Published online May 28, 2013. doi: 10.3748/wjg.v19.i20.3161
Revised: March 13, 2013
Accepted: April 3, 2013
Published online: May 28, 2013
Processing time: 154 Days and 18.4 Hours
We present the first case of an intraductal papillary neoplasm of the bile duct (IPNB) accompanying a mixed adenoneuroendocrine carcinoma (MANEC). A 74-year-old woman presented with fever of unknown cause. Laboratory data revealed jaundice and liver injury. Contrast-enhanced computed tomography revealed a 20 mm polypoid tumor in the dilated distal bile duct, which exhibited early enhancement and papillary growth. Upper gastrointestinal endoscopy revealed mucus production from the papilla of Vater, characterized by its protruding and dilated orifice. Endoscopic ultrasonography visualized the polypoid tumor in the distal bile duct, but no invasive region was suggested by diagnostic imaging. Therefore, the initial diagnosis was IPNB. After endoscopic nasobiliary drainage, a pylorus-preserving pancreaticoduodenectomy was performed. Pathological examination of the resected bile duct revealed papillary proliferation of biliary-type cells with nuclear atypia, indicating pancreaticobiliary-type IPNB. In addition, solid portions comprised of tumor cells with characteristic salt-and-pepper nuclei were evident. Immunohistochemistry revealed expression of the neuroendocrine marker synaptophysin in this solid component, diagnosing it as a neuroendocrine tumor (NET). Furthermore, the MIB-1 proliferation index of NET was higher than that of IPNB, and microinvasion of the NET component was found, indicating neuroendocrine carcinoma (NET G3). This unique case of MANEC, comprising IPNB and NET, provides insight into the pathogenesis of biliary NET.
Core tip: A 74-year-old woman presented with fever and jaundice. Computed tomography revealed a polypoid tumor in the dilated distal bile duct. Pylorus-preserving pancreaticoduodenectomy was performed. Pathological examination revealed the papillary proliferation of biliary-type cells with nuclear atypia in the dilated bile duct, indicating papillary neoplasm of the bile duct. A solid portion comprised of tumor cells with characteristic salt-and-pepper nuclei was found. Immunohistochemistry revealed synaptophysin expression in the solid portion, diagnosing it as a neuroendocrine tumor (NET). This case provides insight into the pathogenesis of biliary NET.
- Citation: Onishi I, Kitagawa H, Harada K, Maruzen S, Sakai S, Makino I, Hayashi H, Nakagawara H, Tajima H, Takamura H, Tani T, Kayahara M, Ikeda H, Ohta T, Nakanuma Y. Intraductal papillary neoplasm of the bile duct accompanying biliary mixed adenoneuroendocrine carcinoma. World J Gastroenterol 2013; 19(20): 3161-3164
- URL: https://www.wjgnet.com/1007-9327/full/v19/i20/3161.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i20.3161
Intraductal papillary neoplasm of the bile duct (IPNB) is a new entity, defined as biliary neoplasms showing papillary or villous proliferation within the dilated lumens of intrahepatic and extrahepatic bile ducts by the 2010 World Health Organization (WHO) Classification of Tumors of the Digestive System[1]. IPNB encompasses several lesions, which were previously categorized as biliary papilloma, papillomatosis, papillary adenocarcinoma of the bile duct, and intraductal growth-type cholangiocarcinoma. The following three key pathologic features are characteristically evident in IPNB in varying combinations: (1) exophytic and papillary proliferation of neoplastic biliary epithelial cells, with delicate fibrovascular stalks within bile duct lumens; (2) mucin hypersecretion (macroscopic mucin is evident in some cases); and (3) variable dilatation or multilocular cystic changes of affected bile ducts.
Neuroendocrine tumors (NETs), including carcinoid tumors, are commonly found in several organs, including the pancreas and gastrointestinal tract. Most biliary NETs exist as a component of mixed adenoneuroendocrine carcinomas (MANECs)[2]. MANECs are found in hepatic hilar cholangiocarcinomas with hepatolithiasis, gallbladder cancers, and extrahepatic cholangiocarcinomas, and show a characteristic histology[3]. Moreover, since the NET components of biliary MANECs define prognosis, it is important to identify them and consider indications for adjunctive therapy, such as somatostatin analogues.
We encountered a patient with IPNB accompanying a NET in the extrahepatic bile ducts. This case is unique, has a different histology from most biliary MANECs, and provides insight into the histogenesis of NETs in biliary tumors.
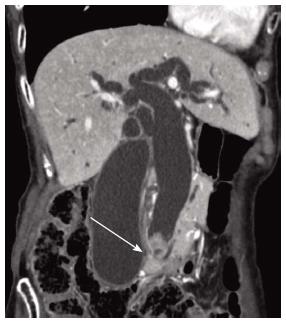
A 74-year-old woman consulted a family physician for fever of unknown cause. Laboratory data revealed jaundice and liver injury. Computed tomography (CT) revealed an elevated lesion in the distal bile duct. She was admitted to our hospital for further examination and treatment. Enhanced CT revealed a 20 mm polypoid tumor, which exhibited early enhancement and papillary growth (Figure 1). Magnetic resonance image (MRI) and magnetic resonance cholangiopancreatography (MRCP) confirmed these findings. Mucus production was evident from the papilla of Vater, characterized by its protruding and dilated orifice. Endoscopic ultrasonography revealed a polypoid tumor in the distal bile duct. No invasive tumor regions were detected using these imaging techniques. Therefore, the initial diagnosis was IPNB. After endoscopic nasobiliary drainage (ENBD), a pylorus-preserving pancreaticoduodenectomy was performed. The postoperative course was uneventful, except for slight pneumonia.
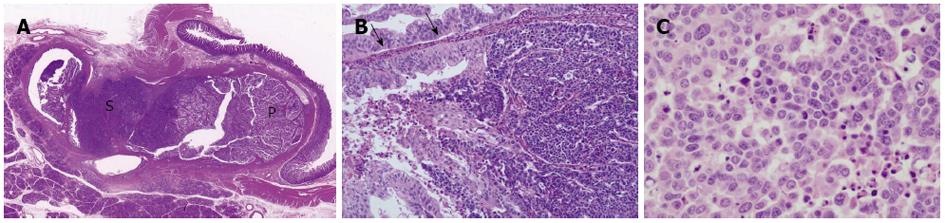
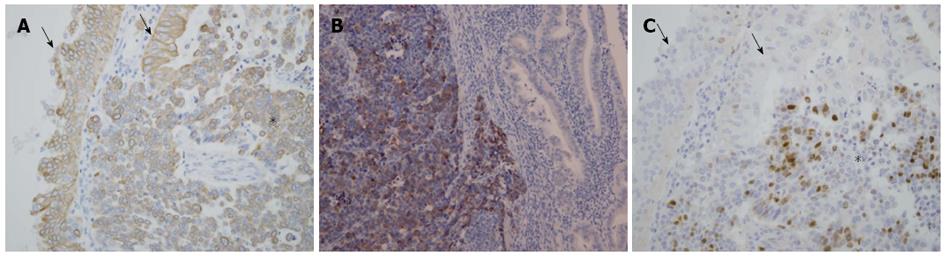
A whitish tumor occupying the dilated lumen of the distal bile duct was found, and the proximal portion of the bile duct was also dilated. As shown in Figure 2A, the tumor was comprised of two different areas: papillary and solid. The papillary proliferating area consisted of cholangiocyte-like columnar epithelial cells covering fine fibrovascular cores, and showed moderate-to high-grade intraepithelial neoplasia, indicating pancreaticobiliary-type IPNB (high-grade intraepithelial neoplasia) according to the WHO classification[1] (Figure 2B). Immunohistochemistry revealed expression of the biliary-type cytokeratin (CK): CK19 (Figure 3A) in these cells, but not CK20, confirming biliary-type IPNB. Conversely, the solid area lacking acinar/glandular structure (Figure 2A and B) was comprised of tumor cells with salt-and-pepper nuclei, a high nucleus-to-cytoplasm ratio, and increased nuclear chromatin (Figure 2C). Immunohistochemistry for neuroendocrine markers revealed synaptophysin expression (Figure 3B), but not chromogranin A and CD56 expression, indicating a NET component of MANEC. Furthermore, NET, as well as IPNB components, expressed CK19 (Figure 3A). The MIB-1 proliferation index of the NET component was significantly higher than that of the IPNB component (Figure 3C). Focal invasion of NET and IPNB components into the ductal wall was evident, but no pancreatic or lymph node metastasis was observed.
IPNB is characterized by a grossly visible, exophytic proliferation of neoplastic cholangiocytes with delicate fibrovascular cores. The WHO classification of digestive tumors[1] recognizes IPNB as a precancerous entity of cholangiocarcinoma. Surgical resection is the most optimal treatment for IPNB because mucin production by these tumors causes recurrent cholangitis and obstructive jaundice, even if these tumors are considered benign. Incomplete surgical resection often causes tumor recurrence; furthermore, recurrence in the remaining bile ducts can develop after apparently complete resection of even noninvasive tumors because of tumor multifocality[4,5].
Radiographic imaging modalities, including CT, MRI, and MRCP, are recommended noninvasive techniques for detecting masses and evaluating the tumor extent. Although cholangiography and cholangioscopy carry risks of complications, these diagnostic techniques are useful for evaluating tumor extent, operative planning, and biliary intervention[6,7]. In this case, it was not difficult to evaluate the degree of tumor invasion using noninvasive radiographic techniques. However, pathological confirmation could not be obtained via biopsies or cytology. Peroral cholangioscopy and intraductal ultrasonography may be necessary for pathological diagnosis. Unfortunately, we were unable to perform these invasive radiographies due to a lack of consent from the patient, who was suffering from pancreatitis after ENBD.
The degree of malignancy and histological atypia of IPNB ranges from borderline or low to moderate in the intraluminal papillary portions, to highly malignant and atypical in the invasive portions. IPNBs without invasive components are comprised of two subgroups: low-grade intraepithelial neoplasia and high-grade intraepithelial neoplasia. Moreover, the neoplastic cells of IPNB are cytologically classifiable into four phenotypes: pancreaticobiliary, intestinal, gastric (clear cell type), and oncocytic types[1]. This case was IPNB with associated pancreaticobiliary-type invasive carcinoma.
NETs in the digestive system, including in the gallbladder and extrahepatic bile ducts, are classified as: NET G1 [carcinoid; mitotic count: < 2 per 10 high-power fields (HPF); and/or ≤ 2% Ki-67 index]; NET G2 (mitotic count: 2-20 per 10 HPF; and/or 3%-20% Ki-67 index); neuroendocrine carcinoma (large- or small-cell type); and MANEC, according to the 2010 WHO classification[2,8]. In biliary MANEC, the adenocarcinomatous component is located on the surface of the main tumor, and the majority of stromal invasion, including vascular invasion and lymph node metastasis, involves the NET component[3]. Therefore, the NET component of biliary MANEC is considered to define prognosis[3]. In this case, contrary to typical biliary MANEC, the adenocarcinomatous component of MANEC was identified as IPNB. NET adjacent to the IPNB component resulted in both being intermingled in one mass (Figure 2B). Moreover, both NET and IPNB were immunophenotypically positive for CK19, suggesting that NET originated from the transformation of biliary tumor cells constituting IPNB. However, the NET component exhibited extensive proliferation and broad stromal invasion of the bile duct wall compared with IPNB, suggesting that the NET component defined the prognosis in this case.
In the pancreas, concomitant intraductal papillary mucinous neoplasm (IPMN; a pancreatic counterpart of IPNB) and pancreatic neuroendocrine tumor (pNET) are frequent, and the prevalence of pNET with IPMN was 2.8%-4.6% according to previous reports[9,10]. However, IPNB accompanying NET has not been previously reported. This case provides important insight into the histogenesis of biliary NET.
In summary, IPNB accompanying biliary MANEC in this patient provided important information for the elucidation of the histogenesis of NET in biliary tumors.
P- Reviewer Folsch UR S- Editor Zhai HH L- Editor Rutherford A E- Editor Lu YJ
| 1. | Nakanuma Y, Curado MP, Franceschi S, Gores G, Paradis V, Sripa B, Tsui WMS, Wee A. Intrahepatic cholangiocarcinoma. WHO Classification of Tumors of the Digestive System, 4th ed. Lyon: IARC Press 2010; 217-224. |
| 2. | Komminoth P, Arnold R, Capella C, Klimstra DS, Kloppel G, Rindi G, Albores-Saavedra J. Neuroendocrine neoplasms of the gallbladder and extrahepatic bile ducts. WHO Classification of Tumors of the Digestive System, 4th ed. Lyon: IARC Press 2010; 274-276. |
| 3. | Harada K, Sato Y, Ikeda H, Maylee H, Igarashi S, Okamura A, Masuda S, Nakanuma Y. Clinicopathologic study of mixed adenoneuroendocrine carcinomas of hepatobiliary organs. Virchows Arch. 2012;460:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, Furukawa K, Mitsuhashi N, Takeuchi D, Takayashiki T. Surgical strategy for mucin-producing bile duct tumor. J Hepatobiliary Pancreat Sci. 2010;17:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Vibert E, Dokmak S, Belghiti J. Surgical strategy of biliary papillomatosis in Western countries. J Hepatobiliary Pancreat Sci. 2010;17:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Lim JH, Jang KT. Mucin-producing bile duct tumors: radiological-pathological correlation and diagnostic strategy. J Hepatobiliary Pancreat Sci. 2010;17:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Tsuyuguchi T, Sakai Y, Sugiyama H, Miyakawa K, Ishihara T, Ohtsuka M, Miyazaki M, Yokosuka O. Endoscopic diagnosis of intraductal papillary mucinous neoplasm of the bile duct. J Hepatobiliary Pancreat Sci. 2010;17:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Rindi G, Klimstra DS, Arnold R, Capella C, Klimstra DS, Kloppel G, Komminoth P, and Solcia E. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. WHO Classification of Tumors of the Digestive System, 4th ed. Lyon: IARC Press 2010; 13-14. |
| 9. | Zhao X, Stabile BE, Mo J, Wang J, French SW. Nesidioblastosis coexisting with islet cell tumor and intraductal papillary mucinous hyperplasia. Arch Pathol Lab Med. 2001;125:1344-1347. [PubMed] |
| 10. | Goh BK, Ooi LL, Kumarasinghe MP, Tan YM, Cheow PC, Chow PK, Chung YF, Wong WK. Clinicopathological features of patients with concomitant intraductal papillary mucinous neoplasm of the pancreas and pancreatic endocrine neoplasm. Pancreatology. 2006;6:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |