choline phospholipid (0.76; P < 0.05). Thus, leptin is present in large quantities in colostrum, smaller and more variable quantities in untreated milk and is likely decreased in skim milk[3,4]. Our results revealed increased levels of serotonin (33.3 ± 0.244), epinephrine (84.25 ± 0.595) and norepinephrine (184.29 ± 8.44) in autistic pups (study 1) due to maternal prenatal exposure to VPA. Furthermore, we observed a significant decrease in serum vitamin D and calcium levels in autistic neonatal rats in study 1 on PND 0 and group 1 on PND 21. Both epinephrine and norepinephrine were significantly increased in autistic pups on PND 0 and group 1 pups on PND 21 compared with the control group.
Colostrum is high in calcium and magnesium, which share bonding characteristics as functional groups. Both organic calcium and organic magnesium in colostrum enhance the absorption of other minerals and vitamins in our body. Colostrum also provides higher levels of vitamin D and vitamin A than normal milk[36]. In addition, vitamin D and IgD can help our body absorb a sufficient amount of calcium to enhance physiological functions[36]. Our investigation revealed that the uptake of human colostrum in group 2 inhibited the decrease in levels of vitamin D and calcium in autistic pups with coeliac disease as shown in Table 4.
An initial mucosal assault due to a gut infection or the toxic effects of gliadin may cause upregulation of tissue transglutaminase (tTG), which causes cross-linking of various proteins, including gliadin. This tTG-gliadin complex represents a “new antigen” (neoantigen), which could trigger the production of tTG antibodies[37]. Tissue TG plays a role in maintaining the integrity of both the intestinal crypt micro-architecture and the dermal-epidermal junction. Therefore, the development of villous atrophy in group 1 could be explained by the production of tTG antibodies (3.1 ± 0.149 g/L) due to repeated administration of gliadin, which may disturb the integrity of the duodenum in breastfeeding pups. However, human colostrum uptake inhibited the increase in the levels of transglutaminase antibody (1.6 ± 1.021 g/L) in autistic pups with coeliac disease.
The outcomes of the present investigation elucidated the effects of early-life nutrition from human colostrum on the functional maturation of the duodenal villi in autistic rats with coeliac disease, which might help to limit of prevent coeliac risk. There are numerous pathways that may lead to the diagnosis of ASD. In some, ASD may begin with genetic susceptibility, and in others, infection or immune abnormalities may play a key role. Further study of the reciprocal actions of the nervous, immune, and endocrine systems may help to unravel the mystery of ASD. Moreover, while many of the observations discussed herein are involved in the pathogenesis of autism, comprehensive studies of autism and coeliac disease with age-matched control individuals and their families are necessary to provide more conclusive results.
Coeliac disease (CD) exhibits a multifactorial aetiology, and both genetic and environmental factors contribute to disease development. CD is a human leukocyte antigen (HLA)-associated disorder, and the majority of CD patients express HLA-DQ2 and HLA-DQ8 to a lesser extent. Disease development is a consequence of the ingestion of immunogenic fragments in gluten by genetically predisposed subjects. The treatment of CD involves a lifelong diet, which may cause difficulties, because avoiding gluten completely is almost impossible.
Many studies have been published regarding the preventive effect of breastfeeding in CD. An important systematic review and meta-analysis of observational studies on breastfeeding and CD by Akobeng et al concludes that breastfeeding offers protection against the development of CD.
Human colostrum may be an ideal antimicrobial for selective targeting in the gastrointestinal tract in autistic rats with coeliac disease.
This investigation revealed that the uptake of human colostrum inhibited the decrease in levels of vitamin D and calcium in autistic pups with coeliac disease
This paper explores the effect of breastfeeding and human colostrums on the prevention of coeliac disease in autistic rats. The paper is well structured and the study is well designed. The article is sufficiently novel and interesting to warrant publication.
P- Reviewers Cheung MC, Khajehei M S- Editor Zhai HH L- Editor A E- Editor Ma S
| 1. | Fombonne E, Simmons H, Ford T, Meltzer H, Goodman R. Prevalence of pervasive developmental disorders in the British nationwide survey of child mental health. J Am Acad Child Adolesc Psychiatry. 2001;40:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, Tildon JT. Gastrointestinal abnormalities in children with autistic disorder. J Pediatr. 1999;135:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 269] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, McTeague M, Sandler R, Wexler H, Marlowe EM. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35:S6-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 498] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 4. | Mearin ML, Biemond I, Peña AS, Polanco I, Vazquez C, Schreuder GT, de Vries RR, van Rood JJ. HLA-DR phenotypes in Spanish coeliac children: their contribution to the understanding of the genetics of the disease. Gut. 1983;24:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 593] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 6. | Brandtzaeg P. Mucosal immunity: integration between mother and the breast-fed infant. Vaccine. 2003;21:3382-3388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Oddy WH. The impact of breastmilk on infant and child health. Breastfeed Rev. 2002;10:5-18. [PubMed] |
| 8. | McLaren DS, Burmad D, Belton NR. Textbook of paediatric Nutrition. 3rd ed. Edinburgh, Scotland: Churchill Livingstone 1991; . |
| 9. | Hanson LA, Korotkova M, Håversen L, Mattsby-Baltzer I, Hahn-Zoric M, Silfverdal SA, Strandvik B, Telemo E. Breast-feeding, a complex support system for the offspring. Pediatr Int. 2002;44:347-352. [PubMed] |
| 10. | Troncone R, Scarcella A, Donatiello A, Cannataro P, Tarabuso A, Auricchio S. Passage of gliadin into human breast milk. Acta Paediatr Scand. 1987;76:453-456. [PubMed] |
| 11. | Ozkan T, Ozeke T, Meral A. Gliadin-specific IgA antibodies in breast milk. J Int Med Res. 2000;28:234-240. [PubMed] |
| 12. | Akobeng AK, Ramanan AV, Buchan I, Heller RF. Effect of breast feeding on risk of coeliac disease: a systematic review and meta-analysis of observational studies. Arch Dis Child. 2006;91:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Binkerd PE, Rowland JM, Nau H, Hendrickx AG. Evaluation of valproic acid (VPA) developmental toxicity and pharmacokinetics in Sprague-Dawley rats. Fundam Appl Toxicol. 1988;11:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Stepánková R, Kofronová O, Tucková L, Kozáková H, Cebra JJ, Tlaskalová- Hogenová H. Experimentally induced gluten enteropathy and protective effect of epidermal growth factor in artificially fed neonatal rats. J Pediatr Gastroenterol Nutr. 2003;36:96-104. [PubMed] |
| 16. | Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:315-323. [PubMed] |
| 17. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 18. | Lang SA, Maron MB, Signs SA. Oxygen consumption after massive sympathetic nervous system discharge. Am J Physiol. 1989;256:E345-E351. [PubMed] |
| 19. | Kluge H, Bolle M, Reuter R, Werner S, Zahlten W, and Prudlo J. Serotonin in Platelets: Comparative Analyses using New Enzyme Immunoassay and HPLC Test Kits and the Traditional Fluorimetric Procedure. J Lab Med. 1999;23:360-364. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Peterlik M, Boonen S, Cross HS, Lamberg-Allardt C. Vitamin D and calcium insufficiency-related chronic diseases: an emerging world-wide public health problem. Int J Environ Res Public Health. 2009;6:2585-2607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Read LC, Francis GL, Wallace JC, Ballard FJ. Growth factor concentrations and growth-promoting activity in human milk following premature birth. J Dev Physiol. 1985;7:135-145. [PubMed] |
| 22. | Playford RJ. Peptides and gastrointestinal mucosal integrity. Gut. 1995;37:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Coffey RJ, Romano M, Goldenring J. Roles for transforming growth factor-alpha in the stomach. J Clin Gastroenterol. 1995;21:S36-S39. [PubMed] |
| 24. | Marchbank T, Playford RJ. Bovine colostrum or TGFβ (a major bioactive constituent of colostrum) are prophylactic against indomethacin induced injury. Gut. 1998;42:A68. |
| 25. | Selim ME, Al-Ayadhi LY. The role of breastfeeding and the intake of bovine colostrum in autistic neonatal rats with coeliac disease. Int J Mol Zool. 2012;2:1-12. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Baxter RC, Zaltsman Z, Turtle JR. Immunoreactive somatomedin-C/insulin-like growth factor I and its binding protein in human milk. J Clin Endocrinol Metab. 1984;58:955-959. [PubMed] |
| 27. | Vacher PY, Blum JW. Age dependency of insulin like growth factor 1, insulin protein and immunoglobulin concentrations and gamma glutamyl transferase activity in first colostrum of dairy cows. Milchwissenschaft. 1993;48:423-425. |
| 28. | Collier RJ, Miller MA, Hildebrandt JR, Torkelson AR, White TC, Madsen KS, Vicini JL, Eppard PJ, Lanza GM. Factors affecting insulin-like growth factor-I concentration in bovine milk. J Dairy Sci. 1991;74:2905-2911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Lo HC, Hinton PS, Yang H, Unterman TG, Ney DM. Insulin-like growth factor-I but not growth hormone attenuates dexamethasone-induced catabolism in parenterally fed rats. JPEN J Parenter Enteral Nutr. 1996;20:171-177. [PubMed] |
| 30. | Dvorák B, Koldovský O. Concerning the physiological role of milk-borne transforming growth factor-alpha for the neonate. Endocr Regul. 1993;27:145-147. [PubMed] |
| 31. | Mix E, Goertsches R, Zett UK. Immunoglobulins--basic considerations. J Neurol. 2006;253:V9-17. [PubMed] |
| 32. | Novak FR, Almeida JA, Silva GO, Borba LM. Human colostrum: a natural source of probiotics? J Pediatr (Rio J). 2001;77:265-270. [PubMed] |
| 33. | Patıroğlu T, Kondolot M. The effect of bovine colostrum on viral upper respiratory tract infections in children with immunoglobulin A deficiency. Clin Respir J. 2013;7:21-26. [PubMed] |
| 34. | Smith-Kirwin SM, O’Connor DM, De Johnston J, Lancey ED, Hassink SG, Funanage VL. Leptin expression in human mammary epithelial cells and breast milk. J Clin Endocrinol Metab. 1998;83:1810-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Morton NM, Emilsson V, Liu YL, Cawthorne MA. Leptin action in intestinal cells. J Biol Chem. 1998;273:26194-26201. [PubMed] |
| 36. | Cizza G, Romagni P, Lotsikas A, Lam G, Rosenthal NE, Chrousos GP. Plasma leptin in men and women with seasonal affective disorder and in healthy matched controls. Horm Metab Res. 2005;37:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Mäki M, Collin P. Coeliac disease. Lancet. 1997;349:1755-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 381] [Article Influence: 13.6] [Reference Citation Analysis (0)] |