Published online May 14, 2013. doi: 10.3748/wjg.v19.i18.2761
Revised: February 11, 2013
Accepted: February 28, 2013
Published online: May 14, 2013
Processing time: 153 Days and 16.4 Hours
AIM: To examine the effect of α-lipoic acid (LA) on mild portal endotoxemia-induced steatohepatitis and associated pancreatic abnormalities in fructose-fed rats.
METHODS: Rats were randomly assigned into two groups with a regular or 60% fructose-enriched diet for 8 wk. After fructose feeding for 4 wk, rats were further divided into four subgroups: with intraportal saline (FPV), with intraportal saline plus administration of LA (FPV + LA), with lipopolysaccharide (LPS) infusion (FPLPS), and with LPS infusion plus administration of LA (FPLPS + LA). Rats were treated with LPS using intraportal infusion while LA was administered orally. Metabolite levels, superoxide levels, inflammatory markers, malondialdehyde content, glutathione content and toll-like receptor 4 (TLR4) gene expression were all measured using standard biochemical techniques. Pancreatic insulin secretion was evaluated by a hyperglycemic clamp technique. Histology of liver and pancreas tissues were evaluated using hematoxylin and eosin staining and immunohistochemistry.
RESULTS: Fructose-induced elevation in plasma C-reactive protein, amylase, superoxide, white blood cell count as well as in hepatic and pancreatic contents of malondialdehyde, tumor necrosis factor alpha and interleukin-6 were increased in animals treated with LPS and reversed with LA administration. The augmented hepatic gene expression of TLR4 in fructose-fed rats was further increased in those with intraportal LPS infusion, which was partially reversed by LA administration. Pathological examination showed inflammatory changes and leukocyte infiltration in hepatic and pancreatic islets of animals treated with LPS but were rarely observed in those with LA treatment. In addition to affects on the liver, impaired pancreatic insulin secretion seen in fructose-fed rats was deteriorated in with LPS treatment and partially reversed with LA administration.
CONCLUSION: These data suggest LA could significantly suppress mild portal-endotoxemia but not fructose-induced liver and pancreatic abnormalities in a rodent model for metabolic syndrome.
Core tip:α-lipoic acid (LA), a potent antioxidant and also an inducer of endogenous antioxidants has been reported to protect the liver and pancreas from injury. Our observations suggest that LA could significantly suppress inflammatory change of steatosis induced by low-dose intraportal lipopolysaccharide infusion and associated deterioration of insulin secretion in this metabolic syndrome rodent model. In addition, our data suggest that hepatic toll-like receptor 4 signaling might not only play a significant role in chronic fructosefeeding-induced hepatic steatosis but also in its subacute inflammatory change induced by mild portal endotoxemia and associated extrahepatic disorders.
- Citation: Tian YF, He CT, Chen YT, Hsieh PS. Lipoic acid suppresses portal endotoxemia-induced steatohepatitis and pancreatic inflammation in rats. World J Gastroenterol 2013; 19(18): 2761-2771
- URL: https://www.wjgnet.com/1007-9327/full/v19/i18/2761.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i18.2761
Nonalcoholic fatty liver disease (NAFLD) is currently the most common liver disease in the world[1]. NAFLD is mainly associated with obesity, diabetes, hyperlipidemia and insulin resistance, which are the main characters of metabolic syndrome[1,2]. The development of liver inflammation in NAFLD, defined as non-alcoholic steatohepatitis (NASH), isone of the crucial steps in causing liver-related morbidity and mortality[3]. Moreover, a pathological link between portal endotoxemia and NASH and also alcoholic liver disease has been speculated in several animal and human studies[4-8]. For instance, genetically obese fatty/fatty rats and ob/ob mice showed increased sensitivity to endotoxin hepatotoxicity, quickly developing steatohepatitis after exposure to low doses of lipopolysaccharide (LPS)[5]. These studies implicate that portal endotoxemia might significantly contribute to the pathogenesis of chronic hepatic inflammation, especially in NAFLD.
The release of liver-derived acute-phase proteins and inflammatory cytokines under chronic liver injury significantly contributes to the extrahepatic effect of inflamed liver and the pathogenesis of metabolic syndrome[9,10]. We have shown that mild portal endotoxemia induced by low-dose intraportal LPS infusion could significantly cause chronic hepatic and pancreatic inflammation, and impair pancreatic insulin secretion in rats[11]. Furthermore, low-dose intraportal LPS infusion could also accelerate the process of NAFLD to NASH in fructose-fed rats, an animal model of metabolic syndrome with NAFLD[4]. However, the possible underlying mechanisms behind the detrimental effects of mild portal endotoxemia on liver and pancreas remain unclear.
Chronic stress such as portal endotoxemia has been documented to activate hepatic Kupffer cells and cause the release of reactive oxygen species, potentially inducing inflammatory changes in the liver and impairing pancreatic functions[5,12]. α-lipoic acid (LA) is a potent antioxidant and an inducer of endogenous antioxidants[13,14]. It also has a protective effect on hepatic and pancreatic injury[15-18]. In this study, we sought to test the potential therapeutic effect of LA on mild portal endotoxemia and fructose-induced inflammatory changes of fatty liver and impaired pancreatic insulin secretion in rats.
Male Sprague-Dawley rats aged five to six weeks were purchased from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan). The rats were housed in an animal center certified by Association of Assessment and Accreditation of Laboratory Animal Care. All animals were handled according to the guidelines and manual of the institutional animal care and use committee of this institute. Rats weighing 250-300 g were randomly assigned into six groups: (1) two control groups: on regular chow diets for 4 wk and then separated into those combined with or without intraportal vehicle (saline) infusion (CPVvs C) for another 4 wk; (2) four experimental groups: on high-fructose enriched diet (60% of the calories from fructose, TD89247, Teklad Primer Labs, Madison, WI) for 4 wk and thereafter (FPV), those combined with intraportal vehicle or low-dose LPS infusion (FPLPS), cotreated with vehicle or LA (α-LA in saline, pH 7.8, Sigma Co. Germany, 60 mg/kg per day, oral gavage)for additional 4 wk (FPV + LA and FPLPS + LA respectively)[19]. In the time-course study, blood samples were taken by tail bleeding method after overnight fasting. At the end of week 8, one set of rats from the above grouping (n = 6 per group) was used for an in vivo hyperglycemic clamp study. The second set (n = 6 per group) was euthanized by overdose pentobarbital injection (100 mg/kg, intraperitoneal) immediately after overnight fasting and blood sampling by cardiac puncture was carried out for the measurement of plasma C-reactive protein (CRP), superoxide, white blood cell (WBC) count and endotoxin levels. Tissue samples were dissected for further analysis. The liver and pancreas dissected from the third set of rats (n = 6 per group) were fixed by perfusion with 4% paraformadehyde. The tissues were embedded in paraffin for further staining. Notably, the metabolic and hemodynamic parameters and histopathological examination of group CPV were similar to those without intraportal vehicle infusion (data not shown). The group CPV was used as the only control group on regular chow diet in the following result section.
A laparotomy was performed under anesthesia (sodium pentobarbital 25 mg/kg, intraperitoneal injection) in rats with intraportal infusion. A catheter (PE-5 tubing, 0.008 inch inner diameter × 0.020 inch outer diameter, SCI micro medical grade polyethylene; Scientific Commodities Inc., Lake Havasu City, AZ, United States) was inserted into the distal end of a colic vein and the tip of the catheter was placed about 3 mm distal to the point at which the catheterized vein enters portal vein. After insertion, the catheters were filled with LPS-saline solution or saline alone and were connected to osmotic mini-pumps (model 2004, Alzet corporation, Cupertino, CA, United States) filled with LPS, 0.42 ng/kg per minute or saline as shown in our previous study[20]. Three days before the surgery, a safe dosage of LA was orally administered once per day for 4 wk[21,22].
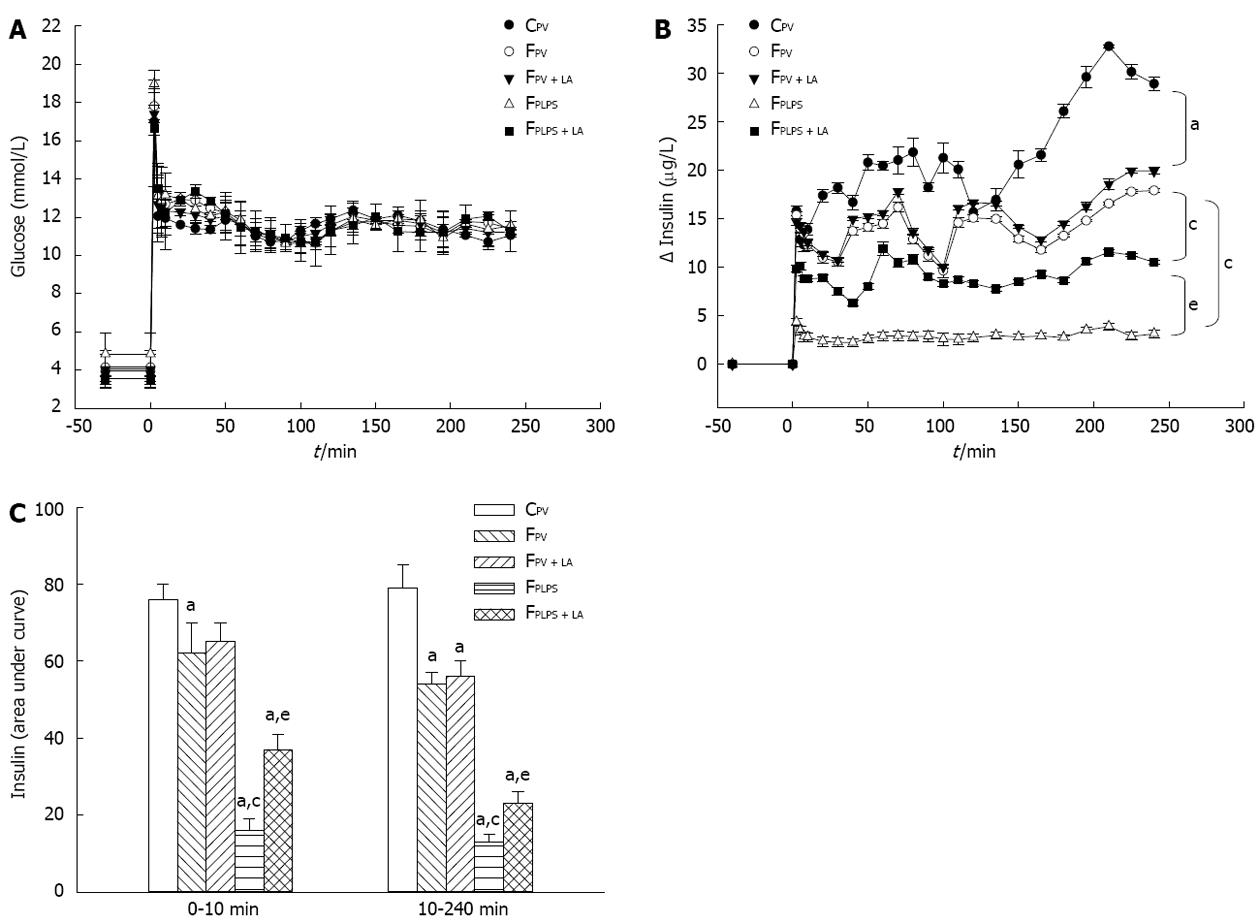
In one set of rats, vascular catheters were placed in the left femoral artery and right femoral vein under anesthesia and their proximal ends were placed in subcutaneous pockets under scapular area at the end of week 8. The hyperglycemic clamp experiment was performed after recovery for 3 to 4 d, as described elsewhere[23,24]. The insulin secretions of the first and second phases were indicated by the incremental plasma insulin values during time 0-10 and 10-240 min in the glucose clamp period, respectively.
The WBC count was determined by using a Bright-Linehemocytometer (Hausser Scientific, Horsham, PA, United States). Whole blood glucose levels were measured by the glucose oxidase method. Plasma and tissue triglyceride levels were determined by using appropriate enzymatic colorimetric method (Randox Laboratories, Antrium, United Kingdom). Plasma insulin levels were determined by commercial rat enzyme-linked immuno sorbent assay (ELISA) kits (Mercodia AB, Uppsala, Sweden). Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), albumin and amylase levels were measured by Randox reagent kits (Randox Laboratories Antrim, United Kingdom). CRP levels were measured by a commercial rat ELISA kit (Alpha Diagnostic, San Antonio, TX, United States). Arterial plasma endotoxin level was assayed by using the limulus amoebocyte lysate test (Kinetic-QLC; Whittaker Bioproducts, Walkersville, MD, United States).
A lucigenin-dependent chemiluminescence assay (Sigma Chemical Co. St. Louis, MO, United States) was used to quantify plasma superoxide levels with the MLA-GOLDS chemiluminescence analyzing system (Tohoku Electronic Industrial Co., Sendai, Japan) as described previously[4]. The total amount of chemiluminescence was calculated by integrating the area under the curve and subtracting it from the background level during the 10 min counting period.
Tissues were prepared as described previously[25]. The supernatant was subjected to tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) assays using commercial rat ELISA kits (R and D Systems, Minneapolis, MN, United States).
Lipid oxidation of the liver and pancreas was detected by a commercial malondialdehyde (MDA) assay kit (Cayman Chemical Co., Ann Arbor, MI, United States). In brief, MDA, the breakdown product of oxidative degradation of lipids, reacted with thiobarbituric acid (TBAR) to form MDA-TBAR, and its levels were expressed as nmol/g protein.
The total glutathione in the liver was measured by using commercial glutathione assay kit (Cayman Chemical Co., Ann Arbor, MI, United States). In brief, the liver was homogenized in RIPA buffer (0.5 mol/L Tris-HCl, pH 7.4, 1.5 mol/L NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mmol/L EDTA) by using a Polytron homogenizer to obtain tissue lysates and centrifuged at 10000 g for 10 min. Subsequently, the supernatant was collected and added HPO3 (v/v = 1/1) to deproteinize. The glutathione (GSH) content of deproteinized supernatant of liver (50 L) was measured by the reductive rate of 5,5’-dithio-bis-2-nitrobenzoic acid to 5-thio-2-nitrobenzoic acid according to the instructions with absorption at 405 nm. The GSH level was expressed as μmol/g protein.
RNA was extracted from the liver and pancreas of experimental rats using Trizol (Ambion, Austin, TX, United States) at the end of the study. TaqMan gene expression assay kits for toll-like receptor 4 (TLR4) (Rn00569848_m1) and the TATA box binding protein (Rn01455648_m1) were used in conjunction with a universal master mix in a 7500 real-time polymerase chain reaction system (Applied Biosystems, Foster City, CA, United States). Gene expression was normalized to the TATA box binding protein expression level measured in each sample and expressed as fold increases or decreases from control values for each gene of interest.
The third set of rats (n = 5-6 per group) was grouping as the hyperglycemic clamp experiment. The perfused liver and pancreas were then fixed in 5% zinc-formalin solution and embedded in paraffin. These tissue slices were then prepared for staining with hematoxylin and eosin (HE) (liver, pancreas) to evaluate pathological changes.
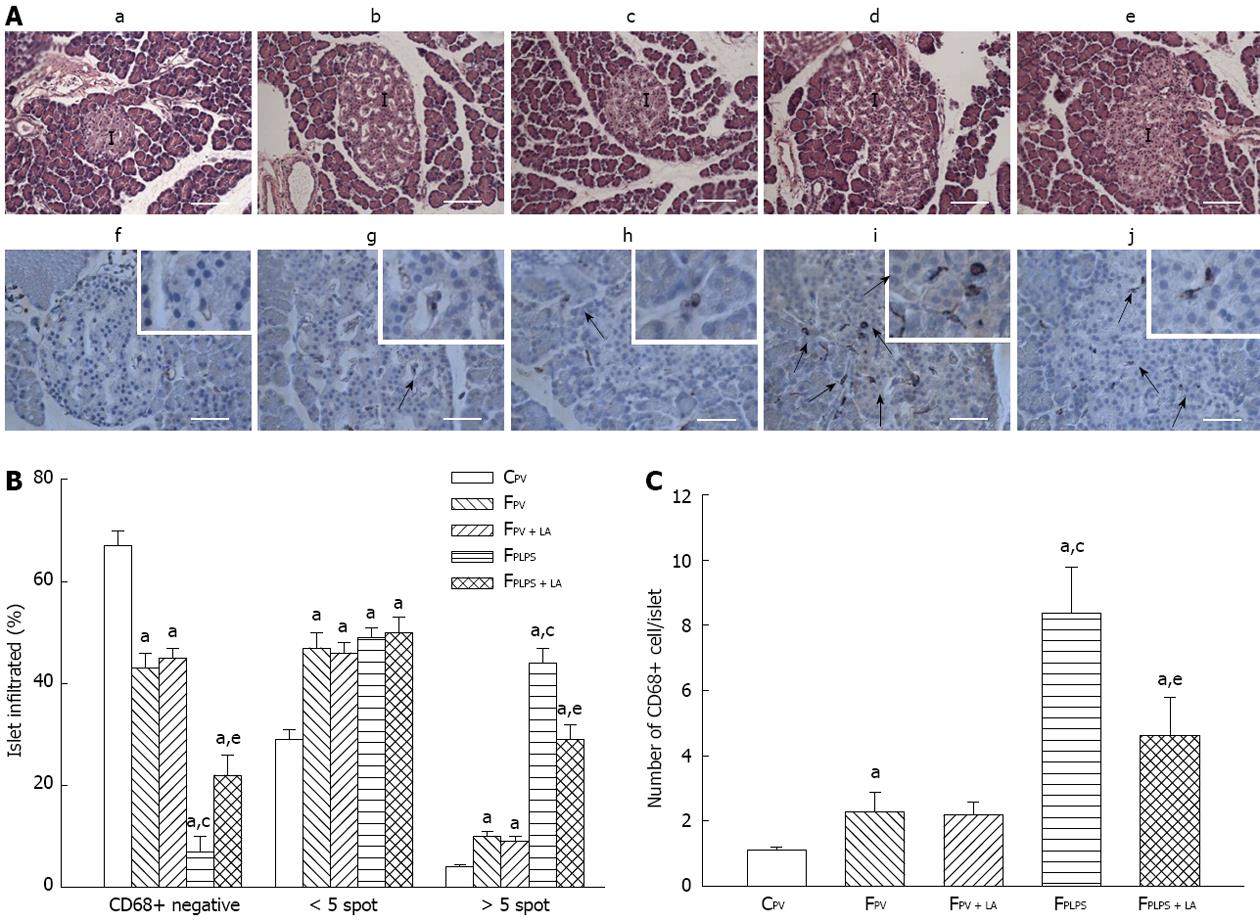
Tissue samples were fixed in formalin, and cut into 4 μm sections. Immunohistochemistry was performed on an automated stainer (Autostainer; Dako, Glostrup, Denmark). Then, sections were incubated with anti-CD68 antibody (ED1 mouse anti-rat CD68, Serotec, 1:100, MorphoSys UK Ltd., Oxford, United Kingdom), followed by incubation with goat anti-mouse secondary antibody and visualized with HRP substrate (REAL EnVision Detection System; Dako).
A total of 100 ± 25 islets per experimental group were blindly scored for CD68-positive cells around the periphery and/or within islets from five or six different animals by two investigators. Islet area was measured as the area of islet capsule in pancreatic section with HE stain and computed using AxioVision LE 4.8.2.0 software.
Statistical analysis was performed according to the repeated measurements of one-way analysis of variance followed by Bonferroni test. A probability of P < 0.05 was taken to indicate a significant difference between means. Values are expressed as mean ± SE.
We first measured the effect of fructose feeding and LPS treatment on rats with and without LPS infusion. After high-fructose feeding for 4 wk, fasting plasma insulin levels in fructose-fed groups were significantly increased as compared with controls, but not different among fructose-fed groups. Plasma CRP, amylase, superoxide and white blood cells were also significantly increased in fructose-fed rats and further increased after intraportal LPS infusion for 4 wk. The above LPS-induced responses were suppressed in those co-treated with LA to levels similar to those in fructose-fed untreated controls. On the other hand, there were no significant differences in fasting plasma glucose, AST, ALT, albumin and endotoxin concentrations among experimental groups (Table 1).
| Pump infusion (wk) | CPV | FPV | FPV + LA | FPLPS | FPLPS + LA | |
| Body weight (g) | 4 | 349 ± 19 | 383 ± 8 | 351 ± 29 | 358 ± 6 | 371 ± 6 |
| 8 | 451 ± 7 | 474 ± 8 | 445 ± 29 | 436 ± 11 | 423 ± 7 | |
| Glucose (mmol/L) | 4 | 5.9 ± 0.3 | 5.9 ± 0.1 | 6.62 ± 1.2 | 6.08 ± 0.1 | 6.1 ± 0.1 |
| 8 | 5.9 ± 0.2 | 6.4 ± 0.2 | 7.8 ± 0.7 | 6.76 ± 0.2 | 6.43 ± 0.1 | |
| Insulin (ng/mL) | 4 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| 8 | 0.6 ± 0.1 | 2.5 ± 0.6a | 2.2 ± 0.3a | 1.9 ± 0.5a | 2.4 ± 0.4a | |
| TG (mg/dL) | 4 | 58 ± 4 | 180 ± 14a | 185 ± 13a | 185 ± 24a | 163 ± 16a |
| 8 | 52 ± 4 | 324 ± 38a | 286 ± 22a | 308 ± 24a | 249 ± 44a | |
| AST (U/L) | 4 | 65 ± 5 | 76 ± 1 | 82 ± 6 | 70 ± 2 | 77 ± 2 |
| 8 | 58 ± 4 | 72 ± 2 | 73 ± 6 | 68 ± 2 | 70 ± 2 | |
| ALT (U/L) | 4 | 53 ± 2 | 57 ± 1 | 56 ± 0 | 52 ± 1 | 51 ± 2 |
| 8 | 56 ± 2 | 49 ± 1 | 44 ± 6 | 46 ± 1 | 51 ± 1 | |
| Albumin (mmol/L) | 4 | 40 ± 1 | 35 ± 1 | 37 ± 1 | 35 ± 0 | 36 ± 0 |
| 8 | 41 ± 1 | 35 ± 0 | 34 ± 1 | 35 ± 0 | 35 ± 0 | |
| CRP (μg/mL) | 8 | 397 ± 10 | 504 ± 10a | 555 ± 39 | 583 ± 13c | 529 ± 22e |
| Amylase (U/L) | 8 | 1278 ± 138 | 1750 ± 23a | 1753 ± 58 | 2149 ± 31c | 1744 ± 16e |
| Superoxide (count/min) | 8 | 1337 ± 68 | 3169 ± 24a | 3860 ± 52 | 6069 ± 60c | 3234 ± 33e |
| WBC count (/mm3) | 8 | 14168 ± 586 | 20290 ± 390a | 22622 ± 630 | 24357 ± 1008c | 21442 ± 767e |
| Plasma endotoxin (EU/mL) | 8 | < 0.02 | < 0.02 | < 0.02 | < 0.02 | < 0.02 |
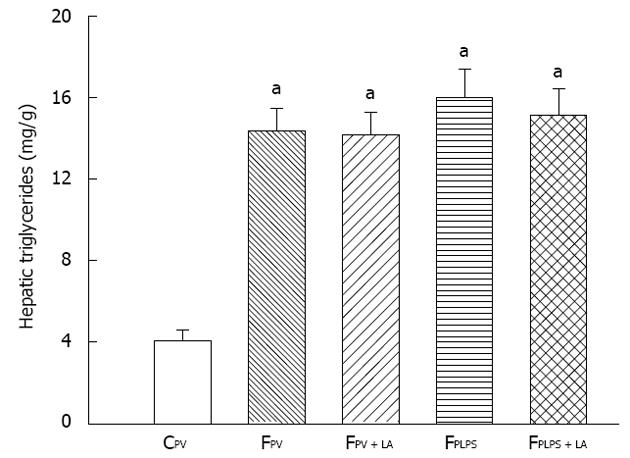
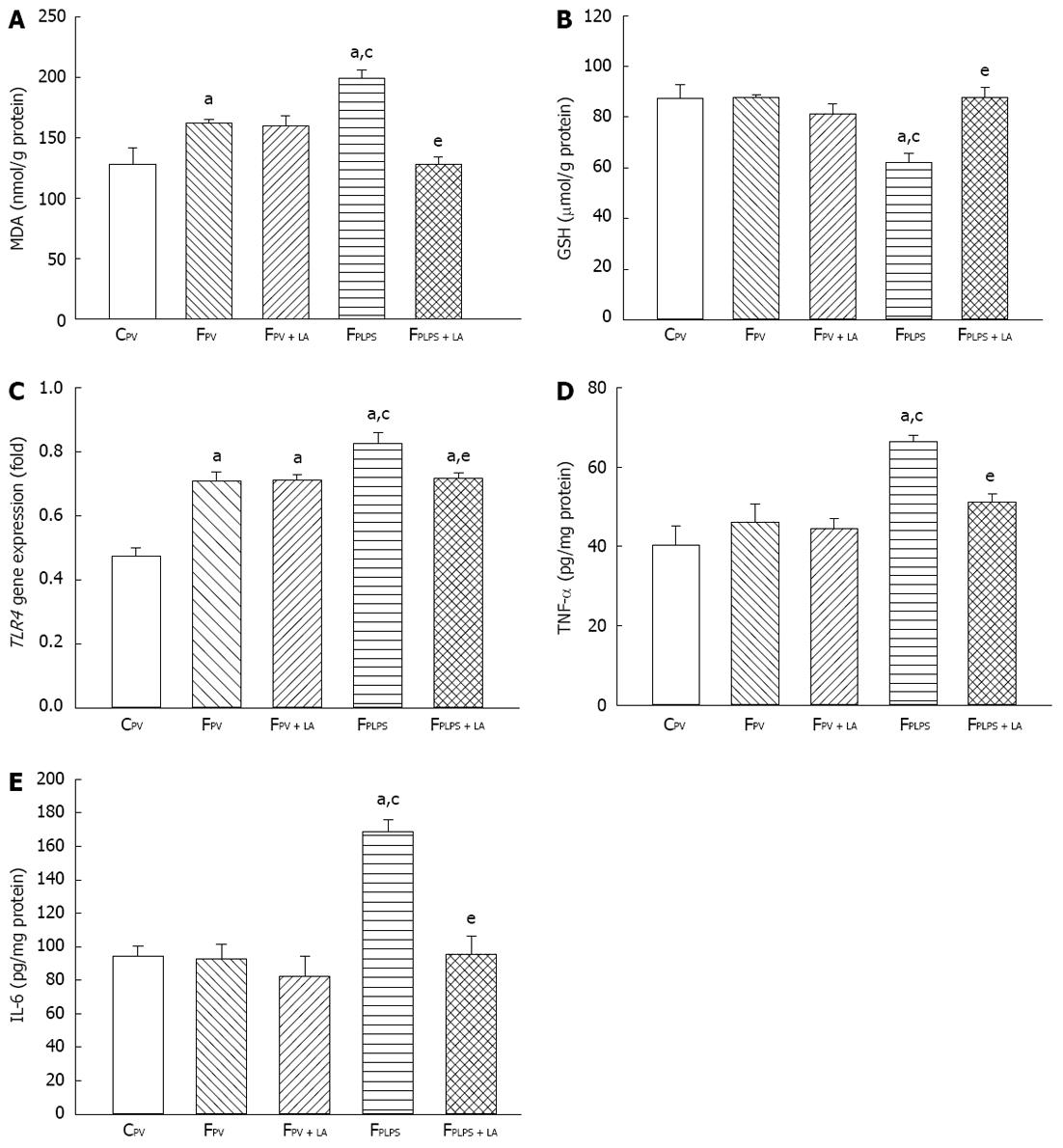
To measure the liver response to our conditions, we measured triglyceride levels, oxidative parameters, and inflammatory parameters. Fructose feeding resulted in elevated triglyceride levels in all groups when compared to the control groups. Administration of LA had no effect on these levels (Figure 1). As shown in Figure 2A, MDA levels in the liver were significantly increased in fructose-fed rats as compared with controls and further elevated in animals treated with LPS. However, the increase of hepatic MDA contents in fructose-fed rats following intraportal LPS infusion was significantly reversed in those with LA administration. Liver GSH levels were significantly decreased following LPS infusion, but were significantly reversed in those treated with LA (Figure 2B). Additionally, the enhanced hepatic TLR4 gene expression by chronic high-fructose feeding was further increased in those with intraportal LPS infusion. LA administration suppressed the augmentation of TLR4 gene expression induced only by mild portal endotoxemia and not by high-fructose feeding (Figure 2C). The significant increase of TNF-α and IL-6 protein levels in the liver of LPS-infused rats was reflective of the hepatic inflammatory response. These levels were significantly suppressed with LA treatment (Figure 2D and E). LPS infusion had no effect on triglyceride levels, but did have an effect on MDA, GSH, TLR4, TNF-α and IL-6. LA administration significantly reversed these effects.
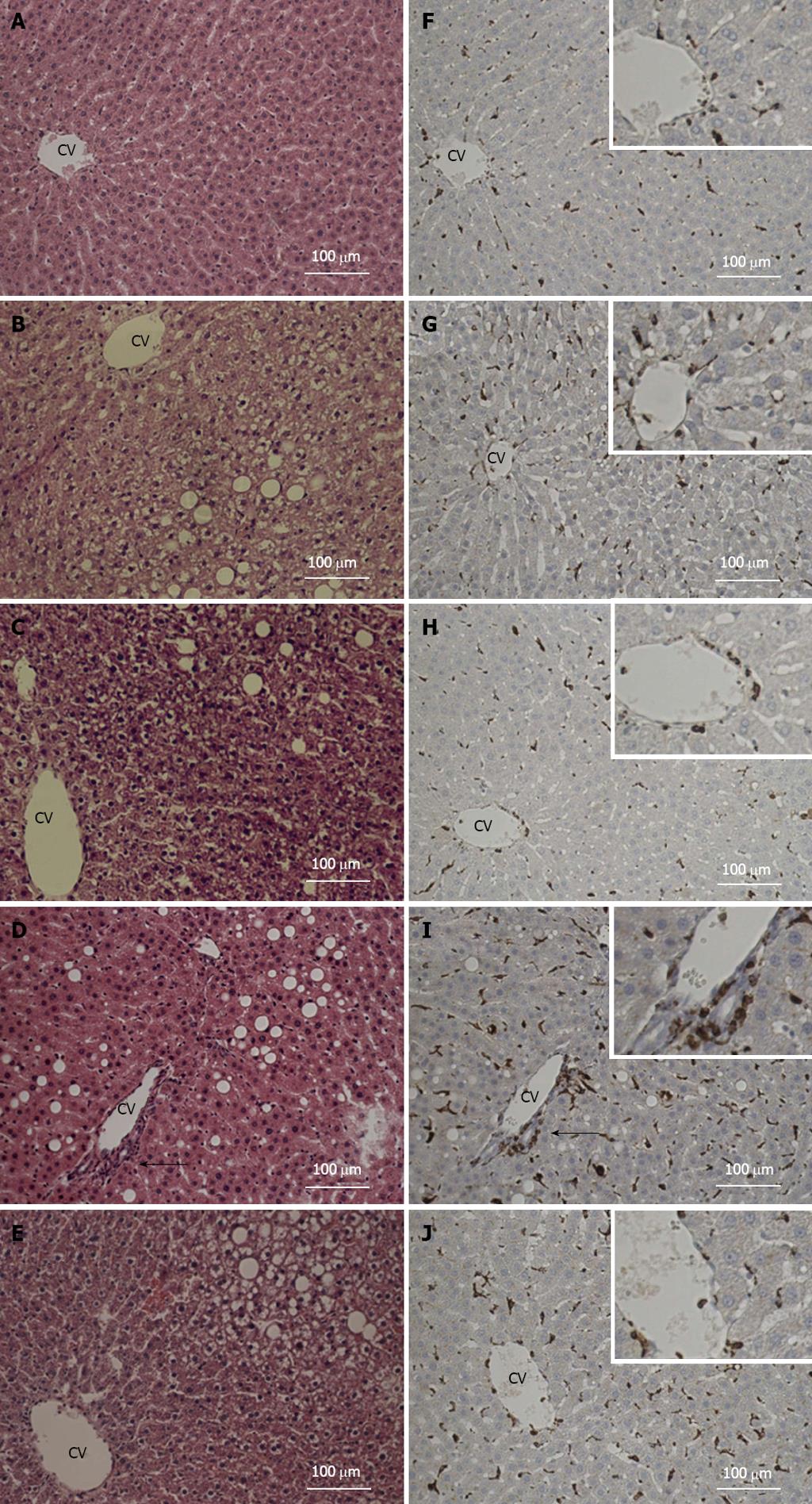
Following analysis of the liver response to our treatments, we sought to examine the liver for histopathological changes. Not surprisingly, steatosis was noted in fructose-fed groups but was not different between groups. On the other hand, the infiltration of monocytes in liver was markedly exhibited in LPS-treated animals (Figure 3D), a phenotype not seen in any other treatment group (Figure 3A-C), and alleviated by the administration of LA (Figure 3E). Accordingly, immunohistochemical staining showed that the observed CD68-positive cell infiltration was observed in areas around the central vein in LPS infused animals (Figure 3I), but significantly attenuated in those with LA administration (Figure 3J). Therefore, LPS treatment resulted in marked histopathological changes in the liver of fructose-fed animals, a phenotype that was reversed by the administration of LA.
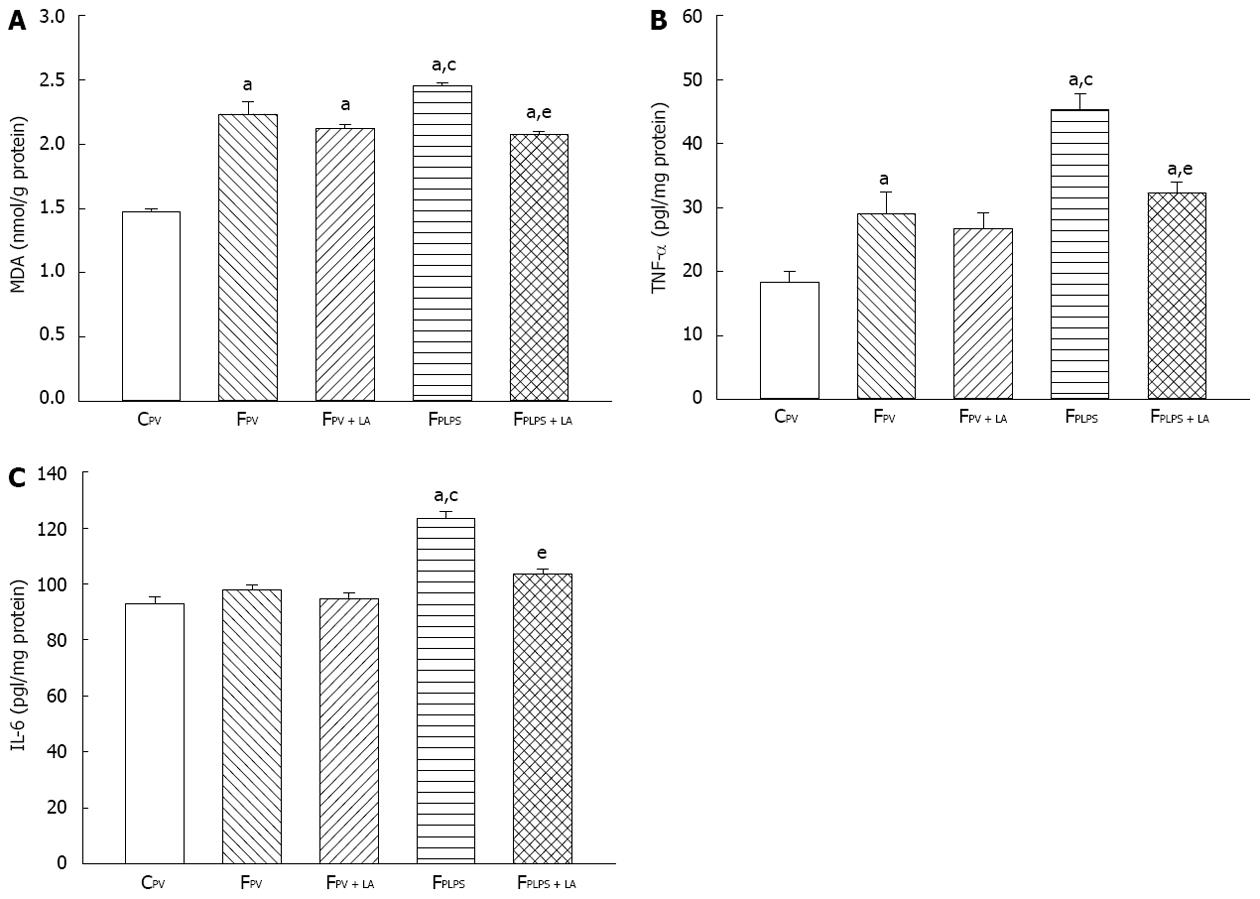
To measure the pancreas response to our conditions, we tested for MDA, TNF-α and IL-6 levels. Fructose feeding increased all three parameters, while fructose feeding with infusion of LPS significantly increased each (Figure 4A-C). Administration of LA resulted in each parameter returning back to original fructose-fed levels. TLR4 gene expression was not detected in pancreas in experimental groups. Therefore, immunological markers including TNF-α and IL-6, but not TLR4, are increased upon fructose feeding and LPS infusion, a phenotype that can be reversed with administration of LA.
We then examined the histopathological response to our treatments in the pancreas. Following LPS treatment, pancreatic islets exhibited damage that was attenuated with LA administration (Figure 5, top panels). Consistently, the frequency and CD68-positive cell infiltration in fructose-fed animals was only slightly increased as compared to controls. However, they were markedly increased in following LPS infusion and attenuated with LA administration (Figure 5, bottom panels). Quantitation of this data shows the frequency of CD68-positive islets and numbers of CD68-positive cells/islet in fructose-fed rats were further elevated following LPS infusion and partially suppressed in those with LA administration.
Hyperglycemic clamp is the gold-standard method to evaluate glucose-stimulated insulin secretion in vivo[26]. Plasma glucose levels were not different during the basal period and maintained similar hyperglycemia during the clamp periods among experimental groups (Figure 6A). Increases in plasma insulin levels from baseline in fructose-fed animals were significantly lower than those in controls and further decreased in those infused with LPS under similar hyperglycemic conditions (Figure 6B). The diminished glucose-stimulated insulin secretion shown in fructose-fed controls was not significantly changed with LA administration. However, intraportal LPS infusion significantly impaired the glucose-stimulated insulin secretion shown in fructose-fed controls, which was significantly reversed in those with LA treatment (Figure 6B). In addition, the first-phase insulin secretion (0-10 min post glucose treatment) and the second-phase insulin secretion (10-240 min post glucose treatment) were significantly lower in fructose-fed animals when compared with controls. This was further exacerbated following intraportal LPS infusion, a phenotype that was partially reversed when treated with LA (Figure 6C).
Nonalcoholic steatohepatitis is not only highly correlated with the development of metabolic syndrome and type 2 diabetes mellitus, but is also a crucial factor in progression to cirrhosis and hepatocellular carcinoma[3]. In addition, mild portal endotoxemia has been speculated as a crucial risk factor to induce hepatic inflammation in the state of steatosis and impaired pancreatic β cell function[4]. This study explored the potential therapeutic role of the potent antioxidant α-LA in liver disease and associated pancreatic abnormalities. Using the animal model for metabolic steathohepatitis, fructose-fed rats, we found administration of LA reversed mild portal endotoxemia-induced inflammation. Further, portal endotoxemia also decreased glucose-stimulated insulin secretion, a phenotype that was reversed following LA treatment.
LA has been reported to scavenge free radicals, chelate metals and restore intracellular GSH, all factors associated with increasing age[13]. In addition, it is used as a therapeutic agent in several liver-related diseases such alcohol-induced liver damage[27] and fatty liver disease[16] through multiple mechanisms. Our observations further establish LA as a therapeutic for liver disease. Specifically, our animal model shows LA improves endotoxin-induced hepatic disorders in addition to its established role as a treatment for chronic high-fructose feeding. These anti-oxidant and anti-inflammatory characteristics may be in the ability of LA to restore tissue GSH-dependent antioxidant defenses during portal endotoxin attack.
Furthermore, these data show LA administration significantly suppressed the augmented TLR4 gene expression induced by mild portal endotoxemia but not chronic fructose feeding. Hepatic TLR4 is activated by gut-derived LPS and attributed to the pathogenesis of liver inflammation[28]. In addition, TLR4 signaling is involved in the development of fructose-induced steatosis in mice[29] and also required for liver steatosis, inflammation and a fibrogenic response after chronic alcohol treatment in TLR4 transgenic mice[30]. In addition to LPS, other potential agents including saturated fatty acids (i.e., palmitate) and alarmins (i.e., HMGB1) have also been shown to stimulate the inflammatory response in a TLR4-dependent manner[31,32]. Hepatic TLR4 signaling occurs not only in Kupffer cells but also in hepatic non-immune cell populations including hepatocytes, biliary epithelial cells, endothelial cells and hepatic stellate cells[33]. In brief, these observations implicate TLR4-mediated inflammatory signaling in hepatic cell populations are crucially involved in the development of NAFLD, affected individually by diet components and portal endotoxemia.
Our observations showed that LA not only significantly improved the inflammatory changes in fructose-induced NASH but also diminished the detrimental inflammatory response of the pancreas. Consistent with our results, LA has a protective effect on cholecystokinin-octapeptide induced acute pancreatitis in rats[15]. LA was reported to have a dose-related cytoprotective effect on hydrogen peroxide-induced oxidative stress on pancreatic beta cells. On the other hand, LA could also directly suppress insulin secretion in pancreatic beta cells at high concentrations by inducing AMP-activated protein kinase activation[34]. Taken together, data from these studies combined with our observations implicate a beneficial effect of LA on impaired pancreatic insulin secretion. This effect might be attributed to its anti-oxidative and anti-inflammatory actions on mild portal endotoxemia-induced liver inflammation and subsequent pancreatic damage, but not its direct effect on pancreatic beta cell secretion.
Nevertheless, the selected dose of intraportal LPS infusion was based on evaluating its effects on the mortality rate and systemic endotoxin levels during 4-wk infusion period in our previous study[11]. Consistently, this low-dose LPS infusion caused subacute hepatic inflammation that could be almost completely be cleared by Kupffer cells once it passed through the liver, so that the arterial plasma endotoxin levels were not different among experimental groups.
The present study showed that administration of LA could protect liver from LPS-induced oxidative stress and inflammation while reversing the subsequent impairment of the pancreas in rats with fructose-associated fatty liver disease. However, LA had no effect on oxidative stress induced by high-fructose feeding. Clinical implications of this observation suggest both the suppression of portal endotoxemia-induced oxidative stress and dietary interventions are crucial for improving symptoms of NASH.
Although both oxidative stress induced by low-dose intraportal LPS infusion and high-fructose feeding have been demonstrated to contribute to the development of steatohepititis in fructose-fed rats, the possible involvement of TLR4 signaling in individual hepatic cell populations remain elusive in the present study.
In conclusion, the present study demonstrates that LA can significantly reduce intraportal LPS-induced liver inflammation and associated deterioration of insulin secretion function in this metabolic syndrome rodent model. In addition, it is also implicated that hepatic TLR4 signaling might not only play a significant role in chronic-fructose-feeding induced hepatic steatosis but also in its subacute inflammatory change induced by mild portal endotoxemia and associated extrahepatic disorders in this model.
The authors also gratefully acknowledge the research assistance of Ms. Kai-Chil Hsieh and Mr. Hung-Che Chien.
α-lipoic acid (LA), a potent antioxidant and also an inducer of endogenous antioxidants has been reported to protect the liver and pancreas from injury.
Chronic stress such as portal endotoxemia has been documented to activate hepatic Kupffer cells and cause the release of reactive oxygen species, potentially inducing inflammatory changes in the liver and impairing pancreatic functions. The potential therapeutic effect of LA on mild portal endotoxemia and fructose-induced inflammatory changes of fatty liver and impaired pancreatic insulin secretion in rats remain controversial.
LA has been documented to have a protective effect on hepatopancreatic damage. However, the effect of LA on lipopolysaccharide-induced non-alcoholic steatohepatitis (NASH) remains unclear. The present result in this manuscript has a significant contribution to clarify this issue.
The present study implicates that LA might suppress inflammatory change of steatosis and associated deterioration of insulin secretion in the patients with metabolic syndrome.
Alpha LA is a potent antioxidant and an inducer of endogenous antioxidants. It also has a protective effect on hepatic and pancreatic injury.
The authors found that LA quenches inflammation and oxidative stress, leading to attenuation of NASH. So far a few reports describe the effect of LA on lipopolysaccharide-induced NASH. The manuscript represents a major effort to fill the gap.
P- Reviewer Yi XW S- Editor Song XX L- Editor A E- Editor Li JY
| 1. | Marchesini G, Marzocchi R. Metabolic syndrome and NASH. Clin Liver Dis. 2007;11:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Moscatiello S, Di Luzio R, Sasdelli AS, Marchesini G. Managing the combination of nonalcoholic fatty liver disease and metabolic syndrome. Expert Opin Pharmacother. 2011;12:2657-2672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Gambino R, Musso G, Cassader M. Redox balance in the pathogenesis of nonalcoholic fatty liver disease: mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2011;15:1325-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Hsieh PS. Inflammatory change of fatty liver induced by intraportal low-dose lipopolysaccharide infusion deteriorates pancreatic insulin secretion in fructose-induced insulin-resistant rats. Liver Int. 2008;28:1167-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557-2562. [PubMed] |
| 6. | Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition. 2001;17:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 343] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518-G525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 646] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 8. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] |
| 9. | Müller S, Martin S, Koenig W, Hanifi-Moghaddam P, Rathmann W, Haastert B, Giani G, Illig T, Thorand B, Kolb H. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptors. Diabetologia. 2002;45:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286-1292. [PubMed] |
| 11. | Hsieh PS, Chan JY, Shyu JF, Chen YT, Loh CH. Mild portal endotoxaemia induces subacute hepatic inflammation and pancreatic beta-cell dysfunction in rats. Eur J Clin Invest. 2008;38:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Reid AE. Nonalcoholic steatohepatitis. Gastroenterology. 2001;121:710-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 352] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 13. | Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790:1149-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 652] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 14. | Bast A, Haenen GR. Lipoic acid: a multifunctional antioxidant. Biofactors. 2003;17:207-213. [PubMed] |
| 15. | Park SJ, Seo SW, Choi OS, Park CS. Alpha-lipoic acid protects against cholecystokinin-induced acute pancreatitis in rats. World J Gastroenterol. 2005;11:4883-4885. [PubMed] |
| 16. | Park KG, Min AK, Koh EH, Kim HS, Kim MO, Park HS, Kim YD, Yoon TS, Jang BK, Hwang JS. Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology. 2008;48:1477-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Abdin AA, El-Hamid MA, El-Seoud SH, Balaha MF. Effect of pentoxifylline and/or alpha lipoic acid on experimentally induced acute pancreatitis. Eur J Pharmacol. 2010;643:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L, Rihn BH. Alpha-lipoic acid in liver metabolism and disease. Free Radic Biol Med. 1998;24:1023-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 235] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4561] [Article Influence: 253.4] [Reference Citation Analysis (1)] |
| 20. | Curat CA, Miranville A, Sengenès C, Diehl M, Tonus C, Busse R, Bouloumié A. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 403] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 21. | Cremer DR, Rabeler R, Roberts A, Lynch B. Long-term safety of alpha-lipoic acid (ALA) consumption: A 2-year study. Regul Toxicol Pharmacol. 2006;46:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Cremer DR, Rabeler R, Roberts A, Lynch B. Safety evaluation of alpha-lipoic acid (ALA). Regul Toxicol Pharmacol. 2006;46:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Wagoner B, Hausman DB, Harris RB. Direct and indirect effects of leptin on preadipocyte proliferation and differentiation. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1557-R1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Marques BG, Hausman DB, Latimer AM, Kras KM, Grossman BM, Martin RJ. Insulin-like growth factor I mediates high-fat diet-induced adipogenesis in Osborne-Mendel rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R654-R662. [PubMed] |
| 25. | Ghanim H, Garg R, Aljada A, Mohanty P, Kumbkarni Y, Assian E, Hamouda W, Dandona P. Suppression of nuclear factor-kappaB and stimulation of inhibitor kappaB by troglitazone: evidence for an anti-inflammatory effect and a potential antiatherosclerotic effect in the obese. J Clin Endocrinol Metab. 2001;86:1306-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1329] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 27. | Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1357] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 28. | Lin Y, Yu LX, Yan HX, Yang W, Tang L, Zhang HL, Liu Q, Zou SS, He YQ, Wang C. Gut-derived lipopolysaccharide promotes T-cell-mediated hepatitis in mice through Toll-like receptor 4. Cancer Prev Res (Phila). 2012;5:1090-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 30. | Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509-1518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2743] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 32. | Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 641] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 33. | Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 551] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 34. | Lee BW, Kwon SJ, Chae HY, Kang JG, Kim CS, Lee SJ, Yoo HJ, Kim JH, Park KS, Ihm SH. Dose-related cytoprotective effect of alpha-lipoic acid on hydrogen peroxide-induced oxidative stress to pancreatic beta cells. Free Radic Res. 2009;43:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |