Published online Jan 14, 2013. doi: 10.3748/wjg.v19.i2.316
Revised: September 30, 2012
Accepted: October 16, 2012
Published online: January 14, 2013
The most frequent cause of pseudomembranous colitis is Clostridium difficile (C. difficile) infection. This type of colitis is characterized by an endoscopic pattern of numerous small, yellowish or whitish plaques diffusely distributed, which typically compromises the rectum extending to proximal colon. Occasionally, the pseudomembranes compromise only the transverse or right colon, but their exclusive localization over polyps has not been reported. In this case report we have described a patient with symptoms compatible with C. difficile infection and positive for C. difficile toxigenic culture. Colonoscopy examination showed two small polyps with a whitish surface, and histopathological analysis confirmed them to be pseudomembranes over tubular adenomas. The rest of the colonic mucosa was normal and no other cause was demonstrated. We suggest that this particular distribution might be due to a higher affinity for dysplastic cells such as adenomatous polyps of colon by C. difficile and/or its toxins.
- Citation: Hernández-Rocha C, Barra-Carrasco J, Guzmán AM, Paredes-Sabja D, Lezcano G, Zoroquiaín P, Álvarez-Lobos M. Atypical presentation of pseudomembranous colitis localized in adenomatous polyps. World J Gastroenterol 2013; 19(2): 316-318
- URL: https://www.wjgnet.com/1007-9327/full/v19/i2/316.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i2.316
The clinical spectrum of Clostridium difficile (C. difficile) infections may vary from mild diarrhea to life-threatening pseudomembranous colitis (PMC), which may localize from the proximal colon to the rectum[1]. Localized forms of PMC have been reported to compromise the transverse and right colon[2], but to the best of our knowledge, we have not found cases with exclusive localization of PMC over polyps. Below, we report a case with the pseudomembranes only covering the surface of adenomatous polyps.
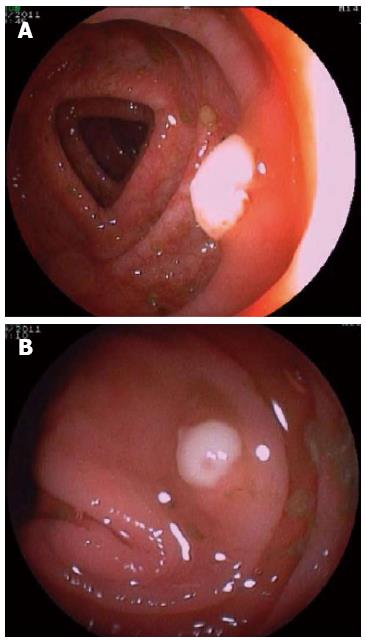
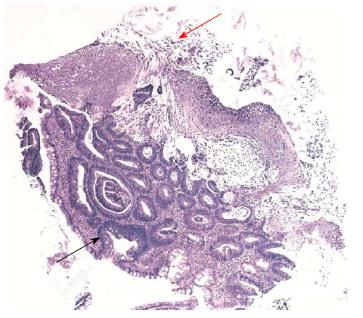
An 86-year-old female receiving anticoagulant therapy due to an aortic valve prosthesis with auricular fibrillation secondary to aortic stenosis was admitted with an incarcerated umbilical hernia. A hernioplasty was performed without complication, and she was treated with sodium piperacillin-tazobactam (4.5 g tid) intravenously for 7 d and discharged 10 d after surgery. Twenty-five days later, she presented hematochezia with hemodynamic compromise. She was admitted to the coronary unit, and an upper endoscopy showed a hiatal hernia with Cameron’s ulcers; she was treated with omeprazole. The next day a colonoscopy showed a clean colonic mucosa, absence of blood, and two sessile 6-mm polyps in the transverse colon and in the right colon. The upper surface of both polyps was covered by an adherent and whitish layer and the surrounding mucosa was normal (Figure 1). The polyps were resected with cold biopsy forceps and sent for histopathological study. Strikingly, the day after colonoscopy, the patient developed fever with no apparent cause in the physical exam, while laboratory results showed a hematocrit of 29%, white blood cell count of 8700 cell/μL without band forms and C reactive protein was 10.2 mg/dL. The chest X-ray was normal and the blood and urine cultures were negative. She was empirically treated with sodium piperacillin-tazobactam, but three days later she presented frequent loose stools and diffuse abdominal pain. An enzyme immune assay (EIA) for C. difficile toxin was positive. The antibiotic was stopped and she was treated with oral metronidazole with an excellent response. Presence of C. difficile was further confirmed by real time-polymerase chain reaction (PCR) and toxigenic culture. PCR-ribotyping indicated that the clinical isolate that caused C. difficile infection was not an epidemic strain (non-ribotype 027 strain). A few days later, the histopathological study of the polyps showed two tubular adenomas with a low-grade epithelial dysplasia and pseudomembranes on their surface with a typical volcanic appearance (Figure 2).
It is our understanding that this is the first documented case in which the pseudomembranes are exclusively localized over adenomatous polyps with the rest of the colonic mucosa being intact. The term PMC is nonspecific and describes an acute mucosal injury characterized by an endoscopic pattern of numerous, discrete and small (2-5 mm), raised, round and yellowish plaques (pseudomembranes) distributed over an erythematous but un-ulcerated mucosa[1]. The rectum is frequently involved, but in a few cases the sigmoid and/or right colon is compromised[2]. The histological pattern is characterized by a neutrophil-rich edema fluid in the lamina propria that bursts through tiny breeches to the surface epithelium, like a volcanic eruption, to form a characteristic punctate inflammatory pseudomembrane[1,3]. The PMC is due, in nearly all cases, to C. difficile. Other infrequent causes are ischemic or infectious colitis[4]. Piperacillin-tazobactam has been associated at lower rates with C. difficile infection than other antibiotics[5]; however, in this case report, the clinical course and the study with EIA, PCR and toxigenic culture confirmed an association with C. difficile and the response to treatment with oral metronidazole was concordant.
The specific type of colonic cells where C. difficile infections starts is unclear, but it is known that at least enterotoxin A, one of its main virulence factors, has remarkable cytotoxicity for cells derived from human or animal cancer, more than for normal colonic epithelial cells. One possible explanation, but by no means proven, is that the surface of neoplastic cells possesses a greater density of specific receptors for toxin A and/or toxin B as compared to less sensitive cell lines[6]. Alternatively, it is well known that members of the Clostridium genus have a tropism for tumors mainly due to the hypoxic environment as a consequence of poor vascular irrigation[7,8]. Thus, it seems plausible that colonic neoplastic cells might favor C. difficile colonization and PMC formation.
In conclusion, we propose that dysplastic changes of the colonic epithelium occurring as adenomatous polyps might represent a site of greater affinity for C. difficile toxin and/or tropism of C. difficile cells with the consequent formation of pseudomembranes, which were observed by an early colonoscopy in this patient.
P- Reviewer Chiarioni G S- Editor Gou SX L- Editor Logan S E- Editor Zhang DN
| 1. | Carpenter HA, Talley NJ. The importance of clinicopathological correlation in the diagnosis of inflammatory conditions of the colon: histological patterns with clinical implications. Am J Gastroenterol. 2000;95:878-896. [PubMed] |
| 2. | Tedesco FJ, Corless JK, Brownstein RE. Rectal sparing in antibiotic-associated pseudomembranous colitis: a prospective study. Gastroenterology. 1982;83:1259-1260. [PubMed] |
| 3. | Price AB, Davies DR. Pseudomembranous colitis. J Clin Pathol. 1977;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 161] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 763] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 5. | Mendez MN, Gibbs L, Jacobs RA, McCulloch CE, Winston L, Guglielmo BJ. Impact of a piperacillin-tazobactam shortage on antimicrobial prescribing and the rate of vancomycin-resistant enterococci and Clostridium difficile infections. Pharmacotherapy. 2006;26:61-67. [PubMed] |
| 6. | Kushnaryov VM, Redlich PN, Sedmak JJ, Lyerly DM, Wilkins TD, Grossberg SE. Cytotoxicity of Clostridium difficile toxin A for human colonic and pancreatic carcinoma cell lines. Cancer Res. 1992;52:5096-5099. [PubMed] |
| 7. | Wei MQ, Ren R, Good D, Anné J. Clostridial spores as live ‘Trojan horse’ vectors for cancer gene therapy: comparison with viral delivery systems. Genet Vaccines Ther. 2008;6:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Minton NP. Clostridia in cancer therapy. Nat Rev Microbiol. 2003;1:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |