Published online Jun 7, 2013. doi: 10.3748/wjg.v19.i21.3255
Revised: March 29, 2013
Accepted: April 27, 2013
Published online: June 7, 2013
Processing time: 134 Days and 10 Hours
AIM: To investigate differences in tolerability and response to treatment in compensated cirrhotic patients affected by hepatitis C virus (HCV) infection before and after liver transplantation.
METHODS: Forty-three HCV non-liver transplanted (LT) cirrhotics (mean age 55 ± 8 years, 65.1% male, Child-Pugh-A, genotype 1-4: 65.1%, 2-3: 34.9%) and 17 LT recipients with recurrent HCV-related cirrhosis (mean age 57 ± 9 years, 88.2% male, Child-Pugh-A, genotype 1-4: 76.5%, 2-3: 23.5%) were included in the analysis from retrospective series. All patients received recombinant or pegylated interferon plus ribavirin at a standard dose and duration. Adverse events were recorded and classified according to the Common Terminology Criteria for Adverse Events. The mean duration of follow-up was of 4.3 ± 1.8 years after the end of the treatment.
RESULTS: An early virological response (EVR) was achieved in 30/43 (69.8%) non-LT and in 8/17 (47.1%) LT cirrhotics, a sustained virological response (SVR) in 18/43 (41.9%) and 5/17 (29.4 %), respectively. No statistical difference was observed in EVR and SVR rates between the two groups. Among HCV non-LT cirrhotics, 6/43 (13.9%) discontinued the treatment prematurely, 11.6% of them receiving ≤ 80% of treatment; 8/17 (47%) LT cirrhotics withdrew the treatment, 35.2% of them receiving ≤ 80% of treatment. If compared with LT-ones (P = 0.015), an higher risk of treatment discontinuation could affect LT cirrhotics, who undergo more frequently ≤ 80% of treatment (P = 0.05). None of the non-LT cirrhotics died after the end of the treatment. With no regards to the achievement of SVR, LT cirrhotic patients showed a reduced survival in respect to non-LT ones (87% at 1 year, 76% at 3 and 5 years after the end of treatment).
CONCLUSION: HCV antiviral treatment is equally effective in compensated cirrhotics both before and after LT, which patients show a higher risk of premature treatment withdrawal and a reduced survival, independently of the achievement of SVR.
Core tip: In patients with hepatitis C virus compensated liver cirrhosis antiviral treatment should be considered in order to prevent short to mid-term complications. However, the results of treatment with pegylated interferon plus ribavirin are worse than in non-cirrhotic patients. Furthermore, in non-liver transplanted (LT) cirrhotics, a sustained virological response to antiviral treatment is associated to improved survival, while, at present, no data regarding LT cirrhotic is available in literature. The present study highlights that cirrhotic patients who undergo antiviral treatment after liver transplantation have a worse prognosis, compared to non-LT ones, independently of the achievement of sustained virological response.
- Citation: Ponziani FR, Annicchiarico EB, Siciliano M, D’Aversa F, Pompili M, Gasbarrini A. Treatment of hepatitis C in compensated cirrhotic patients is equally effective before and after liver transplantation. World J Gastroenterol 2013; 19(21): 3255-3262
- URL: https://www.wjgnet.com/1007-9327/full/v19/i21/3255.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i21.3255
Hepatitis C virus (HCV)-related hepatitis is one of the leading cause of end-stage liver disease worldwide, accounting for half of transplantations in many centers[1]. The natural history of HCV-related liver cirrhosis is characterized by several complications that could affect patients’ survival[2-6]; therefore, a good response to combined antiviral treatment with pegylated interferon (PEG-IFN) and ribavirin (RBV) may produce a benefit on patients quality of life and survival.
According to the recent European Association for the Study of the Liver guidelines[4], in absence of contraindications, the antiviral treatment with PEG-IFN and RBV should be considered for patients with compensated cirrhosis in order to prevent short to mid-term complications. However, results are worse than in non-cirrhotics (30.6%), with a more frequent need for treatment discontinuation and dose reduction[7-11]. However, patients who experience a favorable response to antiviral treatment show an improved survival rate in respect to non-responders [98% vs 86% at 5 years, respectively, patients with sustained virological response (SVR)] and a lower risk of decompensation[7,8].
In liver transplanted (LT) patients, the perspective is quite different. Although several data regarding treatment of post-liver transplant recurrent hepatitis C are already available, data about the treatment of LT cirrhotic patients are scarce. In particular, in different series of LT patients with recurrent hepatitis C, SVR rates range between 30% (genotype 1 HCV) and 60% (genotype 2 and 3 HCV) and the achievement of SVR has resulted in reducing the progression of liver disease and the incidence of decompensation, alongside patients’ survival[12-17]. However, these beneficial effects of antiviral treatment have not been proven yet in LT HCV patients with HCV-related liver cirrhosis.
The aim of this retrospective study is to analyze the safety (incidence of adverse events) and efficacy (rate of SVR, and impact on patients survival) of combined antiviral treatment with PEG-IFN and RBV in a series of LT compensated cirrhotic patients, in comparison with non-LT ones.
Patients with HCV-related compensated liver cirrhosis, either LT or not, treated with combined PEG-IFN and RBV treatment have been included in this retrospective study.
The Agostino Gemelli Hospital database, Division of Internal Medicine and Gastroenterology has been the source for the cirrhotic non-LT patients’ information; further, data about LT cirrhotic patients have been collected by the AISF group for the study of hepatitis C recurrence after liver transplantation (RECOLT-C).
Chronic HCV infection has been diagnosed both on the basis of serum positivity for both anti-HCV antibodies and quantitative HCV-RNA, and the presence of complete or incomplete cirrhosis by histological evaluation of liver tissue specimens performed within 6 mo before the beginning of the treatment (staging of 5 or 6 according to the Ishak scoring system[18]). The population of non-LT cirrhotic patients have received combined treatment with PEG-IFN a2a or a2b, at the dose of 180 mcg/wk or 1.5 mcg/kg per week respectively, and ribavirin 800-1200 mg, based on body weight and HCV genotype, for a whole duration of 24 wk (genotypes 2 and 3 HCV) or 48 wk (genotypes 1 and 4 HCV) according to the international guidelines[4]. On the other hand, the group of LT cirrhotic patients have undergone combined treatment with PEG-IFN a2a or a2b, at the dose of 180 mcg/wk or 1.5 mcg/kg per week respectively, and ribavirin 800-1200 mg based on body weight and HCV genotype, for a whole duration of 48 wk independently of HCV genotype, according to the international guidelines[4]. Patients treated before 2003 (17%) have been subjected to standard IFN alpha2b (3 million units thrice weekly) plus ribavirin.
Quantitative serum HCV-RNA determinations have been made available for all patients at week 12 after the beginning of treatment, at the end of treatment and 6 mo after the end of it. Early virological response (EVR) has been defined as undetectable or more than 2 log drop but detectable HCV RNA level at week 12, end of treatment virological response (EOT-VR) as negative HCV RNA at the end of treatment and SVR as negative HCV RNA 6 mo after the end of treatment.
Safety of antiviral treatment has been evaluated by the occurrence of adverse events, either treatment-related (fatigue, flu-like symptoms and depression, defined as “intolerance”; anemia and neutropenia, defined as “hematological toxicity”) or related to liver disease decompensation (ascites, encephalopathy and gastrointestinal bleeding, defined as “decompensation”). The severity of adverse events has been described according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.0[19] and is shown in Table 1. Grade 1 have been defined as transient, with mild discomfort, no limitation in activity and no need of medical intervention/therapy; grade 2 as a mild to moderate impairment in daily activity, no or minimal medical intervention/therapy required; grade 3 as a markedly reduction in daily activity, requiring medical intervention/therapy and possible hospitalization or hospice care; grade 4 as extreme limitation to daily activity, significant medical intervention/therapy required and hospitalization or hospice care very common; finally, grade 5 of adverse events always causes death.
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Anemia | Hb < LLN-10.0 g/dL | Hb 10.0-8.0 g/dL | Hb < 8.0-6.5 g/dL | Hb < 6.5 g/dL | Death |
| Neutropenia | PMN | PMN | PMN | PMN | Death |
| < LLN-1.5 × 109/L | < 1.5-1.0 × 109/L | < 1.0-0.5 × 109/L | < 0.5 × 109/L | ||
| Intolerance: | |||||
| Fatigue | Mild fatigue over baseline | Moderate or causing difficulty performing some ADL | Severe fatigue interfering with ADL | Disabling | |
| Flu-like symptoms | Symptoms present but not interfering with function | Moderate or causing difficulty performing some ADL | Severe symptoms interfering with ADL | Disabling | Death |
| Depression | Mild mood alteration not interfering with function | Moderate mood alteration interfering with function, but not interfering with ADL; medication indicated | Severe mood alteration interfering with ADL | Suicidal ideation; danger to self or others | Death |
Statistical analysis has been performed by reporting continuous variables as mean and standard deviation and categorical ones as absolute and relative frequencies.
Evaluated end-points were antiviral therapy outcome, overall survival and the occurrence of adverse events. Logistic regression has been used to assess the correlation between each variable and antiviral treatment outcome, and the Fisher’s exact test was applied to categorical variables to assess the differences between the two groups of cirrhotic LT and non-LT patients. The Kaplan-Meier curve has been employed to analyze patients’ survival and the differences between groups have been assessed using log-rank tests.
All tests were 2-sided and P≤ 0.05 has been considered to be statistically significant.
Baseline patients’ characteristics are shown in Table 2. Forty-three patients belonged to the group of non-LT cirrhotics, 17 to the group of LT ones. The majority of patients were male and 9 of them were 65-year-old or more (7 in LT and 2 in non-LT group). All patients were classified in Child-Pugh class A.
| Variable | Non-liver transplanted cirrhotic group frequency(n= 43) | Liver transplanted cirrhotic group frequency(n= 17) | P |
| Age, yr (mean ± SD) | 55 ± 8 (39-69) | 57 ± 9 (38-73) | 0.753 |
| Sex | 0.073 | ||
| Male | 28 (65.1) | 15 (88.2) | |
| Female | 15 (34.9) | 2 (11.8) | |
| Genotype | 0.750 | ||
| 1 | 2 (58.1) | 11(64.7) | |
| 2 | 13 (30.2) | 3 (17.6) | |
| 3 | 2 (4.7) | 31 (5.9) | |
| 4 | 3 (7) | 2 (11.8) | |
| Previous treatment | 16 (37.2) | 4 (23.5) | 0.069 |
| HCV RNA 800000 (UI/mL) | 15 | 9 | 0.198 |
| EVR | 30 (69.8) | 8 (47.1) | 0.100 |
| SVR | 18 (41.9) | 5 (29.4) | 0.371 |
Among non-LT cirrhotics, 16/43 (47.1%) experienced a previous unsuccessful antiviral treatment while the rate was 4/12 (33.3%) among the LT ones, for whom the data was available. Most of patients presented with genotype 1 HCV related chronic hepatitis and a high viral replication; HCV RNA levels ≥ 600000 UI/mL were indeed detected in 20/43 (46.5%) of non-LT cirrhotics and in 11/17 (64.7%) of LT cirrhotics.
An EVR has been reported respectively in 30/43 (69.8%) non-LT cirrhotic patients and in 8/17 (47.1%) LT cirrhotic ones, a SVR in 18/43 (41.9%) non-LT cirrhotic patients and 5/17 (29.4%) in LT cirrhotic ones. Among the investigated factors (age, sex, HCV genotype, viral load, previous treatment, EVR, treatment discontinuation and less than 80% of treatment) only EVR has seemed to have a positive effect on the achievement of SVR in both groups (P = 0.42; OR = 3.3, 95%CI: 0.072-2.287). Statistical analysis has not shown any difference in EVR and SVR rates between the two groups.
Table 3 indicates the general reported adverse events dealing with antiviral treatment safety assessment. No grade 4 or 5 adverse events have been recorded. Six cirrhotic patients (2 non-LT and 4 LT) experienced grade 2 anemia requiring erythropoietin administration, while grade 3 anemia non responsive to RBV dose reduction and erythropoietin administration caused treatment discontinuation in 2 LT cirrhotics. The incidence of grade 3 neutropenia has been reported in 6 non-LT and in 1 LT cirrhotic patients, and has been treated with granulocyte colony stimulating factors (G-CSF), while treatment discontinuation was required in 1 LT cirrhotic patient. Only 1 liver-related adverse event (encephalopathy) has occurred during the antiviral treatment in LT cirrhotic patients group.
| Non-liver transplanted cirrhotics | Liver transplanted cirrhotics | |
| Intolerance (fatigue, flu-like symptoms, depression) | ||
| Grade 2 | 1 | 0 |
| Grade 3 | 3 | 0 |
| Anemia | ||
| Grade 1 | 12 | 0 |
| Grade 2 | 2 | 4 |
| Grade 3 | 0 | 2 |
| Neutropenia | ||
| Grade 2 | 0 | 2 |
| Grade 3 | 6 | 1 |
| Liver decompensation | ||
| Grade 1 (encephalopathy) | 0 | 1 |
| Other | ||
| Hepatocellular carcinoma | 2 | 0 |
| Polytrauma | 1 | 0 |
Overall, among HCV non-LT cirrhotics 6/43 (13.9%) patients prematurely discontinued the treatment: 2 due to the development/recurrence of hepatocellular carcinoma, 3 for grade 3 fatigue, 1 for polytrauma. Then, 5 of them (11.6%) received less than 80% of the intended treatment. None of the 6 patients who discontinued the treatment achieved a SVR, except of 2 of them who received 41 and 35 wk of treatment.
Among HCV LT cirrhotics 8/17 (47%) withdrew the treatment (4 for lack of response and 1 for early relapse, 2 for anemia and 1 for severe neutropenia), 6 of them (35.2%) receiving less than 80% of the intended treatment. None of the 6 patients who discontinued the treatment achieved a SVR, except 2 of them who received 40 and 43 wk of treatment. As a result, we found that LT cirrhotic patients present a higher risk of treatment discontinuation when compared with non-LT ones (P = 0.015, Fisher’s exact test) and undergo more frequently less than 80% of the intended treatment (P = 0.05, Fisher’s exact test).
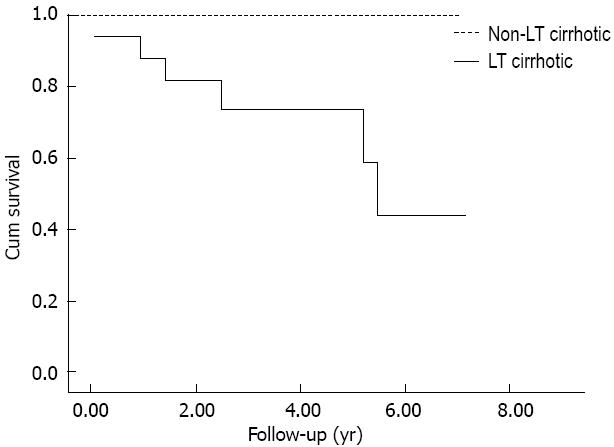
Patients were followed-up until December 2009. None of the non-LT cirrhotics died and the mean follow-up was of 4.3 ± 1.8 years after the end of the treatment; among LT cirrhotics, 6 deaths occurred (one due to extrahepatic causes, 1 due to sepsis, and 4 due to liver insufficiency), with survival rates of 87% at 1 year and of 76% at 3 and 5 years after the end of treatment (mean follow-up of 3.3 ± 2.2 years, Figure 1).
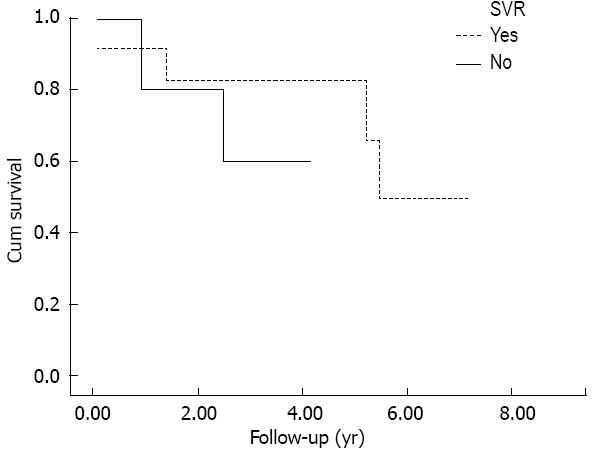
With no regards to the achievement of SVR, LT cirrhotic patients (Figure 1) showed a reduced survival in respect to non-LT ones. Moreover, SVR didn’t show to have a beneficial effect in terms of survival: Kaplan-Meier curve didn’t show any difference between LT-cirrhotic patients who achieved SVR in respect to those who did not (P < 0.463, log-rank test, Figure 2).
International guidelines recommend to treat HCV hepatitis in non-LT cirrhotic patients, especially those with an early stage liver disease (Child-Turcotte-Pugh, CTP, score A or B7), with genotype 2 HCV infection and in absence of contraindications, since the achievement of SVR is possible in about one third of them[4,7-11,20].
Data previously published about the efficacy of HCV treatment in this subgroup of patients have been confirmed by our series, which includes patients affected by compensated liver cirrhosis (CTP A). In non-LT cirrhotic patients, the achievement of a SVR seems related to an improvement of overall survival[8] and 5-year “event-free” survival, also reducing the risk of liver decompensation[7,8]. Moreover, an improvement in liver function has been reported in SVR patients (CTP 7.8 vs 6.4) in respect to non-responders (CTP 8.0 vs 8.7) and to controls (CTP 8.3 vs 9.4)[7]. Finally, a SVR to combined HCV antiviral treatment preempts liver graft re-infection for those patients who will undergo LT[4,9,10,21,22].
However, at present, data regarding the impact of SVR on survival of cirrhotic patients undergoing HCV antiviral treatment after liver transplantation, are scarce. As it can be shown by our analysis, independently of the achievement of SVR, LT cirrhotic patients have a worse survival if compared to non-LT ones (Figure 1, P < 0.0001). In except of 1 case of an infection, which could be easily explained in immunosuppressed patients such as LT ones, the most common reported cause of death in our series was liver insufficiency, in agreement with literature data[14,23].
Furthermore, no difference in survival has been observed between LT cirrhotic patients who achieved a SVR and those who did not. More to the point, it could be interesting to compare our results in terms of survival with the existing data regarding the natural history of post-liver transplant HCV cirrhosis recurrence, in patients who did not receive an antiviral treatment. Berenguer et al[1] followed-up 49 patients with genotype 1b HCV recurrent cirrhosis after liver transplant. The 1-year survival rate of those patients with clinically compensated HCV-graft cirrhosis (39/49) was of 74%, lower than that reported by our study (87%, Figure 1). Firpi et al[23] reported the natural history of 88 HCV LT cirrhotic patients, the major part clinically compensated and of genotype 1; the cumulative probability of survival was 83% at 1 year and 41% at 5 years from the time of diagnosis of compensated cirrhosis. Nevertheless, for those patients who remained free from decompensation, the reported survival was of 90% at 1 year and 70% at 5 years, which is comparable to that reported by our study. Finally, in a series of 55 HCV LT recipients with recurrent cirrhosis, 70 with genotype 1 and 28 with genotype 2-3, Kalambokis et al[24] reported 90% and 74% rates of survival at 1 and 5 years in those subjects who did not developed liver decompensation.
Then, in the subgroup of LT patients with recurrent HCV-related compensated liver cirrhosis, the achievement of SVR to the antiviral treatment is not able to modify the natural course of the liver disease and, therefore, to improve patients’ survival. Probably, the fact that in the liver transplant setting the course of liver cirrhosis has a more rapid and unavoidable progression, difficult to be stopped even with the eradication of HCV infection, is the main reason to support this evidence. Berenguer et al[25] assessed that the rate of post-transplantation fibrosis progression was significantly higher than pre-transplantation [0.2/year (0.09-0.8)]. The suppression of immune system, on one hand, confirmed by the reported histologic improvements due to immunosuppression weaning-off[26], and the tendency to develop a mild, persistent chronic damage due to rejection, on the other hand, may be the main factors affecting the natural course of post-liver transplant HCV-related cirrhosis. However, the impact of immunosuppression weaning-off on liver histology decreases during the time[27]. Moreover, systematic reviews didn’t show any significant relationship between IFN treatment and acute or chronic rejection, with incidence rates comparable to the whole population of LT recipients[28,29]. Pooling the results of 6 studies[17,30-33], rejection rates seem higher (11%-21% acute rejection; 9%-17% chronic rejection), and a recent review by Selzner et al[34] reported acute and chronic rejection rates of 0%-35% and 4%-8%, respectively.
It is also well-known that HCV infection itself could be associated with autoimmune diseases (cryoglobulinemia, Sjogren like syndrome, anti-nucleus or anti-liver kidney microsomes antibodies positivity)[34]. It has been hypothesized that this kind of liver damage could be a variant of rejection rather than the manifestation of a de novo autoimmune hepatitis[35]. Several cases following HCV antiviral treatment have been reported[36,37], probably related to the various IFN and RBV immune-modulating effects and often developing after HCV clearance[34].
In conclusion, HCV infection control in liver allografts is certainly linked to the stimulation of immune system, dealing with the risk of developing acute or chronic rejection or de novo autoimmune liver damage. These kind of damage may impact negatively on the progression of liver cirrhosis, already faster in LT recipients, even in case of SVR to antiviral treatment. We are, at present, still far from a complete knowledge of the mechanism underling allograft acceptance or HCV clearance.
Among non-LT cirrhotic patients, the reported combined antiviral treatment discontinuation rates are widely variable. In a large series of 568 non-LT cirrhotic patients by Fernández-Rodríguez et al[8] a 29.6% rate of treatment discontinuation due to adverse events was reported, while in 19.2% and 17.4% of them, respectively, a PEG-IFN or RBV dose reduction was necessary. The overall reported drop-out rate was 66%. In decompensated cirrhotic patients, rates of treatment withdrawal or dose reduction due to adverse events seems almost comparable: 59% by Iacobellis et al[7], 65% by Everson et al[22], 87% by Crippin et al[11], 30% by Forns et al[10].
With regards to LT cirrhotics undergoing combined antiviral treatment, Berenguer et al[16] reported respectively a 37% rate of discontinuation, and a 45% and a 36% rates of PEG-IFN or RBV dose reduction. In the study by Carrión et al[14], treatment discontinuation rate was of 39% while 67% and 24% of patients experienced RBV or PEG-IFN dose reduction. Angelico et al[17] reported a 33% drop-out rate, and a 45% rate of RBV and a 38% rate of PEG-IFN dose reduction.
In non-cirrhotic LT patients, the most frequently reported causes of treatment withdrawal are cytopenia, in particular anemia, neuropsychiatric symptoms, thyroid dysfunction, intolerance and rejection[12,14,16]. However, these studies included LT patients with HCV hepatitis recurrence after liver transplant but without liver cirrhosis; data about LT cirrhotic patients are scarce, but it is likely to expect a higher incidence of adverse events and a consequent higher rate of treatment dose reduction or discontinuation.
In our study, we did not observe the occurrence of any severe or fatal adverse event (grade 4 or 5). Thirteen patients have received supportive therapy with growth factors due to hematological adverse events, such as anemia and leucopenia, and 3 LT cirrhotics withdrew the treatment. None among LT cirrhotics experienced fatigue, flue-like symptoms and depression, while one grade 2 and three grade 3 adverse events (requiring treatment discontinuation) occurred among non-LT ones.
Overall, as a consequence of reduced tolerance to treatment, LT cirrhotics included in the present analysis reported a higher rate of discontinuation than non-LT ones (47% LT vs 13.9% non-LT; P = 0.015), thus receiving less than 80% of the treatment in a larger proportion of cases (35.2% LT vs 11.6% non-LT; P = 0.05). It is also interesting that one half of non-LT cirrhotic patients discontinued the treatment due to adverse events unrelated to the treatment itself, such as polytrauma or hepatocellular carcinoma recurrence, while after LT treatment discontinuation was almost related to treatment side effects.
Therefore, LT cirrhotic patients have a higher risk to receive an overall treatment dose is not adequate to achieve a SVR. Indeed, none of the 8 LT cirrhotic patients who discontinued the treatment achieved a SVR, in except of two of them who received respectively 40 and 43 wk of treatment; similarly, among non-LT cirrhotics who discontinued the treatment, only the two patients who underwent 41 and 35 wk of treatment achieved a SVR.
Our series shows that the survival of LT compensated HCV cirrhotic patients is lower if compared to non LT ones; nevertheless, the achievement of SVR seems to have no effect on the natural history of these patients and to not improve survival. Moreover, LT HCV cirrhotic patients have a worse tolerance to antiviral treatment than non-LT ones and experience more frequently adverse events. In this era of important changes in the paradigms and drugs employed in HCV antiviral treatment[38,39], some issues about the new incoming drugs could be raised. Indeed, the real gain in risk/benefit ratio of any antiviral treatment should be carefully evaluated. However, the retrospective nature of the present study, and the discrepancy between the sample size of non-LT and LT cirrhotic patients, make possible to provide speculative considerations only. Future prospective studies evaluating the effects of SVR on the long-term outcome of LT HCV cirrhotic patients, and assessing the efficacy and safety of the new therapeutic regimens in LT HCV cirrhotic patients are necessary to “bring light into the dark”, and to develop specific guidelines for clinical practice.
We would like thank AISF RECOLT-C Group: Raffaella Viganò Niguarda Ca’ Granda Hospital-Milan, Rosa Maria Iemmolo University of Modena -Modena, Maria Francesca Donato IRCCS Foundation Ca’ Granda Maggiore Hospital-Milan, Maria Rendina University of Bari-Bari, Pierluigi Toniutto University of Udine-Udine, Luisa Pasulo, Stefano Fagiuoli Ospedali Riuniti-Bergamo, Matteo Cescon Sant’Orsola Malpighi Hospital-Bologna, Patrizia Burra University of Padua-Padua, Eleonora De Martin University of Padua-Padua, Lucia Miglioresi San Camillo Spallanzani Hospital-Rome, Manuela Merli Sapienza University-Rome, Valerio Giannelli Sapienza University-Rome, Daniele Di Paolo University of Torvergata-Rome.
Hepatitis C virus (HCV)-related hepatitis is one of the leading causes of end-stage liver disease worldwide, accounting for half of liver transplanted (LT) in many centers. Antiviral treatment should be considered for patients with compensated cirrhosis in order to prevent short to mid-term complications. The results of antiviral treatment with pegylated interferon plus ribavirin in patients presenting with compensated liver cirrhosis are worse than in non-cirrhotic ones. However, cirrhotics who experience a favorable response to antiviral treatment show an improved survival rate in respect to non-responders (98% vs 86% at 5 years, respectively, for sustained virological response (SVR) and a lower risk of decompensation.
The research hotspot is to compare the efficacy and safety of antiviral treatment in LT and non-LT cirrhotic patients, and to understand how it can affect patients’ survival, especially after liver transplantation.
At present, no data regarding the impact of SVR on survival of cirrhotic patients undergoing HCV antiviral treatment after LT are available in literature. The natural history of the underlying liver disease may heavily affect cirrhotic patients’survival, independently of the achievement of a sustained virological response to antiviral therapy. HCV infection control in liver allografts is certainly linked to the stimulation of immune system, dealing with the risk of developing acute or chronic rejection or de novo autoimmune liver damage. These kind of damage may impact negatively on the progression of liver cirrhosis, already faster in LT recipients, even in case of sustained virological response to antiviral treatment. The present study highlights that cirrhotic patients who undergo antiviral treatment after LT have a worse prognosis, compared to non-LT ones, independently of the achievement of SVR.
The present study suggests that cirrhotic patients have a worse response to antiviral treatment, especially after LT. Probably, a SVR to antiviral treatment could not be able to protect patients with such an advanced liver damage and a complex alteration of the immune response from an unfavorable evolution of the disease.
SVR is the negativization of HCV RNA 6 mo after the end of antiviral treatment.
This is an interesting paper comparing the safety and efficacy of antiviral treatment in patients with HCV infection and cirrhosis before and after transplantation.
P- Reviewers Grattagliano I, Mudawi HMY, Tziomalos K S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Berenguer M, Prieto M, Rayón JM, Mora J, Pastor M, Ortiz V, Carrasco D, San Juan F, Burgueño MD, Mir J. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32:852-858. [PubMed] [DOI] [Full Text] |
| 2. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 3. | Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology. 1999;29:1311-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] |
| 5. | Bartosch B, Thimme R, Blum HE, Zoulim F. Hepatitis C virus-induced hepatocarcinogenesis. J Hepatol. 2009;51:810-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1060] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 7. | Iacobellis A, Siciliano M, Perri F, Annicchiarico BE, Leandro G, Caruso N, Accadia L, Bombardieri G, Andriulli A. Peginterferon alfa-2b and ribavirin in patients with hepatitis C virus and decompensated cirrhosis: a controlled study. J Hepatol. 2007;46:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Fernández-Rodríguez CM, Alonso S, Martinez SM, Forns X, Sanchez-Tapias JM, Rincón D, Rodriguez-Caravaca G, Bárcena R, Serra MA, Romero-Gómez M. Peginterferon plus ribavirin and sustained virological response in HCV-related cirrhosis: outcomes and factors predicting response. Am J Gastroenterol. 2010;105:2164-2172; quiz 2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, Ray C. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 10. | Forns X, García-Retortillo M, Serrano T, Feliu A, Suarez F, de la Mata M, García-Valdecasas JC, Navasa M, Rimola A, Rodés J. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003;39:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Crippin JS, McCashland T, Terrault N, Sheiner P, Charlton MR. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus-infected patients awaiting liver transplantation. Liver Transpl. 2002;8:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Picciotto FP, Tritto G, Lanza AG, Addario L, De Luca M, Di Costanzo GG, Lampasi F, Tartaglione MT, Marsilia GM, Calise F. Sustained virological response to antiviral therapy reduces mortality in HCV reinfection after liver transplantation. J Hepatol. 2007;46:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Berenguer M, Palau A, Aguilera V, Rayón JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008;8:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Carrión JA, Navasa M, García-Retortillo M, García-Pagan JC, Crespo G, Bruguera M, Bosch J, Forns X. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132:1746-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Dumortier J, Scoazec JY, Chevallier P, Boillot O. Treatment of recurrent hepatitis C after liver transplantation: a pilot study of peginterferon alfa-2b and ribavirin combination. J Hepatol. 2004;40:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Berenguer M, Palau A, Fernandez A, Benlloch S, Aguilera V, Prieto M, Rayón JM, Berenguer J. Efficacy, predictors of response, and potential risks associated with antiviral therapy in liver transplant recipients with recurrent hepatitis C. Liver Transpl. 2006;12:1067-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Angelico M, Petrolati A, Lionetti R, Lenci I, Burra P, Donato MF, Merli M, Strazzabosco M, Tisone G. A randomized study on Peg-interferon alfa-2a with or without ribavirin in liver transplant recipients with recurrent hepatitis C. J Hepatol. 2007;46:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3783] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 19. | Available from: http://ctep.cancer.gov. |
| 20. | Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, Rimola A, Rodes J. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 381] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 22. | Everson GT, Kulig CC. Antiviral therapy for hepatitis C in the setting of liver transplantation. Curr Treat Options Gastroenterol. 2006;9:520-529. [PubMed] |
| 23. | Firpi RJ, Clark V, Soldevila-Pico C, Morelli G, Cabrera R, Levy C, Machicao VI, Chaoru C, Nelson DR. The natural history of hepatitis C cirrhosis after liver transplantation. Liver Transpl. 2009;15:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Kalambokis G, Manousou P, Samonakis D, Grillo F, Dhillon AP, Patch D, O’Beirne J, Rolles K, Burroughs AK. Clinical outcome of HCV-related graft cirrhosis and prognostic value of hepatic venous pressure gradient. Transpl Int. 2009;22:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, Córdoba J, Herola A, Ascher N, Mir J. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32:673-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 595] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 26. | Tisone G, Orlando G, Cardillo A, Palmieri G, Manzia TM, Baiocchi L, Lionetti R, Anselmo A, Toti L, Angelico M. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. J Hepatol. 2006;44:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Orlando G, Manzia T, Baiocchi L, Sanchez-Fueyo A, Angelico M, Tisone G. The Tor Vergata weaning off immunosuppression protocol in stable HCV liver transplant patients: the updated follow up at 78 months. Transpl Immunol. 2008;20:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Gurusamy KS, Tsochatzis E, Davidson BR, Burroughs AK. Antiviral prophylactic intervention for chronic hepatitis C virus in patients undergoing liver transplantation. Cochrane Database Syst Rev. 2010;CD006573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Wang CS, Ko HH, Yoshida EM, Marra CA, Richardson K. Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: a review and quantitative analysis. Am J Transplant. 2006;6:1586-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Walter T, Dumortier J, Guillaud O, Hervieu V, Paliard P, Scoazec JY, Boillot O. Rejection under alpha interferon therapy in liver transplant recipients. Am J Transplant. 2007;7:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Stanca CM, Fiel MI, Kontorinis N, Agarwal K, Emre S, Schiano TD. Chronic ductopenic rejection in patients with recurrent hepatitis C virus treated with pegylated interferon alfa-2a and ribavirin. Transplantation. 2007;84:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Saab S, Kalmaz D, Gajjar NA, Hiatt J, Durazo F, Han S, Farmer DG, Ghobrial RM, Yersiz H, Goldstein LI. Outcomes of acute rejection after interferon therapy in liver transplant recipients. Liver Transpl. 2004;10:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Fernández I, Ulloa E, Colina F, Abradelo M, Jiménez C, Gimeno A, Meneu JC, Lumbreras C, Solís-Herruzo JA, Moreno E. Incidence, risk factors, and outcome of chronic rejection during antiviral therapy for posttransplant recurrent hepatitis C. Liver Transpl. 2009;15:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Selzner N, Guindi M, Renner EL, Berenguer M. Immune-mediated complications of the graft in interferon-treated hepatitis C positive liver transplant recipients. J Hepatol. 2011;55:207-217. [PubMed] |
| 35. | Fiel MI, Agarwal K, Stanca C, Elhajj N, Kontorinis N, Thung SN, Schiano TD. Posttransplant plasma cell hepatitis (de novo autoimmune hepatitis) is a variant of rejection and may lead to a negative outcome in patients with hepatitis C virus. Liver Transpl. 2008;14:861-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Cholongitas E, Samonakis D, Patch D, Senzolo M, Burroughs AK, Quaglia A, Dhillon A. Induction of autoimmune hepatitis by pegylated interferon in a liver transplant patient with recurrent hepatitis C virus. Transplantation. 2006;81:488-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Kontorinis N, Agarwal K, Elhajj N, Fiel MI, Schiano TD. Pegylated interferon-induced immune-mediated hepatitis post-liver transplantation. Liver Transpl. 2006;12:827-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Lange CM, Zeuzem S. Perspectives and challenges of interferon-free therapy for chronic hepatitis C. J Hepatol. 2013;58:583-592. [PubMed] |
| 39. | Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 803] [Cited by in RCA: 844] [Article Influence: 60.3] [Reference Citation Analysis (0)] |