Published online May 28, 2013. doi: 10.3748/wjg.v19.i20.3062
Revised: April 4, 2013
Accepted: April 9, 2013
Published online: May 28, 2013
Processing time: 197 Days and 9.2 Hours
AIM: To assess the efficacy of glucomannan (GNN) as the sole treatment for abdominal pain-related functional gastrointestinal disorders (FGIDs).
METHODS: We conducted a double-blind, placebo-controlled, randomized trial. Patients were recruited among children referred to the Department of Paediatrics, Medical University of Warsaw. Included in the study were children aged 7-17 years with abdominal pain-related FGIDs classified according to the Rome III diagnostic criteria. The children were randomly assigned to receive GNN, a polysaccharide of 1,4-D-glucose and D-mannose, a soluble fiber from the Japanese Konjac plant, at a dosage of 2.52 g/d (1 sachet of 1.26 g 2 times a day), or a comparable placebo (maltodextrin) at the same dosage. The content of each sachet was dissolved in approximately 125 mL of fluid and was consumed twice daily for 4 wk.
RESULTS: Of the 89 eligible children, 84 (94%) completed the study. “No pain” and “treatment success” (defined as no pain or a decrease ≥ 2/6 points on the FACES Pain Scale Revised) were similar in the GNN (n = 41) and placebo (n = 43) groups [no pain (12/41 vs 6/43, respectively; RR = 2.1, 95%CI: 0.87-5.07) as well as treatment success (23/41 vs 20/43; RR = 1.2, 95%CI: 0.79-1.83)]. No significant differences between the groups were observed in the secondary outcomes, such as abdominal cramps, abdominal bloating/gassiness, episodes of nausea or vomiting, or a changed in stool consistency. GNN demonstrated no significant influence on the number of children requiring rescue therapy, school absenteeism, or daily activities.
CONCLUSION: In our setting, GNN, as dosed in this study, was no more effective than the placebo in achieving therapeutic success in the management of FGIDs in children.
Core tip: This study focused on abdominal pain-related functional gastrointestinal disorders (FGIDs) which are a common problem in children. The aim of the study was to assess the effectiveness of glucomannan (GNN), a soluble fiber of the Japanese Konjac plant, in alleviating the frequency and the severity of pain in children with FGIDs. We have demonstrated through our prospective, double-blind, placebo-controlled, randomized study that GNN in this setting for 4 wk was not effective in treatment of the FGIDs. The obtained results led us to the conclusion that further studies are needed to explore the role of GNN in the pathophysiology of functional disorders.
- Citation: Horvath A, Dziechciarz P, Szajewska H. Glucomannan for abdominal pain-related functional gastrointestinal disorders in children: A randomized trial. World J Gastroenterol 2013; 19(20): 3062-3068
- URL: https://www.wjgnet.com/1007-9327/full/v19/i20/3062.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i20.3062
Functional abdominal pain (FAP) is one of the most common reasons for a referral to a physician[1]. According to various estimates of the prevalence of FAP, approximately 13% to 38% of children and adolescents report functional abdominal problems[2].
The diagnosis of FAP is symptom-based. The Rome III criteria for abdominal pain-related functional gastrointestinal disorders (FGIDs) have been validated and assist clinicians make diagnoses[2]. The vast majority of children with FGIDs are subsequently diagnosed with functional dyspepsia (FD), irritable bowel syndrome (IBS), and childhood abdominal pain (AP)[3,4]. The symptoms associated with these diagnoses have a great impact on the patients’ quality of life, daily activities, and school absenteeism, which in turn can result in long-term psychological implications[2]. Therefore, patients as well as caregivers and physicians are interested in safe and reliable treatments to relieve troublesome symptoms. Unfortunately, the medical cause for these symptoms remains unclear, thus limiting currently available therapies. FGIDs continue to represent a significant challenge in approaches to patient management.
For most children with FGIDs, no organic causes for their pain are found on physical examination or during investigations[5]. One of the recent hypotheses regarding the pathophysiology of FGIDs proposes that the biological and environmental factors that alter the enteric flora and visceral perception cause the development of functional disturbances[2,6]. The associations between the enteric microbiota, immune activation, and the role of lumen-mucosa interaction have been explored recently[2,7,8]. However, a precise explanation of the etiology of FGIDs remains to be expounded.
Regardless of the lack of a biophysiological model for FGIDs, a variety of therapeutic options have been tried. Unfortunately, studies have proven that most options do not exert any significant influence on the natural history of FGID development[2]. Two recent Cochrane systematic reviews have revealed weak evidence for the benefits of medication or dietary manipulation in children with functional abdominal pain[9,10]. Only 2 small, randomized clinical trials (Christensen 1982, Feldman 1985) have been conducted in children with FGIDs, and they compared the effects of added dietary fiber with placebo[9,11,12]. The results and conclusions of these trials were not consistent. Nevertheless, many clinicians routinely recommend the use of bulking agents or dietary fiber to stimulate regular bowel movements and to improve the symptoms associated with FGIDs[2].
The goal of our study was to assess the efficacy of glucomannan (GNN) as the sole treatment for FGIDs in children. GNN, a polysaccharide of 1,4-D-glucose and D-mannose, is a soluble fiber from the Japanese Konjac plant. GNN exhibits properties that are typical of dietary fiber. Thus, it may provide a positive effect on many aspects of gut physiology and the appropriate functioning of enteric microbiota in patients with FGIDs[8,13].
We conducted a double-blind, placebo-controlled, randomized trial (RCT) from January 2009 to October 2011. The recommendations of the CONSORT 2010 Statement for reporting parallel group randomized trials were followed[14].
Patients were recruited among children referred to the Department of Paediatrics, the Medical University of Warsaw, from January 2009 to October 2011. The RCT included children aged 7-17 years with abdominal pain-related FGIDs classified according to the Rome III diagnostic criteria (Table 1)[5]. Patients with organic gastrointestinal diseases (as established by the medical history, complete blood count, urinalysis, stool examination for occult blood, ova and parasites, blood chemistries, abdominal ultrasound, breath hydrogen testing, and endoscopy, if needed), other chronic disease, or growth failure were excluded from the study. Additionally, during the time of the study, subjects were instructed not to take any medications that could influence the enteric flora, including antibiotics and commercially available probiotic or prebiotic preparations.
| Placebo | Glucomannan | |
| Patients recruited | 45 | 44 |
| Male/female | 21/22 | 21/20 |
| Age mean, yr | 11.3 ± 2.5 | 11.6 ± 3.0 |
| Self-reported frequency of pain1 | ||
| Pain 1-3 times per month | ||
| Pain 1-2 times per week | 12 (27.9) | 9 (21.9) |
| Pain > 2 times per week | 13 (30.2) | 9 (21.9) |
| Pain every day | 14 (32.6) | 14 (34.1) |
| 4 (9.3) | 9 (21.9) | |
| Severity of pain1 | ||
| “face 0” | 0 (0) | 0 (0) |
| “face 1” | 5 (11.6) | 2 (4.8) |
| “face 2” | 18 (40.8) | 13 (31.7) |
| “face 3” | 15 (34.8) | 16 (39.0) |
| “face 4” | 5 (11.6) | 8 (19.5) |
| “face 5” | 0 (0) | 2 (4.8) |
| Self-reported absenteeism from school | 21 | 21 |
| Self-reported alterations in daily activities | 29 | 27 |
| Number of subjects who required rescue therapy | 20 | 25 |
At the randomization visit, the inclusion criteria were checked. Potentially eligible patients were evaluated using a full review of their clinical histories and the results of a physical examination. The included subjects were randomized into a group receiving either GNN (Dicoman Junior, Vitis Pharma, Poland) at a dosage of 2.52 g/d, i.e., 1 sachet of 1.26 g 2 times a day, or a group receiving a comparable placebo (maltodextrin) at the same dosage. The content of each sachet was dissolved in approximately 125 mL of fluid and was consumed twice daily for 4 wk. The manufacturer had no role in the conception, design, or conduct of the study or in the data analysis.
Subjects were instructed to ingest the preparation twice a day, in the morning and in the evening, for 4 wk and to record any symptoms in a questionnaire at the end of the study. At randomization, parents received 28 sachets for the first 2 wk of the study. After this period, the parents were contacted to examine the children’s compliance with the treatment, provide them with the next 28 sachets, and schedule the exact timing of a final visit. The assessment of all outcome measures was based on the patients’ questionnaires collected at the final visit. Additionally, the subjects and/or caregivers were asked to report the following information in their diaries: compliance with consumption of the product and information on any other treatment given to the child during the study. The diary was constructed in a simple, understandable mode. The FACES Pain Scale Revised was used to assess the severity of pain[15]. The scale consists of 6 faces that reflect different levels of pain, ranging from a relaxed face on the left (no pain, indicating a score of 1 points - “face 0”) to a face showing intense pain on the right (highest pain possible, indicating a score of 6 points - “face 5”).
The primary outcome measures included the proportion of patients with self-reported no pain and that of patients with treatment success, which was defined as no pain or a decrease of ≥ 2/6 points on the FACES Pain Scale Revised[15]. The subjective assessment of pain frequency, abdominal cramps, abdominal bloating/gassiness, the number of episodes of nausea or vomiting; changes in stool consistency (loose or constipated stools) during the study were the secondary outcome measures. Furthermore, the frequencies of school absenteeism and changes in daily activities were assessed, as was the percentage of children requiring rescue therapy. Finally, all adverse effects were recorded, and their possible relation to the study product consumption was evaluated. Compliance was assessed by direct questioning of the subjects or their caregivers during clinic visits.
Block randomization, with a block size of 6, was performed using a computer-generated random number list prepared by an investigator with no clinical involvement in the trial. The sequence was concealed until all data were analyzed.
Both the participants and the researchers conducting the study were blinded. The study intervention product was prepared centrally by the hospital pharmacy at the Medical University of Warsaw with the assistance of independent personnel who were not involved in the conduct of the trial. The active product and the placebo were packaged in identical sachets and labeled with one of two codes, each allocated to the experimental product or placebo. This procedure was performed by an independent pharmacist, who was the only person aware of the codes’ meanings. The randomization numbers had been previously generated, and every patient eligible for inclusion received a consecutive number from the list. The appearance and texture of the dry placebo product were identical to those of the active product. When mixed with water, GNN turned into a substance of jelly-like consistency; however, this only occurred if the solution was not consumed within a few minutes, which was the recommended time limit for consumption.
The sample size was based on the treatment success outcome (i.e., the proportion of participants with no pain or a decrease of ≥ 2/6 points on the FACES Pain Scale Revised). It was estimated that an initial sample size of 80 patients would be sufficient to reveal a difference in the treatment effect of 30% (70% of the participants receiving GNN compared with 40% of the participants receiving placebo) considering that alpha = 0.05 and a power (beta) of 80%. The number of 80 for the children accounted for approximately 10% withdrawals or losses.
The computer software “R” [version 2.13.1 (2011-07-08)] was used for the analysis. Analyses of continuous data were performed with a parametric analysis (Student’s t-test) in the case of a normal distribution of variables. The Mann-Whitney test was implemented for non-normally distributed variables. The χ2 or Fisher’s exact test were used, as appropriate, for the analysis of dichotomous outcomes. The RR or the mean difference (MD) with a 95%CI was calculated using the computer software StatsDirect [version 2.7.8b (2011-11-09)]. The differences between the study groups were considered to be statistically significant when the P value was < 0.05 or when the 95%CI: for the RR did not exceed 1.0 or, for the MD, did not exceed 0. The results were analyzed using an Available Case Analysis (ACA).
Parents and children were fully informed about the schedule and the aims of the study. Informed consent was obtained from at least one caregiver of each child included in the study and from all children older than 12 years of age.
The Ethics Committee of The Warsaw Medical University approved the study. The trial was registered at ClinicalTrials.gov (http://clinicaltrial.gov), number NCT 01495806.
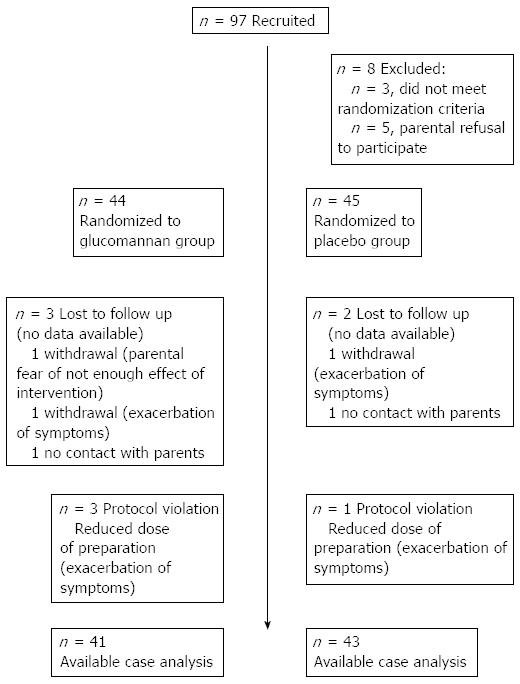
Figure 1 shows a flowchart of the subjects’ progression through the study. Of the 97 eligible children, 89 underwent randomization. Among them, 44 children were assigned to the experimental GNN group, and 45 children were assigned to the placebo group. Of the 89 randomized children, 84 (94%) completed the study. There was no significant difference in the drop-out rate between the 2 groups. The baseline demographic and clinical characteristics were similar between the experimental and placebo groups (Table 1).
Overall, 18 of the 84 (21%) subjects reported the primary outcome measure of ‘no pain’. The subjects in the GNN group were more likely to experience “no pain” (29%) than the subjects in the placebo group (14%); however, the difference was not statistically significant (RR = 2.1, 95%CI: 0.87-5.07).
“Treatment success” (defined as no pain or a decrease ≥ 2/6 points on the FACES Pain Scale Revised) was similar between the study groups. Of the 41 children in the GNN group, 23 (56%) experienced treatment success, compared with 20 of the 43 (47%) in the placebo group (RR = 1.21, 95%CI: 0.79-1.83).
Originally, we had also planned to conduct a subgroup analysis (e.g., FD, FAP, IBS). However, due to the small number of patients included in the subgroups and the overlap of symptoms between the patients with various types of functional disorders, we decided against conducting this analysis.
No significant differences between the GNN and placebo groups were observed in the secondary outcome measures, such as the subjective assessment of gastrointestinal symptoms: (1) Abdominal cramps (32% vs 49%, respectively; P = 0.12); (2) Abdominal bloating/gassiness (44% vs 51%, respectively; P = 0.39); (3) Number of episodes of nausea or vomiting (7% vs 2%, respectively; P = 0.34/24% vs 33%, respectively; P = 0.31); or (4) Change in stool consistency (loose stools: 29% vs 39%, respectively; P = 0.33; constipated stools: 27% vs 21%, respectively; P = 0.53).
The percentage of patients requiring rescue therapy was similar in both groups (GNN 19% vs placebo 14%, respectively; P = 0.53).
The GNN supplementation showed no significant influence on the frequency of school absenteeism (10% vs 14%, respectively; P = 0.56) or on changes in daily activities (27% vs 19%, respectively; P = 0.37) during the study.
The GNN was well tolerated, and no adverse effects were recorded in any of the patients. However, 4 patients in the GNN group complained of an exacerbation of symptoms (1 discontinued the therapy; 3 required a dose reduction) compared with 2 patients in the placebo group (1 discontinued therapy; 1 required a dose reduction). Nonetheless, it was difficult to establish causality in these cases because the course of FGIDs was defined by exacerbations and periods of recovery.
This prospective, double-blind, placebo-controlled, randomized study showed that GNN, a soluble fiber of the Japanese Konjac plant, as used in this study and setting for 4 wk, was not effective in reducing the frequency or severity of pain in children with abdominal pain-related FGIDs. Although “no pain” was more likely to occur in the GNN group, the difference was of borderline statistical significance.
The study groups did not differ with regard to any of the secondary outcomes, such as the proportion of patients with gastrointestinal symptoms during the study period, the need for rescue therapy, and the occurrence of adverse effects.
The optimal management strategy for abdominal pain-related FGDs in children is a matter of ongoing debate. Because of their obscure pathophysiology, the management of FGIDs remains challenging. This difficulty has prompted interest in new and safe treatment options, among them dietary interventions.
Given their safety profiles, prebiotics (especially soluble fiber) appear to be attractive therapeutic options for FGIDs[16]. Dietary fiber is not digested by human enzymes but is instead fermented by the flora of the large intestine[17,18]. There are several reasons why these agents might, in theory, prove to be beneficial in the management of functional disorders. First, some studies have demonstrated the positive effects of prebiotics on changes in the intestinal microbiota through the selective stimulation of the growth of potentially protective bacteria (bifidobacteria and, in part, lactobacilli) and the simultaneous inhibition of potentially pathogenic microorganisms[8,19-23]. Second, fiber increases biomass, feces weight and defecation frequency, which can alter the volume and composition of the stool and gas[8,13,18,24]. These changes in intestinal contents can affect the gastrointestinal symptoms associated with functional disturbances[2,8,18]. Additionally, a reduction in the pH and the release of short-chain organic acids stabilize the intestinal environment[8,16,19,25]. Finally, prebiotic-induced changes could indirectly modulate various parameters of the immune system, such as the NK-cell activity, the secretion of Il-10 and interferon, and the lymphocyte proliferation that may establish intestinal regularity[8,18,26].
Dietary fiber consumption has remained low in many populations, especially in children. The American Academy of Pediatrics recommends a daily dietary fiber intake for children of 0.5 g/kg body weight, up to 35 g/d[27]. Williams et al[28] proposed a minimum daily fiber intake equivalent to the age in years plus 5 g/d for children older than 2 years of age.
Few studies have evaluated the response of functional abdominal disorders to prebiotics. Furthermore, most data on the possible use of prebiotics in the management of functional disorders and the rationale for their use are derived from the studies of adults with IBS[16,19,26]. Until now, there have been only 3 published papers (Christensen 1982, Christensen 1986, Feldman 1985), which described 2 trials and assessed the effect of fiber supplementation on FGIDs in children[9]. These 2 trials, which met the inclusion criteria for the Cochrane systematic review, concerned the dietary interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in children[9]. Both studies involved a total of 92 children with recurrent abdominal pain, and they evaluated the effect of fiber supplementation on the improvement in gastrointestinal complaints[11,12]. According to this meta-analysis, the pooled odds ratio for improvement in the frequency of abdominal pain was 1.26 (95%CI: 0.25-6.29)[9]. Feldman et al[12] (52 children recruited) described changes in the intensity of pain; however, the differences between the fiber and placebo groups were not statistically significant. Christensen et al[11] (40 children recruited) reported the mean number of episodes of pain during the study period, but the differences in the results between groups were also not statistically significant. The lack of recent findings concerning the potential application of fiber in the management of children with FGIDs instigated our decision to examine the effectiveness of GNN, which is composed of a dietary fiber, i.e., water-soluble polysaccharide.
The optimal dose and treatment duration of GNN therapy have not yet been clearly established. Recently, we used GNN to treat functional constipation in children, where it proved to be relatively safe[13]. In the present study, regardless of the dietary recommendations for daily fiber intake, we used only one standard dose of fiber for all participants to provide a relatively easy administration regimen for the clinicians and parents. We applied a daily intake of 2.52 g/d, which exceeded the minimum dose of fiber suggested in the literature for therapeutic purposes[13].
Considering the chronic nature of functional disorders, the chosen duration of treatment (4 wk) appears to be optimal for evaluating GNN’s potential therapeutic influence on gastrointestinal complaints. The administration of GNN, a soluble fiber preparation, was the sole intervention implemented, allowing one to draw conclusions regarding its effectiveness without any confounding influence from other treatments.
Evaluating children as study participants is a complicated issue because they complain of nonspecific chronic abdominal pain and constitute a heterogeneous group of patients. To minimize the heterogeneity of our study’s population, the diagnosis was based on the well-recognized Rome III criteria[5].
The trial was conducted in a pediatric department oriented towards the diagnosis and treatment of children with functional abdominal pain. Nevertheless, the possibility that only less affected patients were included in the study could not be entirely eliminated. The pattern of the response to treatment may differ from that in patients with more severe courses of functional disturbances.
We used the appropriate methods to generate the allocation sequence and allocation concealment. We then strived to maintain the blinding of the selection, treatment, monitoring, data management, and data analyses throughout the study. In addition, the follow-up was appropriate. Data were obtained from more than the 94% of the participants. All of these features minimize the risk of systematic bias.
The lack of an apparent effect of GNN compared with the placebo may be explained by an inadequate dose and an overly brief treatment period. Although the optimal dose and duration of treatment for GNN have not yet been established, a tentative conclusion can be drawn: subjects with abdominal pain-related FGIDs require a modification of the GNN dose and an extension of the period of supplementation to improve their gastrointestinal symptoms. Additionally, nutritional habits can modify the natural course of abdominal complaints. We realized that precise data on the daily fiber intake would be useful. However, collecting that type of information would have required the use of a food diary. We abstained from this process for 2 major reasons. First, our study population of children aged 7-17 years included children who attended school. Collecting reliable information from children in this age group represents a particularly challenging task. Second, the use of a food diary, especially in this group of patients, provides only an approximate evaluation of dietary intake. Moreover, the validity of paper diary records is sometimes questionable. Well-known problems with paper diaries include poor adherence and retrospective recording[29]. As there were no differences between the groups in terms of the baseline characteristics, we may expect that the randomization was implemented properly and that the daily fiber intake was similar in both groups.
It is known that success in treating patients with abdominal pain-related FGIDs depends on the relationship established between a patient and a physician. An “active listening approach” and an enthusiastic, positive, and encouraging attitude towards treatment help improve subjects’ responses to both the therapeutic attempts and the placebo[30]. The placebo effect in adults ranges from 10%-70% for FD and from 0%-84% for IBS[6]. We used a placebo control group, which is considered an essential requirement for interventional studies[31]. According to our previous experiences based on earlier studies conducted in children with functional abdominal pain and functional constipation, we were able to predict the high proportion of children who were responsive to the placebo[3,13]. Considering our positive patient-physician relationship, we expected a drop-out rate of approximately 10% (6% at the end of the trial), in contrast to the 20% drop-out rate estimated for most trials. Thus, paradoxically, these positive relationships can be a potential weakness of our investigation.
Finally, another potential limitation of this trial is that although the overall number of patients was adequate, the study did not allow the analysis of the data for a specific diagnosis of abdominal pain-related FGIDs, e.g., FD, IBS or FAP.
Consequently, an obvious next step in future research should be to identify the characteristics of children with specific abdominal pain-related FGIDs who respond to GNN treatment. Identifying this group of patients will allow for the selection of the optimal GNN dose to improve long-term gastrointestinal symptoms. Further studies are also needed to explore the role of GNN in the pathophysiology of functional disorders.
In our setting, GNN as dosed in this study was no more effective than the placebo in achieving therapeutic success in the management of abdominal pain-related FGIDs in children.
Abdominal pain-related functional gastrointestinal disorders (FGIDs) are widespread complaints in the pediatric population, affecting from 13% to 38% of children and adolescents. The symptoms associated with FGIDs have a great impact on patients’ quality of life and their daily activities, which in turn can result in long-term psychological implications. Therefore a variety of therapeutic options have been considered to date.
Many clinicians routinely recommend the use of bulking agents or dietary fiber to stimulate regular bowel movements and to improve symptoms associated with FGIDs. However, only two small, randomized clinical trials (Christensen 1982, Feldman 1985) conducted in children with FGIDs, which compared the effects of added dietary fiber with placebo, were identified. The results and conclusions of these trials were not consistent.
Development of the optimal management strategy for abdominal pain-related FGIDs in children is difficult. Soluble fiber seems to be an attractive therapeutic option for FGIDs. Only a small number of studies that have evaluated the response of functional abdominal disorders to fiber are available. Additionally, the optimal dose and treatment duration have not been clearly established yet. In the present study, the standard daily dose (2.52 g/d) of glucomannan was used for all participants in order to provide a relatively easy administration regimen for clinicians and parents. The dose was equal to the minimum dose of fiber suggested in the literature for therapeutic purposes. Because of the chronic nature of functional disorders, the 4 wk duration of treatment was chosen to evaluate glucomannan’s potential therapeutic influence on gastrointestinal complaints. The most important contribution of this study and its strength is its methodology. The adequate methods for generation of the allocation sequence, allocation concealment and blinding of selection, treatment, monitoring, data management, and data analyses were used throughout the study. Data were obtained from more than the 94% of the participants. All of these features minimize the risk of systematic bias.
The study results suggest that, glucomannan was no more effective than placebo in achieving therapeutic success in the management of abdominal pain-related FGIDs in children.
Glucomannan, a polysaccharide of 1,4-D-glucose and D-mannose, is a soluble fiber from the Japanese Konjac plant. Glucomannan exhibits properties that are typical of dietary fiber. There are several reasons why these agents might, in theory, prove to be beneficial in the management of functional disorders. Some studies have demonstrated positive effects of fiber on changes in the intestinal microbiota by selective stimulation of the growth of potentially protective bacteria with simultaneous inhibition of potentially pathogenic microorganisms. Fiber increases biomass, feces weight and defecation frequency, which can in turn alter the volume and composition of stool and gas. These changes in intestinal contents can affect the gastrointestinal symptoms associated with functional disturbances.
This study employs a simple, methodologically sound model. The main conclusion of the study is the lack of influence of glucomannan supplementation on the level and duration of pain in children with FGIDs.
P- Reviewers Martellucci J, Sarin YK S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Saps M, Seshadri R, Sztainberg M, Schaffer G, Marshall BM, Di Lorenzo C. A prospective school-based study of abdominal pain and other common somatic complaints in children. J Pediatr. 2009;154:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 2. | Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4:293-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Gawrońska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Bausserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005;147:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1077] [Article Influence: 56.7] [Reference Citation Analysis (6)] |
| 6. | Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S-97S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Saps M, Pensabene L, Di Martino L, Staiano A, Wechsler J, Zheng X, Di Lorenzo C. Post-infectious functional gastrointestinal disorders in children. J Pediatr. 2008;152:812-86, 816.e1. [PubMed] |
| 8. | de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 9. | Huertas-Ceballos AA, Logan S, Bennett C, Macarthur C. Dietary interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database Syst Rev. 2009;CD003019. [PubMed] |
| 10. | Huertas-Ceballos A, Logan S, Bennett C, Macarthur C. Pharmacological interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database Syst Rev. 2008;CD003017. [PubMed] |
| 11. | Christensen MF. [Do bulk preparations help in cases of recurrent abdominal pain in children? A controlled study]. Ugeskr Laeger. 1982;144:714-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Feldman W, McGrath P, Hodgson C, Ritter H, Shipman RT. The use of dietary fiber in the management of simple, childhood, idiopathic, recurrent, abdominal pain. Results in a prospective, double-blind, randomized, controlled trial. Am J Dis Child. 1985;139:1216-1218. [PubMed] |
| 13. | Chmielewska A, Horvath A, Dziechciarz P, Szajewska H. Glucomannan is not effective for the treatment of functional constipation in children: a double-blind, placebo-controlled, randomized trial. Clin Nutr. 2011;30:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5694] [Cited by in RCA: 6416] [Article Influence: 427.7] [Reference Citation Analysis (108)] |
| 15. | Tomlinson D, von Baeyer CL, Stinson JN, Sung L. A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics. 2010;126:e1168-e1198. [PubMed] |
| 16. | Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 383] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 18. | Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401-1412. [PubMed] |
| 19. | Spiller R. Review article: probiotics and prebiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Satokari RM, Vaughan EE, Akkermans AD, Saarela M, De Vos WM. Polymerase chain reaction and denaturing gradient gel electrophoresis monitoring of fecal bifidobacterium populations in a prebiotic and probiotic feeding trial. Syst Appl Microbiol. 2001;24:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 498] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 22. | Malinen E, Krogius-Kurikka L, Lyra A, Nikkilä J, Jääskeläinen A, Rinttilä T, Vilpponen-Salmela T, von Wright AJ, Palva A. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J Gastroenterol. 2010;16:4532-4540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 155] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 23. | Bouhnik Y, Attar A, Joly FA, Riottot M, Dyard F, Flourié B. Lactulose ingestion increases faecal bifidobacterial counts: a randomised double-blind study in healthy humans. Eur J Clin Nutr. 2004;58:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Florent C, Flourie B, Leblond A, Rautureau M, Bernier JJ, Rambaud JC. Influence of chronic lactulose ingestion on the colonic metabolism of lactulose in man (an in vivo study). J Clin Invest. 1985;75:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 173] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Oufir LE, Barry JL, Flourié B, Cherbut C, Cloarec D, Bornet F, Galmiche JP. Relationships between transit time in man and in vitro fermentation of dietary fiber by fecal bacteria. Eur J Clin Nutr. 2000;54:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Whelan K. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care. 2011;14:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Carbohydrate and dietary fiber. In: Kleinman RE, editor. Pediatric nutrition handbook. 4th ed. Elk Grove Village, Il: American Academy of Pediatrics 1998; 203-211. |
| 28. | Williams CL, Bollella M, Wynder EL. A new recommendation for dietary fiber in childhood. Pediatrics. 1995;96:985-988. [PubMed] |
| 29. | Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient non-compliance with paper diaries. BMJ. 2002;324:1193-1194. [PubMed] |
| 30. | Kelley JM, Lembo AJ, Ablon JS, Villanueva JJ, Conboy LA, Levy R, Marci CD, Kerr CE, Kirsch I, Jacobson EE. Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom Med. 2009;71:789-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Veldhuyzen van Zanten SJ. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 244] [Article Influence: 12.8] [Reference Citation Analysis (0)] |