Published online May 28, 2013. doi: 10.3748/wjg.v19.i20.3083
Revised: October 29, 2012
Accepted: January 11, 2013
Published online: May 28, 2013
Processing time: 41 Days and 0.6 Hours
AIM: To analyze whether high-intensity focused ultrasound (HIFU) ablation is an effective bridging therapy for patients with hepatocellular carcinoma (HCC).
METHODS: From January 2007 to December 2010, 49 consecutive HCC patients were listed for liver transplantation (UCSF criteria). The median waiting time for transplantation was 9.5 mo. Twenty-nine patients received transarterial chemoembolization (TACE) as a bringing therapy and 16 patients received no treatment before transplantation. Five patients received HIFU ablation as a bridging therapy. Another five patients with the same tumor staging (within the UCSF criteria) who received HIFU ablation but not on the transplant list were included for comparison. Patients were comparable in terms of Child-Pugh and model for end-stage liver disease scores, tumor size and number, and cause of cirrhosis.
RESULTS: The HIFU group and TACE group showed no difference in terms of tumor size and tumor number. One patient in the HIFU group and no patient in the TACE group had gross ascites. The median hospital stay was 1 d (range, 1-21 d) in the TACE group and two days (range, 1-9 d) in the HIFU group (P < 0.000). No HIFU-related complication occurred. In the HIFU group, nine patients (90%) had complete response and one patient (10%) had partial response to the treatment. In the TACE group, only one patient (3%) had response to the treatment while 14 patients (48%) had stable disease and 14 patients (48%) had progressive disease (P = 0.00). Seven patients in the TACE group and no patient in the HIFU group dropped out from the transplant waiting list (P = 0.559).
CONCLUSION: HIFU ablation is safe and effective in the treatment of HCC for patients with advanced cirrhosis. It may reduce the drop-out rate of liver transplant candidate.
- Citation: Cheung TT, Fan ST, Chan SC, Chok KS, Chu FS, Jenkins CR, Lo RC, Fung JY, Chan AC, Sharr WW, Tsang SH, Dai WC, Poon RT, Lo CM. High-intensity focused ultrasound ablation: An effective bridging therapy for hepatocellular carcinoma patients. World J Gastroenterol 2013; 19(20): 3083-3089
- URL: https://www.wjgnet.com/1007-9327/full/v19/i20/3083.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i20.3083
Deceased donor liver transplantation provides one of the best treatments to patients with hepatocellular carcinoma (HCC) and cirrhosis. The numbers of donations and cases performed are on a rising trend. However, the scarcity of liver grafts in many parts of the world, especially Asia, leads to a significant dropout rate of patients from liver transplant waiting lists, particularly patients with HCC and a low model for end-stage liver disease (MELD) score[1]. In order to reduce the dropout rate, different bridging therapies have been proposed. Among them, transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) are most popular. Despite of treatment applied before liver transplantation, the dropout rate for TACE ranged from 15% to 35% in different studies[1,2]. RFA seems to have produced better results but the dropout rate also ranged from 5.8% to 14% in various studies[3,4].
High-intensity focused ultrasound (HIFU) ablation is one of the latest treatments. It provides a totally non-invasive therapy to HCC and is viable even in patients with severe cirrhosis. In one of our previous studies, it achieved a complete ablation rate of 82.4% for HCCs smaller than 3 cm in with just one treatment session. It is well tolerated even in patients with advanced cirrhosis and age[5]. The current study is the first study that investigates whether HIFU therapy can be safely performed in HCC patients with cirrhosis and whether it can reduce the dropout rate of liver transplant candidates.
From January 2007 to December 2010, 49 consecutive HCC patients were listed for deceased donor liver transplantation (UCSF criteria). The diagnosis of HCC was confirmed by histology, elevated level of serum alpha-fetoprotein (> 400 ng/mL), or typical radiological appearance of lesion shown by contrast computed tomography or contrast magnetic resonance imaging. The median waiting time for transplantation was 9.5 mo. Patients who were listed for transplantation received TACE as a bridging therapy before transplantation. HIFU ablation has been used as a standard local ablative therapy since 2006 for HCC patients who have poor liver function and cannot tolerate hepatectomy[5]. This is a retrospective study performed with prospectively collected data. Informed consent to treatment and to the use of data for research was obtained beforehand.
Five patients received HIFU ablation and 29 patients received TACE as a bringing therapy. Fifteen patients received no treatment before transplantation. Another five patients with the same tumor staging (within the UCSF criteria) who were not on the transplant waiting list but received HIFU ablation were included for comparison. All patients were comparable in terms of Child-Pugh and MELD scores, tumor size and number, and cause of cirrhosis.
Contraindications to TACE included main portal vein thrombosis, arteriovenous shunting, Child-Pugh C cirrhosis, and extrahepatic metastasis. Cisplatin was used as the chemotherapeutic agent and was delivered with Lipiodol, followed by Gelfoam particle embolization. Selective cannulation and embolization of the feeding arteries of the tumors were performed whenever possible. During the procedure, 10 mL of Lipiodol was mixed with 10 mg of cisplatin into a 20 mL emulsion. Depending on the tumor size and number, 4-60 mL of the Lipiodol emulsion was injected into the catheter placed in the artery supplying the tumor, or into the hepatic artery proper beyond the gastroduodenal artery for bilobar disease. Light embolization of the feeding artery was then performed with pellets sized 1 mm × 2 mm mixed with 40 mg of gentamycin. Gelfoam injection was stopped when the blood flow in the artery supplying the tumor slowed down but before occlusion occurred. TACE was repeated every 2 to 3 mo. Patients were monitored every month for hepatic and renal functions and alpha-fetoprotein level. TACE was terminated if there was evidence of further derangement of liver function (bilirubin > 50 μmol/L, ascites not controlled by diuretics, or hepatic encephalopathy), progression of disease, extrahepatic metastasis, or any other major complication.
HIFU ablation was offered to patients with poor liver function or decompensated cirrhosis as documented by (1) presence of gross ascites; (2) disease at Child-Pugh B or above; and (3) tumor located at site considered difficult for percutaneous RFA. The treatment probe can target lesions as deep as 10 cm beneath the skin; any lesion within this range can be ablated. Contraindications to HIFU ablation included serum bilirubin level above 100 μmol/L and subcutaneous tissue thicker than 3.5 cm as adipose tissue would absorb a substantial amount of energy from the energy pathway.
All HIFU treatments were carried out by experienced hepatobiliary surgeons and radiologists. The JC HIFU system (Chongqing Haifu Technology, Chongqing, China) was used. The system comprises a real-time diagnostic imaging unit, a therapeutic unit, a degassed water circulation unit, and a computer system. The real-time diagnostic imaging unit provides direct visualization of the tumor. The therapeutic unit consists of an ultrasound energy transducer which focuses the ultrasound energy at a 12-cm focal point. The degassed water circulation unit provides a medium for ultrasound transmission outside the body. The computer system controls these three units.
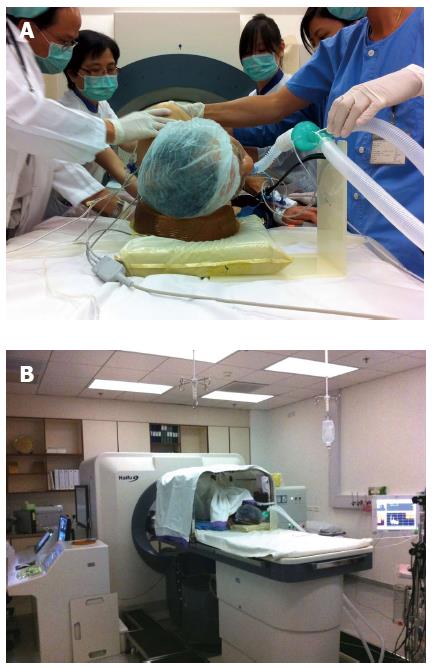
Before operation, the skin of the patient was cleaned by 70% alcohol followed by degassed water to remove all the grease from the skin. A dose of antibiotics (1.2 g of amoxicillin/clavulanic acid) was given on induction. A dose of proton pump inhibitor was also given on induction. Every patient was subjected to general anesthesia to aid comfort as the whole procedure could last for 3 h and the patient had to lie still and endure long periods of breath-holding. In addition, general anesthesia allowed manipulation of tumor location by the Valsalva maneuver during the procedure. If the tumor was at the dome of the liver, artificial right pleural effusion was induced before treatment. If the tumor was located at the right lobe of the liver, the patient was put in a right lateral position (Figure 1A). For better ultrasound conduction, the patient was put in a water bath. The surgeon and the radiologist controlled the operation from the control panel next to the treatment unit (Figure 1B). The treatment was performed under real-time ultrasound image guidance. The lesion was localized by a 3.6-MHz diagnostic ultrasound probe (Philips) incorporated at the center of the transducer. Parallel slices of the target tumor with 5-mm separations were planned and then ablated slice by slice with focused ultrasound energy produced by the transducer operating at 0.8 MHz. Grey-scale changes of the ablated sites were observed during the ablation procedure, indicating the temperature change inside the target lesion.
After the procedure, the patient was usually sent to the general ward and was closely monitored for vital signs, particularly body temperature. Transient hypothermia could occur as the patient had been immersed in a water bath. For patients who had received artificial pleural effusion, radiograph of the chest was taken to check for pneumothorax.
Tumor response was categorized according to the RECIST criteria: (1) complete response was denoted by disappearance of all target lesions; (2) partial response was denoted by at least a 30% decrease in the sum of the largest diameters of the target lesions; (3) progressive disease was denoted by at least a 20% increase in the sum of the largest diameters of the target lesions or appearance of one or more new lesions; and (4) stable disease was denoted by the absence of sufficient shrinkage of tumor qualified as partial response and the absence of sufficient increase of tumor qualified as progressive disease[6]. Contrast computed tomography or contrast magnetic resonance imaging was performed one month after the HIFU treatment and then every three month to evaluate tumor response before transplantation.
The baseline characteristics of patients were expressed as medians with range. The Mann-Whitney U test was used to compare continuous variables and a χ2 test was used to compare discrete variables. Statistical significance was denoted by P < 0.05. All statistical calculations were made with the SPSS/PC + computer software (SPSS, Chicago, IL, United States).
The TACE group and the HIFU group had no difference in age, hepatitis B virus infection, or hepatitis C virus infection. The two groups of patients had similar liver function in terms of serum levels of albumin, aspartate aminotransferase and alanine aminotransferase, prothrombin time, indocyanine green retention rate, and international normalized ratio. One patient in the HIFU group had gross ascites before treatment. No patient in the TACE group had gross ascites. The two groups showed no difference in terms of tumor size and tumor number (Table 1). Table 2 shows the Child-Pugh and MELD scores of the patients. The median number of sessions of TACE was 3 (range, 1-7).
| HIFU (n = 10) | TACE (n = 29) | P value | |
| Age (yr) | 59.5 (49-76) | 57 (43-65) | 0.107 |
| Sex (male/female) | 7/3 | 24/5 | 0.399 |
| Child-Pugh A disease | 3 (30) | 17 (58.6) | 0.267 |
| Child-Pugh B disease | 6 (60) | 12 (41.4) | |
| Child-Pugh C disease | 1 (10) | 0 | |
| Carrier of hepatitis B virus | 5 (50) | 28 (96.5) | 0.002 |
| Carrier of hepatitis C virus | 4 (40) | 1 (3.4) | 0.011 |
| Serum bilirubin (μmol/L) | 14.5 (6-36) | 25 (4-49) | 0.074 |
| Serum albumin (g/dL) | 32 (27-38) | 34 (20-43) | 0.606 |
| Platelet count (109/L) | 67 (28-166) | 59 (23-144) | 0.688 |
| Aspartate transaminase (U/L) | 52 (29-141) | 47 (15-104) | 0.440 |
| Alanine transaminase (U/L) | 44 (26-109) | 36 (9-132) | 0.376 |
| Alpha-fetoprotein (ng/mL) | 8 (2-160) | 24 (1-1151) | 0.101 |
| International normalized ratio | 1.25 (0.9-1.5) | 1.3 (1.0-1.5) | 0.960 |
| Largest tumor size (cm) | 2.6 (1.2-4.0) | 2.0 (0.8-4.3) | 0.252 |
| Tumor number | 1 (1-2) | 1 (1-3) | 0.172 |
| HIFU (n = 10) | TACE (n = 29) | |
| MELD score (P = 0.687) | ||
| 14 | 0 | 2 |
| 13 | 1 | 4 |
| 12 | 1 | 4 |
| 11 | 4 | 7 |
| 10 | 1 | 3 |
| 9 | 1 | 3 |
| 8 | 0 | 1 |
| 7 | 1 | 4 |
| 6 | 1 | 1 |
| Child-Pugh score (P = 0.096) | ||
| 5 | 0 | 10 |
| 6 | 3 | 7 |
| 7 | 5 | 4 |
| 8 | 1 | 5 |
| 9 | 0 | 2 |
| 10 | 1 | 1 |
The median hospital stay was 1 d (range, 1-21 d) in the TACE group and two days (range, 1-9 d) in the HIFU group (P < 0.000). Seven patients in the TACE group dropped out from liver transplant waiting list. One of them developed extrahepatic metastasis and six of them had local progression of disease rendering them unqualified for transplantation. No patient in the HIFU group dropped out during the study period (P = 0.559).
According to the RECIST criteria, nine patients (90%) had complete response and one patient (10%) had partial response in the HIFU group. In the TACE group, only one patient (3%) had response to the treatment while 14 patients (48%) had stable disease and 14 patients (48%) had progressive disease (P = 0.00).
Three out of the five patients in the HIFU group subsequently received transplantation. The median waiting time was nine months (range, 3-36 mo). Histopathological examination showed coagulation necrosis with no active tumor cells in two of the excised livers. One patient had 90% necrosis of the HCC. None of the patients who had received HIFU ablation as a bridging therapy developed complication due to intolerance of the procedure. The other two patients who were still waiting for transplantation had stable disease during the study period.
The incidence of HCC is increasing throughout the world. The annual incidence of HCC in hepatitis B carriers is around 0.5%. The incidence in patients with liver cirrhosis is even higher at around 2.5% annually[7,8]. Hepatitis-B-related cirrhosis is common in Asia, where HCC is endemic. Other risk factors for the development of HCC include hepatitis C infection, alcoholic cirrhosis, genetic hemochromatosis, and primary biliary cirrhosis. These patients should be offered regular surveillance in order to identify small tumors that may be potentially treatable. However, most patients with small HCCs have no symptoms. Resection is the main hope of cure for HCC but is only possible in 25% of the patients because the disease is usually so advanced at presentation and is frequently associated with cirrhosis[9,10]. For patients with unresectable HCC, liver transplantation appears to be the only viable option. The chance of receiving a liver graft varies worldwide. Liver donation rate is highest in Spain where there are 33.7 donations per one million of the population. In contrast, the donation rates in Asia range from only 0.05 to 4.3 donations per one million of the population. The general lack of suitable deceased donors makes successful liver transplantation for HCC difficult[11,12]. In order to maximize the benefit of utilizing this scarce resource, different liver graft allocation systems are adopted worldwide. The principle of allocation is to prioritize the sickest and yet maintain the highest survival rate possible. As a corollary, patients with very high MELD scores have priority. In most countries, patients with unresectable HCC and yet lower MELD scores have a low priority.
The results of liver transplantations in the early period of development were not satisfactory, with a 5-year survival rate below 40%. This urged recognition of poor prognostic factors in liver transplantation in patients with HCC[13]. Mazzaferro et al[14,15] showed that a subgroup of patients with radiological evidence of a single tumor smaller than 5 cm in diameter or two to three tumors each smaller than 3 cm in diameter had better survival outcome. The Milan criteria were established in 1996 and have led to the improvement of the 5-year survival rate to 83%. At many transplant centers, patients with tumor status beyond the Milan criteria are not accepted for transplantation and those on transplant waiting lists are delisted if their tumors enlarge to beyond the criteria, ensuring that liver grafts are allocated to patients predicted to have longer survival[16].
In order to make sure patients receive appropriate treatment before transplantation so as to remain listed, different bridging therapies have been tried. This is particularly important for patients whose treatment options are limited by poor liver reserve and portal hypertension. TACE and RFA are the most popular bridging therapies.
TACE is a standard bridging therapy at some centers, achieving a rate of down-staging of tumors of around 40%. However, about 20% of patients develop tumor progression after TACE, rendering them delisted[1,2,17,18]. RFA is an effective thermal ablative treatment modality and is widely practiced to treat small HCCs. RFA is also used as a bridging therapy. Successful tumor down-staging is observed in 70%-85% of patients. However, the dropout rate after RFA is around 14%[3,19-21]. TACE and RFA seem to be effective bridging therapies, but only for selected patients. They are not safe for patients with liver decompensation such as gross ascites and thrombocytopenia.
The concept of using ultrasound energy as a penetrating force to destroy something remote originated in last century and was summarized by Kremkau[22]. In the 1950’s, researchers bought the phenomenon of piezoelectricity to the clinical setting, treating Parkinson’s disease and other neurological conditions with focused ultrasound energy[23-26].
Nowadays clinical HIFU ablation for liver tumors utilizes a unique frequency of ultrasound wave, 0.8-3.5 MHz, which can be focused at a distance from the therapeutic transducer. The accumulated energy at the focused region induces necrosis of the target lesion by elevating the tissue temperature to above 60 °C[27,28]. Temperature outside the focus point remains static as particle oscillation remains minimal. This is an advantage of HIFU over RFA in which inadvertent collateral damage is unavoidable. Patients with derangement of liver function and thrombocytopenia usually show intolerance of RFA[29]. A bridging therapy must not cause further liver decompensation. The slow process of heating by HIFU energy propagation followed by resting leads to little tissue destruction beyond the focused point.
The presence of gross ascites facilitates HIFU treatment. As ultrasound energy travels much better in water than in air, ascites encourages energy propagation to the target lesion. In addition, the presence of ascites acts as a cushion of coolant inside the peritoneal cavity and prevents the muscle wall and skin absorbing too much energy from the beam pathway where subcutaneous tissue burn could happen.
HIFU ablation is a totally extracorporeal non-invasive treatment modality using focused ultrasound energy that is capable of causing coagulative necrosis of the target lesion via intact skin without the need of surgical incision.
The unique needleless design of the HIFU system makes HIFU ablation superior to RFA, as percutaneous needle penetration may induce hemorrhage from a hypervascular tumor in a patient with coagulopathy and a low platelet count. Furthermore, without needle puncture, there is no risk of direct tumor seeding to the surrounding major vessels[30]. For tumors located at the dome of the liver, open RFA would be required if HIFU ablation is not used.
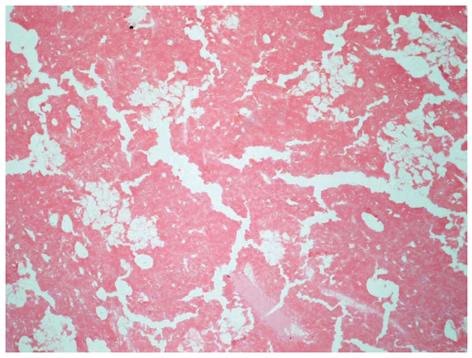
Figure 2 demonstrates the effect of HIFU ablation on the HCC in one of the excised liver in the series in the present study. Coagulative necrosis of the tumor was observed microscopically on almost the whole cut surface. Although histological examination showed the presence of viable tumor cells at a few focuses, there was no gross tumor progression. HIFU ablation was a successful bridging therapy in this case in which TACE and RFA were not considered acceptable treatment due to the poor liver reserve.
Repeated sessions of HIFU or adjuvant TACE should be performed to enhance the effect of tumor necrosis when liver function allows and the waiting time is prolonged.
Wu et al[31] reported the results of HIFU treatment for 68 patients with liver tumors. Thirty patients subsequently received liver resection. In histological examination, all the lesions showed complete coagulation necrosis.
We recently published the results of HIFU treatment for 49 patients with HCC. The complete ablation rate was comparable to that of RFA, ranging from 79.5% to 82.4%. The complication rate was around 8.2%. The complications were mainly mild skin edema and injury due to energy accumulation at the ultrasound beam pathway. The treatment was well tolerated in most of the patients. The median hospital stay was 4 d[5].
In conclusion, to the best of our knowledge, we are the first liver transplant center investigating HIFU ablation as a bridging therapy before liver transplantation. In this study, HIFU ablation was shown to be an effective thermal ablation method. The treatment stopped gross tumor progression in a patient with severe cirrhosis when TACE and RFA were contraindicated. HIFU treatment can potentially reduce the dropout rate of patients from the transplant waiting list. In a broader sense, HIFU may prolong the survival of selected patients with decompensated cirrhosis for which liver transplantation is not an option.
The lack of liver grafts in many places, particularly Asia, is the major obstacle to liver transplantation for patients with hepatocellular carcinoma (HCC). An effective bridging therapy which is well tolerated by patients with decompensated liver cirrhosis is important and much needed.
High-intensity focused ultrasound (HIFU) ablation is a relatively new technique which provides a non-invasive treatment for HCC. However, its efficacy and safety in candidates of liver transplantation have not been known. In this study, the authors demonstrated that it is an effective treatment modality for liver transplant candidates who have decompensated cirrhosis.
This is the first original study on the effect of HIFU ablation as a bridging therapy for liver transplantation. Unlike radiofrequency ablation, HIFU ablation does not require any needle puncture and so eliminates the risk of disease dissemination. It can even be performed in patients with gross ascites without the risk of precipitating liver decompensation. In the study, the ablation caused no adhesion. The absence of adhesion renders the subsequent liver transplantation easier.
With its advantages, safety and efficacy as demonstrated in this study, HIFU ablation should be considered as a treatment option for HCC. This study already proved that it is a safe and effective bridging therapy before liver transplantation for HCC.
HIFU ablation utilizes a unique frequency of ultrasound wave of 0.8 to 3.5 MHz, causing a cavitation effect in the target lesion.
The authors studied the effect of HIFU ablation on HCC performed before liver transplantation. The study demonstrated that HIFU ablation is an effective ablation modality which is totally non-invasive. It can effectively reduce the drop-out rate of liver transplant candidates by giving effective control on their tumors. The histological examination of the excised livers provided evidence that necrosis is effective in an in vivo model, which makes this article unique of its kind.
P- Reviewer Mearini L S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, Miller CM, Schwartz ME. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 318] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Maddala YK, Stadheim L, Andrews JC, Burgart LJ, Rosen CB, Kremers WK, Gores G. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transpl. 2004;10:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Brillet PY, Paradis V, Brancatelli G, Rangheard AS, Consigny Y, Plessier A, Durand F, Belghiti J, Sommacale D, Vilgrain V. Percutaneous radiofrequency ablation for hepatocellular carcinoma before liver transplantation: a prospective study with histopathologic comparison. AJR Am J Roentgenol. 2006;186:S296-S305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 4. | Lu DS, Yu NC, Raman SS, Lassman C, Tong MJ, Britten C, Durazo F, Saab S, Han S, Finn R. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Ng KK, Poon RT, Chan SC, Chok KS, Cheung TT, Tung H, Chu F, Tso WK, Yu WC, Lo CM. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg. 2011;253:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Duffaud F, Therasse P. New guidelines to evaluate the response to treatment in solid tumors. Bull Cancer. 2000;87:881-886. [PubMed] |
| 7. | Poon RT, Fan ST, Lo CM, Liu CL, Ng IO, Wong J. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol. 2000;18:1094-1101. [PubMed] |
| 8. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 682] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 9. | Fan ST, Ng IO, Poon RT, Lo CM, Liu CL, Wong J. Hepatectomy for hepatocellular carcinoma: the surgeon’s role in long-term survival. Arch Surg. 1999;134:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Fuster J, Bruix J. Prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2002;49:7-11. [PubMed] |
| 11. | Lo CM, Fan ST, Liu CL, Chan SC, Wong J. The role and limitation of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2004;10:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Ríos A, López-Navas A, Ayala-Garcia MA, Sebastián MJ, Martínez-Alarcón L, Ramírez EJ, Muñoz G, Camacho A, Rodríguez JS, Martínez MA. Donation and transplantation among personnel in the hospital emergency department: a multicenter study conducted in Spain and Mexico. Transplant Proc. 2011;43:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 419] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Mazzaferro V, Rondinara GF, Rossi G, Regalia E, De Carlis L, Caccamo L, Doci R, Sansalone CV, Belli LS, Armiraglio E. Milan multicenter experience in liver transplantation for hepatocellular carcinoma. Transplant Proc. 1994;26:3557-3560. [PubMed] |
| 15. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5292] [Article Influence: 182.5] [Reference Citation Analysis (0)] |
| 16. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1270] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 17. | Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 18. | Hayashi PH, Ludkowski M, Forman LM, Osgood M, Johnson S, Kugelmas M, Trotter JF, Bak T, Wachs M, Kam I. Hepatic artery chemoembolization for hepatocellular carcinoma in patients listed for liver transplantation. Am J Transplant. 2004;4:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, Sarli D, Schiavo M, Garbagnati F, Marchianò A. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 379] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Fisher RA, Maluf D, Cotterell AH, Stravitz T, Wolfe L, Luketic V, Sterling R, Shiffman M, Posner M. Non-resective ablation therapy for hepatocellular carcinoma: effectiveness measured by intention-to-treat and dropout from liver transplant waiting list. Clin Transplant. 2004;18:502-512. [PubMed] |
| 21. | Pompili M, Mirante VG, Rondinara G, Fassati LR, Piscaglia F, Agnes S, Covino M, Ravaioli M, Fagiuoli S, Gasbarrini G. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: Assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Kremkau FW. Cancer therapy with ultrasound: a historical review. J Clin Ultrasound. 1979;7:287-300. [PubMed] [DOI] [Full Text] |
| 23. | Barnard JW, Fry WJ, Fry FJ, Krumins RF. Effects of high intensity ultrasound on the central nervous system of the cat. J Comp Neurol. 1955;103:459-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Fry WJ, Barnard JW, Fry EJ, Krumins RF, BRENNAN JF. Ultrasonic lesions in the mammalian central nervous system. Science. 1955;122:517-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Fry WJ, Barnard JW, Fry FJ, Brennan JF. Ultrasonically produced localized selective lesions in the central nervous system. Am J Phys Med. 1955;34:413-423. [PubMed] |
| 26. | Fry WJ, Mosberg WH, Barnard JW, Fry FJ. Production of focal destructive lesions in the central nervous system with ultrasound. J Neurosurg. 1954;11:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 267] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, Cranston D. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics. 2004;42:931-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 28. | Wu F, Wang ZB, Chen WZ, Wang W, Gui Y, Zhang M, Zheng G, Zhou Y, Xu G, Li M. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 29. | Cheung TT, Ng KK, Poon RT, Fan ST. Tolerance of radiofrequency ablation by patients of hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Nicoli N, Casaril A, Abu Hilal M, Mangiante G, Marchiori L, Ciola M, Invernizzi L, Campagnaro T, Mansueto G. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, Wang ZB. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol. 2001;27:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 239] [Article Influence: 10.0] [Reference Citation Analysis (0)] |