Published online May 21, 2013. doi: 10.3748/wjg.v19.i19.2941
Revised: April 1, 2013
Accepted: April 9, 2013
Published online: May 21, 2013
Processing time: 143 Days and 10.8 Hours
AIM: To study gastric mucosal interleukine-8 (IL-8) mRNA expression, the cytotoxin-associated gene A (cagA) mutation, and serum pepsinogen (PG) I/II ratio related risk in Thai gastric cancer.
METHODS: There were consent 134 Thai non-cancer volunteers who underwent endoscopic narrow band imaging examination, and 86 Thais advance gastric cancer patients who underwent endoscopic mucosal biopsies and gastric surgery. Tissue samples were taken by endoscopy with 3 points biopsies. The serum PG I, II, and Helicobacter pylori (H. pylori) immunoglobulin G (IgG) antibody for H. pylori were tested by enzyme-linked immunosorbent assay technique. The histopathology description of gastric cancer and non-cancer with H. pylori detection was defined with modified Sydney Score System. Gastric mucosal tissue H. pylori DNA was extracted and genotyped for cagA mutation. Tissue IL-8 and cyclooxygenase-2 (COX-2) mRNA expression were conducted by real time relative quantitation polymerase chain reaction. From 17 Japanese advance gastric cancer and 12 benign gastric tissue samples, all were tested for genetic expression with same methods as well as Thai gastric mucosal tissue samples. The multivariate analysis was used for the risk study. Correlation and standardized t-test were done for quantitative data, P value < 0.05 was considered as a statistically significant.
RESULTS: There is a high non cagA gene of 86.8 per cent in Thai gastric cancer although there are high yields of the East Asian type in the positive cagA. The H. pylori infection prevalence in this study is reported by combined histopathology and H. pylori IgG antibody test with 77.1% and 97.4% of sensitivity and specificity, respectively. The serum PG I/II ratio in gastric cancer is significantly lower than in the non-cancer group, P = 0.045. The serum PG I/II ratio of less than 3.0 and IL-8 mRNA expression ≥ 100 or log10≥ 2 are significant cut off risk differences between Thai cancer and non-cancer, P = 0.03 and P < 0.001, respectively. There is a significantly lower PGI/II ratio in Japanese than that in Thai gastric cancer, P = 0.026. Serum PG I/II ratio at cut off less than 3.0 and IL-8 mRNA expression Raw RQ > 100 or log10 > 2 are significantly difference between Thai cancer group when compared to non-cancer group, P = 0.013 and P < 0.001, respectively. In the correlation study, low PG I/II ratio does not associate with chronic atrophic gastritis severity score in Thais non-cancer cases. However, there is a trend, but not significant convert correlation between IL-8 mRNA expression level and low PG I/II ratio in Thai positive H. pylori infection. The high expression of IL-8 gene demonstrates a poorer prognosis by stage and histology.
CONCLUSION: Predominant gastric mucosal IL-8 mRNA expression level, H. pylori infection, and low PG I/II ratio are relative risks for Thai gastric cancer without correlation with cagA mutation.
Core tip: A high level of interleukine-8 (IL-8) mRNA expression was detected in more than eighty percent of Thai gastric cancer patients and nearly two fold in the normal Thai population. The majority of northern Thai gastric cancer patients who had negative cagA gene Helicobacter pylori infection even with or without its mutation, still have a high IL-8 mRNA expression level. The pathogenesis of Thai gastric cancer may primarily involve another gate-way besides the bacterial factor. The results show that there is a predominantly cancer inflammation state regulated by IL-8 mRNA expression level that can be detected in Thai gastric cancer patients.
-
Citation: Yamada S, Kato S, Matsuhisa T, Makonkawkeyoon L, Yoshida M, Chakrabandhu T, Lertprasertsuk N, Suttharat P, Chakrabandhu B, Nishiumi S, Chongraksut W, Azuma T. Predominant mucosal
IL-8 mRNA expression in non-cagA Thais is risk for gastric cancer. World J Gastroenterol 2013; 19(19): 2941-2949 - URL: https://www.wjgnet.com/1007-9327/full/v19/i19/2941.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i19.2941
Gastric cancer pathogenesis is a well-known worldwide multifactorial condition. The gastric cancer incidence rate in Thailand ranks ninth by 4.1:100000 in males and 2.1:100000 in females. Despite being a low incidence country, northern Thailand has a higher gastric cancer incidence rate with 6.6:100000 in males that ranks fifth of overall cancer in the northern Thai region, and 4.5:100000 in females[1]. The author was interested in the carcinogenesis of gastric cancer in Thais, and why the incidence in Thais is much different from other East Asian countries. The interleukin-8 (IL-8) gene is one of the principal mediators for the inflammatory response gate way that was first reported in 1970s, and it is one of factors that are possible to affect gastric cancer carcinogenesis[2]. A recent case-controlled surveillance study in northern Thailand on cytokine gene IL-1b-511 mutations in three East Asian populations showed no predominantly correlated specific causative factor responsible for differences among ethnics and histologic types[3,4]. Therefore, the author proposed the study on other gate-ways of cytokine expression in the human gastric mucosal cell.
Recently, an in vitro study showed the association of the mucosal tissue IL-8 mRNA expression related to the Helicobacter pylori (H. pylori), positivity cytotoxin-associated gene A (cagA) gene. The East Asian genotype was reported in Japanese gastric cancer in about 85% of the cases. Many in vitro studies showed this toxicity gene related to gastric mucosal cell injury, inflammation, and oncogenic potential[5-7]. The cagA, East Asian genotype is commonly detected in chronic gastritis and gastric cancer of the Japanese[8,9]. There is reported data that a low serum pepsinogen (PG) I/II ratio of less than 3.0 with a PG I level of less than 70 ng/dL was considered as a high risk factor for Japanese gastric cancer[10,11]. There is no recent in vivo study reporting a correlation among these above factors, especially IL-8 and cyclooxygenase-2 (COX-2) mRNA expression level in Thais.
The author hypothesized that gastric mucosal tissue IL-8 mRNA expression may be different among ethnicities, and it may correlate to other reference pathogenesis factors. This study aimed to look for the risk and correlation of these factors in Thai gastric cancer. The level of IL-8, COX-2 mRNA expression, and cagA gene mutation distribution were also to be the first report in Thai gastric cancer.
Research methodology was considered and permitted by Thai and Japanese local ethical committees, the NRCT and Japan Society for the Promotion of Science code ID-NRCT 10726.
An experimental based cross-sectional study was conducted in the Gastrointestinal Surgery and Endoscopy Unit, Chiang Mai University Hospital from 2007 through 2010. Informed consents were obtained from 86 Thai gastric cancer patients who underwent narrow band imaging (NBI) endoscopy and gastric surgery during year 2007-2010, and 134 Thai non-cancer volunteers who underwent NBI endoscopic examination from 2006 to 2008. All gastric cancer patients in this study had locally advanced gastric cancer, and underwent examinations by endoscopy before curative gastric resection. Seventeen advanced stage Japanese gastric cancer and 12 non-cancer surveillance patients were recruited. Peptic ulcer disease was excluded in this study. Gastric mucosal tissue samples were taken by endoscopy with three biopsy sites for pathology and bimolecular genetic tests before surgical treatment. In cancer cases, biopsy points were specified from non-necrotic areas of the tumor. The histopathology description of the tumor and histologic type were defined. For pathological examination in both groups, chronic gastritis and metaplasia with H. pylori detection were classified with a modified Sydney Score System.
A 5 cc sample of venous blood was collected from each study participant. The red blood cell and serum separation was done, and preserved at -20 °C. The serum PG I, II, and immunoglobulin G (IgG) antibody for H. pylori were tested by the standard enzyme-linked immunosorbent assay technique. The standard cut off value used was a PG I level of more than 70 ng/mL or PG I/II ratio more than 3.0 for no atrophy or positive Grade 1, PG I < 70 ng/mL and PG I/II ratio < 3.0 excluding severe atrophy for moderate atrophy or positive Grade 2, and PG I < 30 ng/mL and PG I/II ratio < 2.0 for severe atrophy or positive Grade 3, respectively[10,11]. All samples were tested twice for reliability confirmation (Toyobo, co, Ltd., Japan)
The tissue H. pylori DNA extracted from the lower antral position in the stomach was examined by the polymerase chain reaction method, and genotyped for cagA mutation in all samples by the author (Samples were also examined by double blinded test by Toyobo, co, Ltd). The H. pylori positive control of cagA positive strain number 11638 (Western), 26695 (Western), and F57 (East Asian) were provided by the collaborative institute. The bacterial tissue DNA and genotyping method with primers used in this study were conducted as recently described. The specific oligonucleotide primers forward (5’-AAAAGCGACCTTGAAAATTCC-3’; nucleotides 2299-2319), reverse-1 (5’-CTTCATTTTTTTGAGCTTGTTGAGC-3’; nucleotides 2488-2463) and reverse-2 (5’-ATTAATGCGTATGTGGCTGTTAGTAGC-3’; nucleotides 3222-3195, were originally described by Azuma et al[12].
The AGS cell line was grown before cell collection for mRNA extraction at a cell count of 2 × 106-4 × 106. They underwent a total mRNA extraction protocol. The technique followed was a reverse transcriptase reaction using a commercial high capacity RNA-to-cDNA kit (Applied Biosystems)[13].
We conducted the experiment from three positions of gastric mucosal biopsies in all Thais and Japanese study participants. All of gastric mucosal tissue samples were transformed to cDNA after total mRNA extraction. The analysis was substantially correctable by analysis both in raw relative quantitation (RQ) and log10 value for adjusted normal distribution curve. All Human TaqMan probe primer express that was used in this study had 81-base pairs (bp) IL-8 specific human primer assay ID number Hs99999034_m1, 111- base pairs (bp) COX- 2 assay ID number Hs01573471_m1, and 121- base pairs (bp) specific human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Hs99999905_m1 those designed and supplied by Applied Biosystems, United States. The internal control was performed by GAPDH of a matched number template. The real-time relative quantitation value was measured by comparing to the base line value of AGS cell line subject control before making the analysis.
A student t-test was used for quantitative data, IL-8 and COX-2 mRNA expression level, and PG level. The χ2 test was used for qualitative data. The correlation study for pair factors was done in subgroup analysis for defined groups of ethnic, cancer and non-cancer populations. The multivariate analysis was used for risk study for both non-normal distribution and normal distribution curve data bases. STATA 11.0, United States and SPSS 16, United States were used for statistical analysis, and the P value of less than 0.05 was considered statistically significant.
There were 86 cases of advanced gastric cancer and 45 (33.8%) normal control cases, 46 (34.6%) non-peptic disease benign lesions without recent history of any treatment, and 42 (31.6%) chronic gastritis cases among 134 non-cancer control cases who were included in the genetic expression experiment. Thai male and female cancer incidences are 60.5% (52/86) and 34.0% (39/86), respectively. Males are also the predominant gender in Japanese. Both nations have significantly high incidence of gastric cancer at age 40 years old or above.
The H. pylori infection prevalence is reported by combined histopathology, H. pylori IgG antibody level, and 23S rDNA results that have 77.1% and 97.4% of sensitivity and specificity, respectively. Among Thai cancer patients and non-cancer volunteers, H. pylori prevalence was 72.1% and 71.6%, respectively. Meanwhile, Thai gastric cancer cases had a cagA genotype demonstrated in only 7/62 (12.3%) in positive H. pylori infection cases by 23S rDNA that yields six cases of East Asian type and one case of Western type. In non-cancer volunteers, there were 62/98 (63.9%) of positive cagA and 34/98 (36.1%) of negative cagA genotyping in positive H. pylori infection cases that yielded 47.7% of East Asian, 27.4% of Western, and 24.9% of Mixed genotype. For the six year follow up of 18 cases of high grade chronic atrophic gastritis (CAG group) in non-cancer Thais who had long term H. pylori cagA East Asian type infection, no one has developed gastric cancer.
The enzyme PG results, showed a significantly lower PG I/II ratio with a mean of 3.3 ± 1.7 in gastric cancer patients than one in non-cancer volunteers, P = 0.045, and of other CAG, P = 0.002. There is a significantly lower PG I/II ratio in Japanese gastric cancer than in Thai gastric cancer, P = 0.026.
For IL-8 and COX-2 mRNA expression results, 86 Thai gastric cancers were tested successfully in comparison with 134 Thai non-cancer volunteers. The detection rates of IL-8 mRNA expression were 77/86 (89.5%) in Thai gastric cancer and 102/134 (74.6%) in Thai non-cancer volunteers. Thai population characteristic data that was examined for IL-8 mRNA expression is demonstrated in Table 1. Serum enzyme PG I, II level, and H. pylori infection status are demonstrated in the cancer population and non-cancer volunteers in Thais is demonstrated in Table 2. We found a remarkable number of Thai gastric cancers with a negative cagA; therefore, IL-8 mRNA expression was examined and the cut-off point of expression value difference is demonstrated in Table 3. Serum PG I/II ratio at cut-off point of less than 3.0 and raw RQ ≥ 100 or log10≥ 2 of IL-8 mRNA expression level showed the significantly different between the Thai gastric cancer group and the non-cancer group, P = 0.045 and P < 0.001, respectively. In the multivariate analysis application, the four co-factors related to gastric cancer risk including IL-8 mRNA expression in Thais are shown in Table 4.
| Variable | Cancer (n = 86) | Benign (n =134) |
| Sex | ||
| Male | 52 (60.5) | 41 (30.6) |
| Female | 34 (39.5) | 93 (69.4) |
| Age (yr) | ||
| < 40 | 5 (5.8) | 28 (20.9) |
| ≥ 40 | 81 (94.2) | 106 (79.1) |
| mean ± SD | 56 ± 11.3 | 48.5 ± 11.2 |
| Alcohol drinking | ||
| No | 50 (58.1) | 80 (59.7) |
| Yes | 36 (41.9) | 54 (40.3) |
| Smoking | ||
| No | 62 (72.1) | 122 (91.0) |
| Yes | 24 (27.9) | 12 (9.0) |
| Diseases | ||
| Normal | - | 45 (33.8) |
| Benign lesion (polyps, erosion, mild superficial gastritis) | - | 46 (34.6) |
| Chronic active gastritis | - | 42 (31.6) |
| Cancer | 86 (100.0) | - |
| Variable | Cancer (n = 86) | Benign (n = 134) | P value |
| PG I/II, (ng/μL), mean ± SD | |||
| I | 57.39 ± 46 | 54.86 ± 68.5 | 0.780 |
| II | 19.42 ± 21 | 15.42 ± 11.7 | 0.090 |
| PG I/II ratio | |||
| ≤ 3 | 28 (39.4) | 34 (27.9) | 0.0451 |
| > 3 | 43 (60.6) | 88 (70.1) | |
| H. pylori pathology | |||
| Negative | 31 (36.8) | 60 (44.8) | 0.001 |
| Positive | 55 (63.2) | 74 (55.2) | |
| Serum IgG | |||
| Negative | 31 (46.3) | 57 (44.2) | 0.8201 |
| Positive | 36 (53.7) | 72 (55.8) | |
| CagA genotyping in positive 23S rDNA | |||
| Negative | 55 (88.7) | 62 (64.6) | < 0.0011 |
| Positive | 7 (12.3) | 34 (35.4) | |
| H. pylori Infection status | |||
| True negative | 20 (24.4) | 37 (36.0) | 0.1201 |
| True positive | 62 (75.6) | 96 (64.0) | |
| Variable | Cancer (n = 86) | Benign (n = 134) | P value |
| COX2 raw RQ | |||
| No expression detection | 30 (34.90) | 43 (53.10) | < 0.0011 |
| Expression detection | 56 (65.10) | 38 (46.90) | |
| COX2 raw RQ | |||
| mean ± SD | 41.69 ± 4.90 | 5.37 ± 4.20 | < 0.0011 |
| COX2 log10 (N, %) | |||
| mean ± SD | 1.62 ± 0.96 | 0.73 ± 0.62 | < 0.0011 |
| IL-8 raw RQ | |||
| No expression detection | 9 (10.47) | 32 (25.37) | < 0.001 |
| Expression detection | 77 (89.53) | 102 (74.63) | |
| IL-8 raw RQ | |||
| ≤ 100 or undetected | 33 (38.37) | 105 (78.36) | < 0.001 |
| > 100 | 53 (61.63) | 29 (21.64) | |
| mean ± SD | 9615.64 ± 49715.00 | 2262.29 ± 10454.60 | < 0.010 |
| IL-8 log10 | |||
| ≤ 2 or undetected | 32 (37.21) | 105 (78.36) | < 0.001 |
| > 2 | 54 (62.79) | 29 (21.64) | |
| mean ± SD | 2.62 ± 1.10 | 1.49 ± 1.20 | < 0.010 |
| Variable | OR | 95%CI | P value |
| Male | 4.32 | 2.06-9.04 | < 0.001 |
| H. pylori infection status | 0.98 | 0.96-0.99 | 0.020 |
| PG II/I ratio ≤ 3 | 2.06 | 0.94-4.47 | 0.060 |
| IL-8 mRNA expression | 7.97 | 3.75-16.97 | < 0.001 |
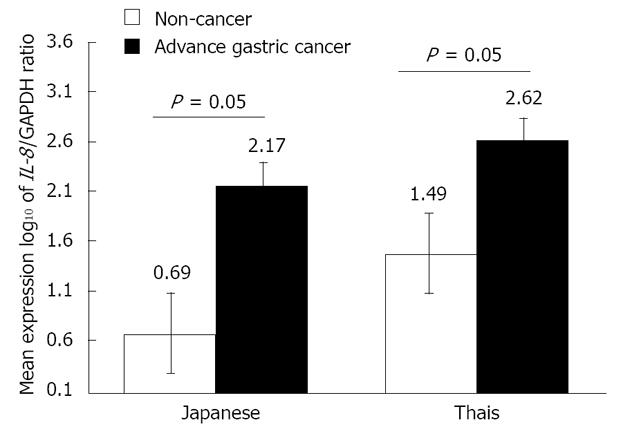
At the same stage of advanced gastric cancer, the mean levels of IL-8 mRNA expression in Thai cancer and Japanese cancer were 9615.65 (log10 = 2.62) and 1509.11 (log10 = 2.17), respectively, P = 0.014. For gastric cancer risk at cut-off IL-8 expression level by log10 greater than two 2 in Thais and Japanese, odds ratio (OR) = 7.97 (95%CI: 3.75-16.97, P < 0.001) and OR = 4 (95%CI: 1.29-12.40), respectively. In the non-cancer group, we found that the IL-8 mRNA expression level was lower than cancer population with a significant difference, P < 0.001. The total mean IL-8 mRNA expression in non-cancer Thais was 2262 (log10= 1.49) while that in Japanese non-cancer was 10.79 (log10 = 0.69), P < 0.001. In comparison within the same ethnic group, the mean levels of IL-8 mRNA expression in Thai and Japanese cancer were higher than those in non-cancer, P = 0.05 as showed in Figure 1.
The COX-2 mRNA expression did not indicate significant rising level with detection rate of 65% in Thai and Japanese gastric cancer. In comparison with IL-8 mRNA expression, although the level of COX-2 mRNA expression was slightly higher in gastric cancer than normal gastric mucosal tissue, there were much lower levels than those of IL-8 mRNA expression.
In the correlation study, low PG I/II ratio was not associated with the CAG severity score in Thai non-cancer cases because of a few number of CAG in both Thai gastric cancer and non-cancer populations in this study. There was no significant difference for the IL-8 mRNA expression level in cancer between positive and negative H. pylori infection. There was no direct correlation of IL-8 mRNA expression level and serum IgG levels. In subgroup analysis, there was a significant difference of higher levels in groups of poorly differentiated histopathology in comparing both nations. For the diffuse histologic type, the IL-8 mRNA expression level is about 1.5 times higher than that of intestinal histologic type with a statistically significant difference in Japanese.
High IL-8 mRNA expression was primarily found in the non-cagA Thai gastric cancer population. There was a significantly different mean IL-8 mRNA expression level between groups of negative cagA by log10 = 2.46 (± 1.04) and positive cagA by log10 = 3.29 (± 1.68) in the Thai gastric cancer group. However, there were few numbers of Thai gastric cancers with positive cagA. In other subgroup analysis of 18 Thais who had high grade CAG, some level of IL-8 mRNA expression in the 12 Japanese non-cancer patients appeared which an equivalently lower level.
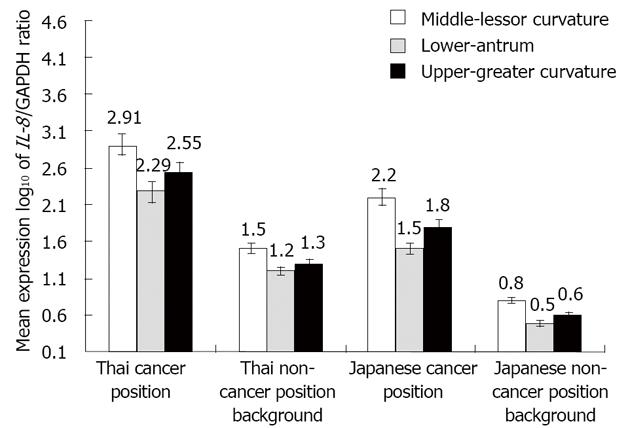
Gastric cancer mucosal tissue IL-8 mRNA expression in the cancer position had a significantly higher mean level than its level at the non-cancer background position in both Thai and Japanese shown in Figure 2. There was significantly different IL-8 mRNA expression level between intestinal (favorable) and diffuse (unfavorable) histologic cell types. In Thai gastric cancer, the poorly differentiated gastric adenocarcinoma and signet ring cell were predominantly found in this study. The log10IL-8 mRNA mean expressions in unfavorable cell type were 2.55 and 2.85 in Japanese and Thais, respectively, as shown in Table 5. There is a significantly higher level of IL-8 mRNA expression in diffuse cell type than that in a differentiated histologic cell type, P = 0.04. The differentiated histologic cell type in Thais has higher expression level than that in Japanese with a statistically significant difference, P = 0.013 The RT-PCR results of gastric mucosal tissue and AGS cell line IL-8 mRNA expression were demonstrated in Figure 3. and Figure 4. However, there was no significant difference of IL-8 mRNA expression level between positive and negative H. pylori infection in subgroup analysis of non-cancer background positions.
| Histopathology | Thai (n = 77) | Japanese (n = 17) | P value |
| Diffuse type | 55 (71.4) | 4 (23.5) | 0.01 |
| mean ± SD | 2.85 ± 1.10 | 2.55 ± 0.52 | 0.95 |
| Intestinal type | 22 (28.6) | 13 (76.5) | |
| mean ± SD | 2.52 ± 1.11 | 1.56 ± 1.06 | 0.01 |
In summary, IL-8 mRNA expression level is predominantly found, and trend toward an inverted correlation to PG I/II low ratio in Thai gastric cancer patients. There is no direct correlation of IL-8 mRNA expression level with the cagA gene mutation in Thai gastric cancer.
In the present in vivo study, there is a significant risk of IL-8 mRNA expression level predominantly found rising up more than 80% of northern-Thai gastric cancer. There is significantly higher level of IL-8 mRNA expression in poorly differentiated than in well differentiated carcinoma in both Thailand and Japan. There is a trend of converted independent correlation with the very low PG I/II ratio in gastric cancer as well as a few numbers of Thai severe CAG, but no direct correlation with positive H. pylori infection or cagA genotypes.
Higher level IL-8 mRNA expression in non-cancer Thais comparing with non-cancer Japanese demonstrated the difference of gastric mucosal defense and genetic expression between the two nations. Thai gastric cancer has less background of CAG. In this study, there is no evidence that showed a direct correlation of IL-8 mRNA expression with cagA mutation genotype in H. pylori positive cases. Predominant IL-8 mRNA expression level resulted in non-atrophic mucosa of both gastric cancer and non-cancer Thais. The result is different from previous in vitro or some in vivo studies in high incidence gastric cancer countries, such as Japan, China, and South Korea.
In Thai gastric cancer patients, IL-8 mRNA expression level at the lessor curvature is the most represented location. Its predominantly high level may represent relatively vascular invasion as well as major gastric mucosal inflammation. However, the area of gastric antrum in Thai gastric cancer patients has less activity than the lessor curvature because atrophy occurs more frequently.
Also, a high level of IL-8 mRNA expression is matched with the poor prognosis by histopathology cell type and tumor stage. Long-term bacterial infection has less effect to change the gastric mucosa into CAG. In our recent six year followed up study in non-cancer cases controlled with positive H. pylori infection and without eradication of 500 new non-cancer cases, a few people developed severe gastritis and still have a high PG I/II ratio. In this study, the number of high PG I/II ratio in Thai cancer is still about 45% which is nearly the same percentage in our recently published study[4].
A few gastric cancer preventive models on natural Thai products were reported[14]. There is one study on diet consumption in Thais showing a linkage of gastric cancer risk. The factors which were found to be a higher risk but not statistically significant were low intake of vegetables and fruits (OR = 1.2, 95%CI: 0.74-1.96) and Jeaw prik (mainly chilly with Plara broth or pickled fish), a kind of preserved food in North and North-eastern regions of Thailand (OR = 1.2, 95%CI: 0.76-2.01)[15]. The consensus of the Asian Pacific guideline on gastric cancer prevention is still debated in some experts’ opinions[16]. H. pylori infection screening in a low risk gastric cancer population is not recommended, but serum PG may be helpful to screen the high risk population in northern Thailand. We reported its different characteristics that rely on H. pylori IgG antibody and PG I/II ratio in our cancer populations comparing to the data in a Japanese report[17].
The Japanese study primarily reported the relation of cagA genotype and a low PG I/II ratio. Nevertheless, approximately half of the Thai cancer population demonstrates a low ratio of PG I/II similar to the Japanese. There are still a small number of Thais who had a severe atrophy score related to H. pylori infection though in the positive cagA Thai population.
In this study, the negative cagA gene is found in the majority of Thai gastric cancer unlike the Japanese. In Thais, the poorly differentiated cell type gastric adenocarcinoma occurred mainly in the negative cagA gene H. pylori infection, not in Western type cagA.
There is a small number of the Thai population who had a severe atrophy score of gastric mucosa found in our recent and present collaborative study[18]. Reduction of the fundic gland in chronic gastritis was also related to the low level of PG I/II ratio in Japanese[19,20]. In our recent study of subgroup analysis on the PG I/II ratios, there was no significant difference between CAG and gastric cancer group, P = 0.12. However, the low PG I/II ratio was significantly related to gastric cancer when compared to normal population in a recent match-case control study by OR of 2.3 (95%CI: 1.10-4.80), P = 0.025[4]. The tumor location demonstrated locations mainly at the upper portion and corpus in both of our studies. In the present study, the author found risk for cancer by OR 2.06 (95%CI: 0.94-4.47), P = 0.059 that seems to be close to the result of our recent study. The low PG I/II ratio was not found to be a high percentage in Thais unlike in Japanese gastric cancer[9].
For other genetic host factors, in one interesting study, two of the four gastric carcinoma cell lines expressed vascular endothelial growth factor (VEGFR-3) mRNA. In 17 of 36 gastric carcinoma specimens, VEGFR-3-specific immune activity was detected in tumor cells. These angiogenesis and lymphangiogenesis were also detected in VEGF-C-transfected tumors than in control tumors[21]. IL-8 mRNA expression is found to be the gate way mechanism of the vascular epithelial growth factor. For IL-1 gene, it is a pro-inflammatory cytokine, and the T/T genotype of IL-1β-511 is suspected as the risk factor of both hypochlorhydria related H. pylori infection and gastric cancer in a case-control population in the United States[22]. The author reported that c/c genotype was a risk in Japanese, and a lower number of c/c genotype was found as a minor risk related to Thai gastric cancer[4].
Recent in vivo animal models studies showed the expressions of IL-8 and COX-2 had linkage to the epithelial cell which was co-infected with H. pylori. However, our preliminary study reported that there was no difference of IL-8 mRNA expression level between a cell line which was co-infected with H. pylori and the Thai gastric mucosa tissues which had positive or negative H. pylori[23,24]. The toleration, remarkable host response to cancer inflammatory process, healing of stomach mucosal turnover rate, and re-healing process of ulcer in Thais may be different and caused by host susceptible differences to the virulence bacteria.
The theory regarding inflammatory cytokine’s influence on cancer development was first contributed by Rudolph Virchow around 150 years ago[25]. Many studies regarding IL-8 gene expression remarkably found significant relation with H. pylori infection and many cag pathogenicity island both in vitro[26,27] and in some number in in vivo study of Japanese cancer[28,29]. However, no study has demonstrated differences of expression level in the individualized host[30].
In this study, Japanese gastric cancer has a lower IL-8 mRNA expression on average than that in Thai gastric cancer patients at the same stage of disease and H. pylori infection status. However, Japanese gastric adenocarcinoma cases are mostly an intestinal type and infected by positive cagA strain H. pylori. In contrast with northern Thai gastric adenocarcinoma cases, they are mostly diffuse histologic cell type, and infected by negative cagA strain H. pylori. Therefore, the authors speculate that the predominant level of IL-8 mRNA expression found in non-cagA gene H. pylori infection is not directly related to atrophic gastritis mucosa in Thai gastric cancer.
Some results in this study are unexpected outcomes and different from our recent knowledge of in vitro and in vivo study in Japanese atrophic gastric mucosa which almost easily infected by positive cagA H. pylori infection. The cagA was also the suspected cause of the IL-8 gene expression rising.
This result in Thais showed that gastric mucosal tissue IL-8 mRNA expression has a higher level in the advanced stage and poorer differentiated cell type than in favorable histology or differentiated cell type. The author was suspicious that the less atrophic background of Thai stomach cancer and non-cancer gastric mucosa may be caused by non-cagA H. pylori infection. However, the high level of mRNA IL-8 gene expression in Thai gastric cancer cases may be explained by the cancer inflammation carcinogenesis that may not be directly related to only H. pylori infection in Thais.
The level of IL-8 mRNA expression in Thai gastric cancer or poorly differentiated gastric carcinoma may be regulated by other factors besides of H. pylori infection. Also, unknowns remain regarding how long IL-8 mRNA expression has been high before the occurrence of gastric cancer or after becoming a more advanced stage. Although the author analyzed the level of IL-8 and COX-2 mRNA expression level in normal mucosa and of advanced gastric cancer, this occurrence could not be shown in early gastric cancer. The environmental factors and bacterial virulence effect cannot be excluded.
In this study, the signet ring cell type is predominantly found in Thai gastric cancer population. The poor prognostic histological cell type may have different disease carcinogenesis related to gastric mucosal tissue IL-8 mRNA high expression level and severity of the disease. The COX-2 mRNA expression level is directly correlated with only the H. pylori infection, and tended to be suppressed unlike IL-8 mRNA expression. The author supposed that extremely high gastric mucosal IL-8 expression level may relate to other factors, such as VEGF that could not be demonstrated in this study and should be explored further.
In conclusion, this present in vivo study shows results of new factual data on predominant gastric mucosal IL-8 mRNA expression level in Thai gastric mucosal biopsy tissues in both non-cancer and gastric cancer volunteers. In the present study, the northern Thai gastric cancer population has a high incidence of signet ring cell by the nature of histologic type. The positive H. pylori may be one of the co-factors, though the host is infected with non-cagA gene and still has an extremely high IL-8 mRNA expression level. This cytokine expression may represent the individual host defense in both high and low incidence gastric cancer ethnics. The factual results in an experimental based study demonstrated how prevalence of the northern Thai gastric cancer host had active co-infection or had been recently infected by non-cagA gene H. pylori infection. The IL-8 mRNA expression level does not directly correlate to non-cagA H. pylori infection in Thai gastric cancer. However, there is a trend of converted correlation between IL-8 mRNA expression and low ratio PG I/II without statistical significance, and it seems to be an independent correlation. By these preliminary results, the author expected to do further study on IL-8 mRNA expression that may act as one of prognostic genetic biomarkers in clinical practice and for chemotherapy application in the nearby future. A study on the current cancer chemotherapy with northern Thai gastric cancer population is on-going.
This study with preliminary results was presented in a free oral paper symposium session of Oncology and Stomach session, International Surgical Week 2011, in Yokohama during August 28-September 1.
The incidence of gastric carcinoma is very low although the incidence of Helicobacter pylori (H. pylori) seemed not to be low in Thais. However, the northern Thai population still has the highest gastric cancer incidence in Thailand. Currently, the disease incidence is rising faster than in the past and still the second cause of cancer death worldwide. The cause and risk factors for gastric cancer carcinogenesis in Thais is unclear especially the risk related with H. pylori infection or other cofactors.
Interleukine-8 (IL-8) mRNA expression is a common event found in some epithelial malignancies and in gastric adenocarcinoma either due to H. pylori caused chronic inflammation or other causes by unrelated carcinogens. It is not clear how the level of this expression related to gastric adenocarcinoma. The authors demonstrated that the predominant overexpression of IL-8 mRNA could be a potential relative risk for gastric adenocarcinoma in Thais and demonstrated the difference of its level in relationship with the histologic type of gastric cancer.
Recent reports have highlighted the importance of cytotoxin-associated gene A (cagA) H. pylori infection and its mutation type that is shown predominantly in Japanese gastric cancer carcinogenesis. Particularly in the well differentiated histologic type, the IL-8 mRNA expression level in the Japanese seems to be much lower than in Thais. However, its level in poorly differentiated cell type of Thai gastric adenocarcinoma has less atrophic background and a higher level of expression than in well differentiated gastric adenocarcinoma. This is the first study to report how measurement of IL-8 mRNA expression level demonstrates risk in non-cagA H. pylori infection of Thai gastric cancer and the trend of differences in carcinogenesis related to H. pylori infection between high and low incidence ethnics. Furthermore, their in vivo studies would suggest that the IL-8 mRNA expression level yields high prevalence detection in gastric adenocarcinoma and may be a useful tool for gastric cancer prognostic or therapeutic study.
By understanding different IL-8 mRNA expression levels, this study may represent a future study with a tissue molecular biomarker for gastric cancer.
IL-8 mRNA expression is pro-inflammatory cytokines that is detected in gastric epithelial mucosa and gastric cancer cell lines, such as Kato III and AGS cells.
The authors examined the expression of IL-8 and cyclooxygenase-2 in AGS cell line, normal gastric mucosa, gastritis, and gastric adenocarcinoma tissues. It revealed that IL-8 mRNA expression predominantly increased in poorly differentiated or signet ring cell gastric adenocarcinoma that showed the trend of a poorer prognosis. The expression was not directly correlated to cagA H. pylori infection and its mutation type. The results are interesting and may represent a different carcinogenesis of Thai gastric cancer in comparison to recent Japanese studies.
P- Reviewer Aoyagi K S- Editor Wen LL L- Editor A E- Editor Xiong L
| 1. | Khuhaprema T, Srivatanakul P. Stomach. Gastric Cancer. In: Cancer in Thailand Vol. IV, 1998–2000. Bangkok: Bangkok Medical Publisher 2007; 32-33. |
| 2. | Kozlov SV. Inflammation and cancer. Methods and protocols. Volume 1: Experimental models and practical approaches. Preface. Methods Mol Biol. 2009;511:v-viii. [PubMed] |
| 3. | Matsukura N, Yamada S, Kato S, Tomtitchong P, Tajiri T, Miki M, Matsuhisa T, Yamada N. Genetic differences in interleukin-1 betapolymorphisms among four Asian populations: an analysis of the Asian paradox between H. pylori infection and gastric cancer incidence. J Exp Clin Cancer Res. 2003;22:47-55. [PubMed] |
| 4. | Yamada S, Matsuhisa T, Makonkawkeyoon L, Chaidatch S, Kato S, Matsukura N. Helicobacter pylori infection in combination with the serum pepsinogen I/II ratio and interleukin-1beta-511 polymorphisms are independent risk factors for gastric cancer in Thais. J Gastroenterol. 2006;41:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Crabtree JE, Wyatt JI, Trejdosiewicz LK, Peichl P, Nichols PH, Ramsay N, Primrose JN, Lindley IJ. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61-66. [PubMed] |
| 6. | Wu K, Crusius JB, Fan D, Peña AS. The immunogenetics and pathogenesis of gastric cancer. Highlights of the First Sino-European Workshop on the Immunogenetics and Pathogenesis of Gastric Cancer. Drugs Today (Barc). 2002;38:391-417. [PubMed] |
| 7. | Aihara M, Tsuchimoto D, Takizawa H, Azuma A, Wakebe H, Ohmoto Y, Imagawa K, Kikuchi M, Mukaida N, Matsushima K. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN45. Infect Immun. 1997;65:3218-3224. [PubMed] |
| 8. | Sasazuki S, Inoue M, Iwasaki M, Otani T, Yamamoto S, Ikeda S, Hanaoka T, Tsugane S. Effect of Helicobacter pylori infection combined with CagA and pepsinogen status on gastric cancer development among Japanese men and women: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2006;15:1341-1347. [PubMed] |
| 9. | Kikuchi S, Wada O, Miki K, Nakajima T, Nishi T, Kobayashi O, Inaba Y. Serum pepsinogen as a new marker for gastric carcinoma among young adults. Research Group on Prevention of Gastric Carcinoma among Young Adults. Cancer. 1994;73:2695-2702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Azuma T, Yamazaki S, Yamakawa A, Ohtani M, Muramatsu A, Suto H, Ito Y, Dojo M, Yamazaki Y, Kuriyama M. Association between diversity in the Src homology 2 domain--containing tyrosine phosphatase binding site of Helicobacter pylori CagA protein and gastric atrophy and cancer. J Infect Dis. 2004;189:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Jones KR, Joo YM, Jang S, Yoo YJ, Lee HS, Chung IS, Olsen CH, Whitmire JM, Merrell DS, Cha JH. Polymorphism in the CagA EPIYA motif impacts development of gastric cancer. J Clin Microbiol. 2009;47:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Azuma T. Helicobacter pylori CagA protein variation associated with gastric cancer in Asia. J Gastroenterol. 2004;39:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Keith WN, Hoare SF. Detection of telomerase hTERT gene expression and its splice variants by RT-PCR. Methods Mol Med. 2004;97:297-309. [PubMed] |
| 14. | Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Suwanrungruang K, Sriamporn S, Wiangnon S, Rangsrikajee D, Sookprasert A, Thipsuntornsak N, Satitvipawee P, Poomphakwaen K, Tokudome S. Lifestyle-related risk factors for stomach cancer in northeast Thailand. Asian Pac J Cancer Prev. 2008;9:71-75. [PubMed] |
| 16. | Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, Xiao SD, Lam SK, Goh KL, Chiba T. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 17. | Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 373] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 18. | Matsuhisa TM, Yamada NY, Kato SK, Matsukura NM. Helicobacter pylori infection, mucosal atrophy and intestinal metaplasia in Asian populations: a comparative study in age-, gender- and endoscopic diagnosis-matched subjects. Helicobacter. 2003;8:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Miki K, Ichinose M, Shimizu A, Huang SC, Oka H, Furihata C, Matsushima T, Takahashi K. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn. 1987;22:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 216] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Matsuhisa T, Yamada S. The Helicobacter Pylori Infection in Asia (Japanese-English). Chiyoda-ku, Tokyo: Nishinura Shoten 2009; 58-59, 167-176. |
| 21. | Kodama M, Kitadai Y, Tanaka M, Kuwai T, Tanaka S, Oue N, Yasui W, Chayama K. Vascular endothelial growth factor C stimulates progression of human gastric cancer via both autocrine and paracrine mechanisms. Clin Cancer Res. 2008;14:7205-7214. [PubMed] |
| 22. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [PubMed] |
| 23. | Yamada S, Makonkawkeyoon L, Jukrabandhu T, Lertprasertsuk N, Matsuhisa T, Azuma T. Correlation of cytokine gene IL-8 expression, cag A mutation of H. pylori infection and pepsinogen I/II ratio result: Reflection of host response in proximal gastric cancer. Ann Oncol. 2008;19:vi29-vi105. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Sutharat P, Kato S, Yamada S, Matsuda N, Matsukura N, Sandhu T, Tajiri T. Racial variations for the risk of stomach carcinogenesis depend on. Helicobacter pylori infection and mucosal conditions of stomach (oral presentation abstract symposium). The 100st AACR meeting;. 2009;Apr 18-22; Denver, Colorado. |
| 25. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11272] [Article Influence: 490.1] [Reference Citation Analysis (2)] |
| 26. | Chiou CC, Chan CC, Sheu DL, Chen KT, Li YS, Chan EC. Helicobacter pylori infection induced alteration of gene expression in human gastric cells. Gut. 2001;48:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Boonjakuakul JK, Canfield DR, Solnick JV. Comparison of Helicobacter pylori virulence gene expression in vitro and in the Rhesus macaque. Infect Immun. 2005;73:4895-4904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Lee KH, Bae SH, Lee JL, Hyun MS, Kim SH, Song SK, Kim HS. Relationship between urokinase-type plasminogen receptor, interleukin-8 gene expression and clinicopathological features in gastric cancer. Oncology. 2004;66:210-217. [PubMed] |
| 29. | Park MJ, Kim KH, Kim HY, Kim K, Cheong J. Bile acid induces expression of COX-2 through the homeodomain transcription factor CDX1 and orphan nuclear receptor SHP in human gastric cancer cells. Carcinogenesis. 2008;29:2385-2393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Subramaniam D, Ramalingam S, May R, Dieckgraefe BK, Berg DE, Pothoulakis C, Houchen CW, Wang TC, Anant S. Gastrin-mediated interleukin-8 and cyclooxygenase-2 gene expression: differential transcriptional and posttranscriptional mechanisms. Gastroenterology. 2008;134:1070-1082. [PubMed] [DOI] [Full Text] |