Published online May 28, 2013. doi: 10.3748/wjg.v19.i20.3069
Revised: March 19, 2013
Accepted: April 13, 2013
Published online: May 28, 2013
Processing time: 149 Days and 19.1 Hours
AIM: To evaluate the efficacy and safety of sodium hyaluronate solution (SH) in endoscopic submucosal dissection (ESD) of gastric neoplasms.
METHODS: A prospective multicenter randomized, double blind, controlled trial was designed and utilized in this study. A total of 76 patients with 5-20 mm sized gastric neoplasms were enrolled at three academic hospitals in South Korea from June 2011 to October 2011. Patients were randomly assigned to the 0.4% sodium hyaluronate or control groups. All lesions underwent endoscopic ESD. ESD was performed with 0.4%SH and normal saline (NS) solution for submucosal injection. Efficacy was assessed using en bloc resection and the number of additional injections. Secondary evaluation variables were the volume of injection material, steepness of mucosal elevation, bleeding rate, procedural time and operator satisfaction. Finally, the safety was assessed by analyzing adverse events during the study.
RESULTS: The usefulness rate in the 0.4%SH group and the controlled group had statistically significant difference under intention to treat (ITT) analysis (90.91% vs 61.11% P = 0.0041). Under per protocol (PP), the usefulness rate is statistically significant different (93.10% vs 61.76%, P = 0.0036). The difference in volume of the solution injected between 0.4%SH group and the controlled group and NS group was also statistically significant under intention to treat and per protocol analysis (ITT: 0.03 ± 0.02 mL vs 0.06 ± 0.03 mL, P = 0.0003, PP: 0.03 ± 0.02 mL vs 0.06 ± 0.03 mL, P = 0.0004). Satisfaction above the grade good was significantly higher in the SH group under intention to treat and per protocol analysis (ITT: 90.91% vs 61.11%, P = 0.0041, PP = 93.11% vs 61.77%, P = 0.0022). Adverse events above grade 3 were not noticed in either group. All adverse events were treated and were judged as not associated with the submucosal injection solutions.
CONCLUSION: 0.4%SH solution is a safe and effective agent that doesn’t cause any significant adverse events and is useful for submucosal injection during ESD.
Core tip: Saline-assisted endoscopic mucosal resection is an established method for excision of nonpolypoid early neoplastic lesions of the gastrointestinal tract. However, it is sometimes difficult to maintain a desired level of tissue elevation after injection of saline, especially when using a one-channeled endoscope. Adequate elevation of the mucosa and sufficient elevation time is achieved more effectively when a material more viscous than normal saline (NS) is used. The 0.4% sodium hyaluronate solution (SH) used in this study provides a more effective and prolonged cushion effect for large lesions without serious adverse events compared to NS. Therefore, endoscopic submucosal dissection (ESD) with SH is more useful than ESD with NS.
- Citation: Kim YD, Lee J, Cho JY, Kim SW, Kim SH, Cho YK, Jang JS, Han JS, Cho JY. Efficacy and safety of 0.4 percent sodium hyaluronate for endoscopic submucosal dissection of gastric neoplasms. World J Gastroenterol 2013; 19(20): 3069-3076
- URL: https://www.wjgnet.com/1007-9327/full/v19/i20/3069.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i20.3069
Endoscopic mucosal resection (EMR) using submucosal saline injection is an established method for excision of nonpolypoid early neoplastic lesions of the gastrointestinal tract[1,2]. Numerous resection methods have been developed using the submucosal injection technique. In 1998, Hosokawa developed an IT knife (insulated tipped electrosurgical knife) useful for endoscopic submucosal dissection (ESD)[3], which has made en bloc resection of not only elevated lesions but depressive lesions without ulcers and flat lesions possible, compared to EMR. The procedure has minimal limitations regarding location and size of the lesions. The technical advancement of ESD combined with development of many endoscopic accessories has made ESD a standard treatment of early gastric cancer in selected cases.
The submucosa is a thin connective tissue layer with a lax structure compared to the mucosa or the muscularis propria. The injection of solutions to this layer forms a bulla and lifts the lesion above. Submucosal injection during ESD facilitates the removal of the lesions, provides a safety cushion during resection, and prevents perforations during the procedure. The most commonly used material is normal saline (NS). However, it is sometimes difficult to maintain a desired level of tissue elevation after an injection of saline, especially when using a one-channeled endoscope[4].
An ideal submucosal injection solution would have a prolonged cushion effect, and be easily available and inexpensive, nontoxic, and easy to inject[5]. Sodium hyaluronate solution (SH) is a macromolecular polysaccharide composed of D-glucuronate and N-acetyl-D-glucosamine. The high viscosity, elasticity, and lack of antigenicity or toxicity[6-8] have led to its extensive use in ophthalmologic surgical procedures and intra-articular injections. Since Yamamoto et al[9] reported the use of SH in porcine stomach yielding a more distinct and prolonged submucosal elevation compared to NS, numerous studies have used SH for difficult cases.
The authors aimed to compare the usefulness rate and safety of 0.4%SH in ESD as compared to NS in a multicenter prospective randomized double-blind control study.
From June 2011 to October 2011, 76 patients with less than 20 mm sized early gastric cancer or gastric adenoma were enrolled in 3 independent academic hospitals in South Korea. Experienced endoscopists with more than 300 cases of ESD experience performed the ESD. Criteria for inclusion in the study were: (1) patients between the ages of 20 and 80; (2) patients with 5- to 20-mm early gastric cancer or gastric adenoma; and (3) patients who gave written informed consent. The limitation of the lesion size to less than 20 mm was based on the absolute indication criteria of ESD. Exclusion criteria of the study were: (1) residual or recurrent lesion; (2) lesions accompanied by ulcers; (3) undifferentiated cancer; (4) advanced malignant neoplasm; (5) patient with pacemakers; (6) history of hypersensitivity to hyaluronic acid; (7) serum creatinine ≥ 1.5 mg/dL or creatine clearance ≤ 50 mL/min; (8) patients with severe liver disease; (9) severe cardiovascular disease; (10) patient taking immunosuppressants including prednisolone or anti-cancer agents; (11) alcohol- or drug-addicts; (12) pregnant or lactating patients; and (13) patients judged by a physician as inappropriate for inclusion.
The clinical trial was approved by the Korean Food and Drug Administration (KFDA) and the study protocol was approved by the Institutional Review Board of each center. Written informed consent was obtained from all the patients.
The study was double-blinded by having an independent investigational device manager that supplied the solutions used for the study. The endoscopist was blind to the material used. During the procedure, an experienced assistant did the injection of fluids to the submucosa. Although the endoscopist could visualize the elevation effect, the difference in pressure during injection of the material was known only to the injecting assistant. The injecting assistant did not take any part in evaluating the satisfaction grade of the materials. All lesions were resected through ESD. The margin of the lesion was marked through electrocautery and submucosal injection was done at the normal mucosa adjacent to the lesion. ESD was initiated after an adequate amount of solution was injected to lift the lesion. The maximum amount of 0.4%SH solution (Endo-Mucoup, BMI Korea, Co., Ltd) for the procedure was limited to 40 mL, which is 1/10 the nontoxic level administered into the peritoneum. The amount of SH solution supplied for the procedure was limited to 40 mL and any additional amount of injection needed used NS. Necessary medication or procedure for the treatment of coexisting disorders of the patients was allowed. Except for the use of epinephrine and indigo carmine, any other material for submucosal injection solution was prohibited.
The study was designed as a multicenter, randomized, double blind, and placebo-controlled trial. The primary outcome of the study was en bloc complete resection and additional injections of the solution used. En bloc complete resection was defined as en bloc resection with negative resection margins confirmed histopathologically. Additional resection was defined as the number of additional injects required to maintain the mucosal lifting for the procedure. The usefulness rate was defined as the percentage of en bloc complete resection with 1 or less additional injection during the procedure (Table 1). The 40 mL of SH was decided for the efficacy evaluation as it is 1/10 the nontoxic level administered into the peritoneum.
| Completeen blocresection | Additional injection | Usefulness | Satisfaction |
| Complete | 0 | Usefulness | Excellent |
| Complete | 1 | Usefulness | Good |
| Complete | ≥ 2 | Useless | Moderate |
| Incomplete or not evaluated | - | Useless | Poor |
Secondary outcomes of the study included: (1) volume of the solution injected; (2) the steepness of the lift; (3) presence or absence of bleeding; (4) procedure time; and (5) satisfaction of the solution. The volume of the solution used for evaluation was the total amount of the solution divided by the area of the lesion (long diameter × short diameter). The steepness of the lift was graded as steep, mild, or non lifted. The percentage of each grade was used for evaluation. Bleeding was defined as the need for electrocauterization before the incision and after injecting the submucosal solution. The procedure time was defined as the period from the marking of the margins to the completion of the excision. Satisfaction rate of the solution was comprehensively assessed by evaluating the en bloc complete resection rate and the number of additional injections (Table 1).
The safety of the solution was assessed through 5 grades of symptoms and signs unwarranted during the study. Grade 1 adverse event was defined as symptoms or signs that do not require treatment and do not inhibit daily activities. Grade 2 adverse events were defined as symptoms or signs that required treatment but did not inhibit daily activities. Grade 3 adverse events were when the patient experienced substantial discomfort leading to limitation of daily activities and requiring admission. Life-threatening adverse events were defined as grade 4. Death was defined as grade 5. For each adverse event, the clinician assessed the association of the response to the submucosal injection solution as definitely related, probably related, possibly related, probably not related, definitely not related, or unknown.
This multi center randomized, double-blind, placebo-controlled study was designed with a level of significance α = 0.05 and detection power 1 - β = 0.8 to test the superiority of the 0.4%SH solution compared to NS for the lift and maintenance of the submucosa during ESD. The calculated sample size was 76 patients. Thirty-four patients were analyzed in each group assuming a 10% rate of non-comparability in the two groups.
Statistical analysis was done using SAS software 9.1 version (SAS Institute, Cary, NC). Intention to treat analysis and per protocol analysis was used in the study. The clinical usefulness was assessed using both methods, with intention to treat as the primary method. Per protocol analysis was used for demographic data and adverse effects. Continuous variables for the two groups were compared using t-test and χ2 test or the Fisher’s exact test, which was used for categorical variables. The primary outcome, the usefulness rate, was analyzed using the χ2 test and 95%CIs are given. The secondary outcomes, volume of the solution used and procedure time were analyzed using t-test. Presence or absence of bleeding and satisfaction of the solution was assessed using χ2 test. Safety evaluation using the total number of the adverse effects and the rate of patients with more than 1 adverse effect was done using the χ2 test. Null hypotheses of no difference were rejected if P-values were less than 0.05.
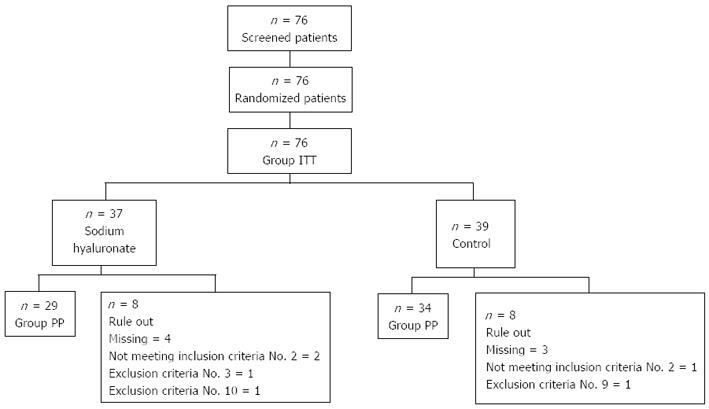
Seventy-six patients who underwent screening examination and who gave informed written consent were included in this study. Using randomization, 37 patients were assigned to the 0.4%SH group and 39 patients were assigned to the control group that used NS for submucosal injection. All these patients were included in the intention to treat analysis. Eight patients from the 0.4%SH group and 5 patients from the control group were either lost to follow up or had a clinical trial protocol violation; the remaining 63 patients were included in the per protocol analysis (Figure 1). There were no statistically significant differences in the patients’ age, sex, percentage of gastric cancer, or location of the lesion between the two groups (Table 2).
| Sodium hyaluronate | Control | P-value | |
| Age | 62.59 ± 9.23 | 62.44 ± 9.93 | 0.943 |
| Sex (male/female) | 25/12 | 26/13 | 0.933 |
| Diagnosis, n (%) | |||
| Adenocarcinoma | 5 (13.51) | 7 (17.95) | 0.869 |
| Adenoma | 31 (83.78) | 31 (79.49) | |
| Atypia | 1 (2.7) | 1 (2.56) | |
| Size (mm) | 14.2 ± 5.47 | 13.5 ± 4.35 | 0.293 |
| Location, n (%) | |||
| Antrum | 23 (62.16) | 29 (74.35) | 0.465 |
| Angle | 3 (8.10) | 3 (7.69) | |
| Body | 11 (29.72) | 7 (17.94) |
Usefulness, the primary outcome of the study, was assessed using both intention to treat analysis and per protocol analysis, with intention to treat as the primary method. Under intention to treat analysis, the usefulness rate of the 0.4%SH group (90.91%, 30/33) was found to be significantly greater than the control group (61.11%, 22/36) (P = 0.0041). Using the per protocol analysis, the usefulness rate of the 0.4%SH group and the control were 93.10% (27/29) and 61.76% (21/34), respectively. The difference of the usefulness rate using per protocol analysis was also statistically significant (P = 0.0036). The usefulness according to the site of the gastric neoplasm, using the intention to treat (ITT) analysis revealed that 0.4%SH was statistically significantly useful when used during procedures for gastric neoplasms at the body and angle. However, there was no statistically significant difference in procedures done at the antrum. Using a per-protocol analysis, 0.4%SH showed statistically significant usefulness in all the sites of the stomach.
Secondary outcomes of the study were analyzed including volume of the solution injected, the steepness of the lift, presence or absence of bleeding, procedure time, and satisfaction of the solution (Table 3).
| Sodium hyaluronate | Control | P-value | |
| ITT | |||
| Usefulness rate | 30/33 (90.91) | 22/36 (61.11) | 0.004 |
| Volume of injection | 0.03 ± 0.02 | 0.06 ± 0.03 | 0.000 |
| Procedural time | 23.42 ± 16.76 | 21.64 ± 16.52 | 0.658 |
| Steepness | 0.228 | ||
| Steep | 26 (78.79) | 27 (75.00) | |
| Mild | 6 (18.18) | 4 (11.11) | |
| Non lifted | 1 (3.03) | 5 (13.89) | |
| Absence of bleeding | 32/33 (96.97) | 32/36 (88.89) | 0.359 |
| Satisfaction | 30/33 (90.91) | 22/36 (61.11) | 0.002 |
| PP | |||
| Usefulness rate | 27/29 (93.10) | 21/34 (61.76) | 0.004 |
| Volume of injection | 0.03 ± 0.02 | 0.06 ± 0.03 | 0.000 |
| Procedural time | 23.79 ± 17.51 | 19.71 ± 12.65 | 0.288 |
| Steepness | 0.474 | ||
| Steep | 24 (82.76) | 26 (76.47) | |
| Mild | 4 (13.79) | 4 (11.76) | |
| Non lifted | 1 (3.45) | 4 (11.76) | |
| Absence of bleeding | 29/29 (100.00) | 30/34 (88.24) | 0.118 |
| n (%) | 27/29 (93.11) | 21/34 (61.77) | 0.002 |
The volume of the solution used for evaluation was the total amount of the solution divided by the area of the lesion (long diameter × short diameter). The volume of the solution injected for the 0.4%SH group (0.03 ± 0.02 mL) was significantly less compared to the control group (0.06 ± 0.03 mL) (P = 0.0003). The difference in volume of the solution injected between the 0.4%SH group and NS group using per protocol analysis was also statistically significant (P = 0.0004).
The procedure time was defined as the period from the marking of the margins to the completion of the excision. The procedure time analyzed by intention to treat showed 23.42 ± 16.76 min in the 0.4%SH group and 21.64 ± 16.52 min in the control group. Using the per protocol analysis the procedure times were 23.79 ± 17.51 min in the 0.4%SH group and 19.71 ± 12.65 min in the control group. Although the procedure time for the 0.4%SH group was shorter compared to the control group, the difference was not statistically significant.
The steepness of the lift was graded as steep, mild, or non lifted in the two study groups and showed no statistically significant differences.
The absence of bleeding during injection of the submucosal solution was higher in the 0.4%SH group (96.97%, 32/33) compared to the control group (88.89%, 32/36) using the intention to treat analysis. Using the per protocol analysis, the absence of bleeding was 100% (29/29) in the 0.4%SH group and 88.24% (30/34) in the control group. However, the difference was not statistically significant in both analyses.
Satisfaction with the solution was comprehensively assessed by evaluating the en bloc complete resection rate and the number of additional injections. ESD with en bloc complete resection and no additional submucosal injection was defined as excellent. En bloc complete resection with 1 additional submucosal injection was defined as good. Satisfaction above the grade good was significantly higher in the 0.4%SH group (90.91%, 30/33) compared to the control group (61.11%, 22/36) using intention to treat analysis (P = 0.0175). Using per protocol analysis showed statistically significant difference in the satisfaction above the grade good in 93.11% (27/29) and 61.77% (21/34) in the 0.4%SH group and control group, respectively (P = 0.0022).
The ESD that had additional submucosal injections was analyzed by the site of the lesion using the usefulness rate, volume of solution injection and procedure time (Table 4).
| Sodium hyaluronate | Control | P-value | |
| ITT | |||
| Usefulness rate | |||
| Antrum | 18/20 (90.00%) | 18/27 (66.67%) | 0.086 |
| Body/angle | 12/13 (92.30%) | 6/9 (66.67%) | 0.007 |
| Volume of injection | |||
| Antrum | 0.03 ± 0.02 | 0.06 ± 0.04 | 0.003 |
| Body/angle | 0.03 ± 0.02 | 0.05 ± 0.02 | 0.047 |
| Procedural time | |||
| Antrum | 19.40 ± 7.46 | 18.41 ± 11.67 | 0.741 |
| Body/angle | 28.08 ± 25.47 | 31.33 ± 24.68 | 0.768 |
| PP | |||
| Usefulness rate (%) | |||
| Antrum | 15/16 (93.75%) | 17/26 (65.38%) | 0.036 |
| Body/angle | 12/13 (92.30%) | 3/8 (37.50%) | 0.007 |
| Volume of injection | |||
| Antrum | 0.03 ± 0.02 | 0.06 ± 0.04 | 0.011 |
| Body/angle | 0.03 ± 0.02 | 0.05 ± 0.03 | 0.039 |
| Procedural time | |||
| Antrum | 19.06 ± 6.78 | 18.23 ± 11.88 | 0.800 |
| Body/angle | 28.08 ± 25.47 | 24.50 ± 14.70 | 0.723 |
The sites of the lesion were divided into the antrum group and body/angle group. Under intention to treat analysis on antrum, there is no significant difference of the usefulness rate in the 0.4%SH group (90.00%, 18/20) compared to the control group (66.67%, 18/27) (P = 0.086). But using per protocol analysis, the antrum had a significantly higher usefulness rate in the 0.4%SH group (93.75%, 15/16) compared to the control group (65.38%, 3/8) (P = 0.036). The usefulness rate analyzed in the lesions located at the body/angle using intention to treat analysis was 92.30% (12/13) in the 0.4%SH group compared to the 66.67% (6/9) in the control group. The difference was statistically significant (P = 0.007). Per protocol analysis also revealed a statistically significant higher usefulness rate of 92.30% (12/13) in the 0.4%SH group compared to 37.50% (3/8) in the control group (P = 0.007).
The amount of volume injected at the antrum using intention to treat analysis was 0.03 ± 0.02 mL in the 0.4%SH group and 0.06 ± 0.04 mL in the control group. The smaller amount used in the 0.4%SH group was statistically significant (P = 0.003). Using per protocol analysis, the difference in the amount of volume injected 0.4%SH group (0.03 ± 0.02 mL) and control group (0.06 ± 0.04 mL) was statistically significant (P = 0.011).
The amount of volume injected at the body/angle using intention to treat analysis was 0.03 ± 0.02 mL in the 0.4%SH group and 0.05 ± 0.02 mL in the control group. The smaller amount used in the 0.4%SH group was statistically significant (P = 0.047). Using per protocol analysis, the difference in the amount of volume injected 0.4%SH group (0.03 ± 0.02 mL) and control group (0.05 ± 0.03 mL) was statistically significant (P = 0.039).
The procedure time using both intention to treat analysis and per protocol analysis based on the site of the lesion showed no statistically significant differences.
Adverse events were seen in 8 out of the 76 patients, with 13 incidents in the intention to treat analysis. Grade 1 adverse events were noticed in 12.50% (5/37) of the SH group and 2.44% (1/39) of the control group. Grade 2 adverse events were seen in 7.50% (3/37) and 9.76% (4/39) of the 0.4%SH group and control group, respectively. Adverse events above grade 3 were not noticed in both groups. Gastrointestinal symptoms were the most common adverse events with 10 out of the 13 incidents. Five cases of nausea, 3 cases of vomiting, and each 1 case of dyspepsia and hematemesis were noticed. All adverse events were treated and were judged as not associated with the submucosal injection solutions.
ESD is now a standard treatment for early gastric neoplasms in the gastrointestinal tract. The EMR used currently are strip-biopsy method or lift and cut EMR[10], endoscopic resection after local injection of a solution of hypertonic saline and epinephrine[11], endoscopic double-snare polypectomy[12], EMR using an over-tube[13], strip-biopsy using two small diameter endoscopes[14], EMR with a cap-fitted panendoscope[15], an EMR using a ligation device[16,17]. The submucosal injection of solutions during the resection of gastrointestinal neoplasm is an essential part of the resection procedure[18-21]. Tanabe et al[10] introduced strip biopsy with submucosal injection using NS as a safety cushion. Since Ikeda et al[22] injected a mixture of hypertonic saline and epinephrine to lift the lesion in 1986, lifting the lesion with a safety cushion underneath the lesion has become an essential procedure in EMR. ESD can resect a larger lesion without regard to the location of the lesion compared to EMR. To achieve an en bloc resection of a large lesion, adequate lift of the lesion is needed for a prolonged period. Numerous solutions are under research and have been used for mucosal resection. These agents include NS, mixture of NS and epinephrine, hypertonic saline (3.8%), hypertonic glucose solution (20%, 50%), 10% glycerin + 5% fructose + 0.9% NS, SH, hydroxypropyl methycellulose, and a fibrinogen mixture. NS is easily available, cheap, and causes minimal tissue injury due to its isotonic property. However, NS is easily absorbed to the tissue and multiple injections are needed. The mixture of NS and epinephrine (1:10000) is most widely used. The steepness and maintenance of the cushion is not significantly different from NS, but the mixture of epinephrine causes vasoconstriction of the vessels leading to hemostasis. The addition of indigo carmine to the mixture assists in identifying the submucosa and muscularis propria during resection of the deep margins. However, during ESD, the cushion is easily dissipated and multiple injections are needed. This leads to a prolonged procedure time and the risk of perforation is high when resecting the lesion without adequate cushion. Therefore, an ideal agent would have a higher viscosity to maintain the cushion longer and require fewer additional injections during the procedure. Although hypertonic saline and hypertonic glucose solution had a steeper elevation compared to NS, this was not statistically significant. The hypertonic solution has a propensity to cause tissue injury and may delay healing of the artificial ulcer after the procedure.
SH has a prolonged cushion-effect and causes minimal tissue injury. However, the solution is expensive and has special requirements for storage. Hydroxypropyl methycellulose has a similar viscosity and tensile strength compared to SH and has an adequate cushion-effect, is inexpensive, and is easy to store. However, the synthetic material can cause cross-antigen reaction in the body. Fibrinogen mixture is a 340 kDa high molecular glycoprotein separated from blood. The viscosity is high and has a microvascular hemostatic effect. However, the high viscosity requires a large diameter injection tip. Lee et al[23] report that the fibrinogen mixture is superior to NS in the procedure time, total volume of solution injection, and additional injection rates in ESD.
SH is considered the best solution for submucosal dissection. However, its use is limited due to the high cost of the solution. The molecular weight and dilution rate of SH is continuously researched to decrease the cost and find the dilution rate for effective lifting. Fujishiro et al[24,25] report that the optimal viscosity and tensile strength was 1% 1900-kDa solution. However, 1% 800-kDa, 0.5% 1900-kDa, 0.5% z800-kDa, and 0.25% 1900-kDa solutions all showed similar results. Concentration of the solution below 1% still maintained adequate viscoelasticity while not increasing osmolarity or causing tissue injury. Despite the fact that it is an ideal substance for submucosal injection, the high cost and special requirements for storage led to a mixture of it with other hypertonic solutions.
This study evaluated the clinical usefulness and safety of 0.4%SH compared to NS. All procedures for the resection of the lesions were ESD. The size of the lesion was limited to 2cm due to the absolute indication of ESD for early gastric cancers without lymph node metastasis[14]. This led to the limitation of gastric adenoma size to less than 2cm. The primary outcome, usefulness rate, analyzed using intention to treat and per protocol analysis revealed higher and statistically significant rates in the 0.4%SH group. Secondary outcome of the study including volume of the solution injected, the steepness of the lift, presence or absence of bleeding, procedure time, and satisfaction with the solution were analyzed. The volume of the solution injected was statistically significantly decreased and satisfaction of the solution was significantly superior in the 0.4%SH group. The limitation of the size in the inclusion criteria may have limited the analysis of the resection rate in the two groups. The usefulness rate may have showed a more significant difference in the two groups as the size of the lesion is increased. The procedure time of the two groups in this study showed no statistically significant difference. This may have been due to the fact that the size of the lesion was limited to less than 2 cm. The experienced clinicians participating in this study may have decreased the difference in time required for the 2 groups. However, if larger lesions with scars were included in the study, even experienced clinicians may show a difference in the procedure time for the 2 groups due to the multiple injections that may be required to maintain the cushion-effect during the procedure. Therefore, a prospective study including gastric adenoma that has no limitation of size in en bloc resection using ESD may show a difference in the usefulness rate, volume of solution injected and satisfaction with the solution. The procedure time may show a significant difference because 0.4%SH had a prolonged cushion-effect and requires less additional injections compared to NS. There were grade 1 and grade 2 adverse events observed in this study. However, there was no statistically significant difference in the two groups and clinicians judged that the adverse events were not related to the solution injected. Therefore, the use of 0.4%SH for submucosal injections during ESD is considered safe.
This study has a size limitation of 2 cm. Gotoda et al[26] reported that due to the lack of lymph node metastasis, mucosal gastric cancer less than 3 cm accompanied by an ulcer and submucosal gastric cancer invading the SM1 layer less than 3 cm are also indications of endoscopic therapy. This has formed the basis of the expanded criteria for endoscopic therapy in early gastric cancer. However, the current study was approved by the KFDA for absolute indications of ESD for early gastric cancer. The size of the gastric adenomas was also limited for evaluation of the two materials without bias.
The recent development of endoscopic accessories with more skilled clinicians has led to en bloc resection of lesions without regard to the location and size. This has led to a more extended indication of ESD and a solution with a long lasting cushion-effect is needed. Using NS for lesions that are easily approachable and small is cost-effective. However, for lesions that are large and difficult to approach, the procedure time could be prolonged with more frequent complications. In these cases, using 0.4%SH for the submucosal injection would be more cost-effective.
In conclusion, the ideal agent would have a prolonged cushion effect, be easily available, nontoxic, easy to inject, and inexpensive. SH has a long-lasting cushion effect compared to the widely used NS and leads to a safe and effective procedure. SH has no significant adverse events compared to NS and is safe. With the recent advances in the indication of ESD in early gastric cancer, development of endoscopic accessories, and more trained clinicians, the resection of large lesions without concern regarding the location of the lesion is possible. Therefore, with appropriate measures taken regarding price and storage issues, 0.4%SH may further enhance the ease and safety of ESD.
Endoscopic submucosal dissection (ESD) has made en bloc resection of early gastric neoplasms possible. A sufficient amount of submucosal fluid cushion is one of the important requisites for ESD. The ideal injection agent should provide a long-lasting submucosal cushion.
The submucosal injection during ESD is a requisite for safety during and after ESD. An ideal submucosal injection solution would have a prolonged cushion effect, be easily available, nontoxic, easy to inject, and inexpensive. Numerous solutions are being studied to determine if they are safe and effective in the procedure.
There are no studies evaluating the efficacy and safety of sodium hyaluronate solution (SH) solution in ESD for gastric neoplasm in a Korean population.
0.4%SH solution gives gastric mucosa long lasting lifting compared to normal saline (NS), thereby ESD using it is more effective compared to the control group. The solution is safe when comparing adverse events to the NS group. Therefore, 0.4%SH is a useful agent for submucosal injection during ESD.
This is a randomized controlled trial on efficacy and safety of 0.4% sodium hyaluronate for gastric ESD. It is worthy pubishing.
P- Reviewer Mishra PK S- Editor Huang XZ L- Editor A E- Editor Lu YJ
| 1. | Takekoshi T, Baba Y, Ota H, Kato Y, Yanagisawa A, Takagi K, Noguchi Y. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 135] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 367] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1148] [Article Influence: 47.8] [Reference Citation Analysis (4)] |
| 4. | Sakal P, Maluf Filho F, Iryia K, Moura EG, Tomishigue T, Scabbia A, Ishioka S. An endoscopic technique for resection of small gastrointestinal carcinomas. Gastrointest Endosc. 1996;44:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kwon R, Mamula P, Rodriguez S, Shah RJ, Wong Kee Song LM. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 6. | Laurent TC, Fraser JR. The properties and turnover of hyaluronan. Ciba Found Symp. 1986;124:9-29. [PubMed] |
| 7. | Johnson ME, Murphy PJ, Boulton M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch Clin Exp Ophthalmol. 2006;244:109-112. [PubMed] [DOI] [Full Text] |
| 8. | Miyauchi S, Sugiyama T, Machida A, Sekiguchi T, Miyazaki K, Tokuyasu K, Nakazawa K. The effect of sodium hyaluronate on the migration of rabbit corneal epithelium. I. An in vitro study. J Ocul Pharmacol. 1990;6:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Yamamoto H, Yube T, Isoda N, Sato Y, Sekine Y, Higashizawa T, Ido K, Kimura K, Kanai N. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Tanabe S, Koizumi W, Kokutou M, Imaizumi H, Ishii K, Kida M, Yokoyama Y, Ohida M, Saigenji K, Shimao H. Usefulness of endoscopic aspiration mucosectomy as compared with strip biopsy for the treatment of gastric mucosal cancer. Gastrointest Endosc. 1999;50:819-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 264] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Takekoshi T, Takagi K, Fujii A, Kato Y. [Treatment of early gastric cancer by endoscopic double snare polypectomy (EDSP)]. Gan No Rinsho. 1986;32:1185-1190. [PubMed] |
| 13. | Shimada H, Nishi T, Makuuchi H, Ozawa S, Chino O. [EEMR-tube method]. Nihon Rinsho. 2011;69 Suppl 6:231-235. [PubMed] |
| 14. | Takechi K, Mihara M, Saito Y, Endo J, Maekawa H, Usui T, Moriwaki H, Muto Y. A modified technique for endoscopic mucosal resection of small early gastric carcinomas. Endoscopy. 1992;24:215-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Inoue H. Endoscopic mucosal resection for the entire gastrointestinal mucosal lesions. Gastrointest Endosc Clin N Am. 2001;11:459-478. [PubMed] |
| 16. | Suzuki H, Masuda K, Fujisaki J, Okuwaki S. [Endoscopic treatment of gastrointestinal cancers--indication and limitation]. Nihon Rinsho. 1996;54:1699-1704. [PubMed] |
| 17. | Chaves DM, Sakai P, Mester M, Spinosa SR, Tomishige T, Ishioka S. A new endoscopic technique for the resection of flat polypoid lesions. Gastrointest Endosc. 1994;40:224-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Ishiguro A, Uno Y, Ishiguro Y, Munakata A, Morita T. Correlation of lifting versus non-lifting and microscopic depth of invasion in early colorectal cancer. Gastrointest Endosc. 1999;50:329-333. [PubMed] |
| 19. | Kim MH, Lee SK, Seo DW, Won SY, Lee SS, Min YI. Tumors of the major duodenal papilla. Gastrointest Endosc. 2001;54:609-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Fleischer D. Endoscopic mucosal resection: (not) made in the USA (so commonly). A dissection of the definition, technique, use, and controversies. Gastrointest Endosc. 2000;52:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Appropriate use of gastrointestinal endoscopy. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 2000;52:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Ikeda Y, Takakuwa R, Hatakeyama H, Kawashima H, Sugihara T, Hosokawa Y, Hirao M. [Evaluation of endoscopic local injection of hypertonic saline- epinephrine solution and surgical treatment on hemorrhagic gastroduodenal ulcer]. Nihon Geka Gakkai Zasshi. 1989;90:1545-1547. [PubMed] |
| 23. | Lee SH, Park JH, Park do H, Chung IK, Kim HS, Park SH, Kim SJ, Cho HD. Clinical efficacy of EMR with submucosal injection of a fibrinogen mixture: a prospective randomized trial. Gastrointest Endosc. 2006;64:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Matsuura T, Enomoto S, Kakushima N, Imagawa A, Kobayashi K. Different mixtures of sodium hyaluronate and their ability to create submucosal fluid cushions for endoscopic mucosal resection. Endoscopy. 2004;36:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 25. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1895] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 26. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1324] [Article Influence: 53.0] [Reference Citation Analysis (0)] |