Published online May 7, 2013. doi: 10.3748/wjg.v19.i17.2697
Revised: October 17, 2012
Accepted: November 24, 2012
Published online: May 7, 2013
Processing time: 129 Days and 23.7 Hours
AIM: To investigate the expression of distal-less homeobox 2 (DLX2) in gastric adenocarcinoma and its clinicopathological significance.
METHODS: Gastric adenocarcinoma tissues were obtained from gastrectomy specimens of 129 patients from the Department of Surgery and Pathology, the Second Affiliated Hospital of Kunming Medical University. Sixty cases of normal gastric tissues were collected from gastrectomy specimens of adjacent gastric cancer margins greater than 5 cm. Patient diagnosis was established pathologically, and no patient had received chemotherapy or radiotherapy before surgery. All tissue specimens were formalin-fixed and paraffin-embedded. Immunohistochemistry was carried out to investigate the expression of DLX2 in 129 gastric adenocarcinoma tissues and 60 adjacent normal tissues. The immunostaining reaction was semiquantitatively evaluated based on the proportion of positive cells and the median staining intensity in normal gastric epithelial cells or tumor cells. All patients had follow-up records for more than 5 years. Correlations of DLX2 expression with clinicopathological features and prognosis of patients with gastric adenocarcinoma were analyzed. All statistical analyses were performed using the SPSS 17.0 software.
RESULTS: The positive expression of DLX2 was detected in 68 (52.7%) cases of 129 gastric adenocarcinoma tissues and 14 (23.3%) cases of 60 adjacent normal tissues. The difference in DLX2 expression between gastric adenocarcinoma tissues and adjacent normal tissues was statistically significant (χ2 = 14.391, P < 0.001). Moreover, high expression of DLX2 was detected in 48 (37.2%) cases of 129 human gastric cancer tissues, but not in adjacent normal tissues. The expression of DLX2 correlated with the size of tumor (P = 0.001), depth of invasion (P = 0.008), lymph node metastasis (P = 0.023) and tumor-node-metastasis stages (P = 0.020), but was not correlated with age, gender, histological differentiation and distant metastasis. The Kaplan-Meier survival analysis revealed that survival time of patients with high DLX2 expression was significantly shorter than that with low DLX2 expression. However, the multivariate analysis showed that invasion depth (P < 0.001), lymph nodes metastasis (P = 0.001) and distant metastasis (P < 0.001) were independent prognostic factors for patients with gastric adenocarcinoma, but DLX2 expression, tumor location and tumor size were not.
CONCLUSION: These results suggest that increased expression of DLX2 may correlate with the advanced stage of gastric adenocarcinoma, and it may contribute to tumor development.
- Citation: Tang P, Huang H, Chang J, Zhao GF, Lu ML, Wang Y. Increased expression of DLX2 correlates with advanced stage of gastric adenocarcinoma. World J Gastroenterol 2013; 19(17): 2697-2703
- URL: https://www.wjgnet.com/1007-9327/full/v19/i17/2697.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i17.2697
Gastric cancer is one of the leading causes of cancer-related death worldwide due to its frequency, poor prognosis and limited treatment options[1]. Although the incidence of gastric cancer has been declining for several decades in most Western countries, it remains a crucial public health problem in developing countries[2,3]. In China, gastric cancer is the second most common malignancy and was the third leading cause of death from cancer in 2007, representing a major disease burden on health services[4]. Several studies have shown that various genetic and epigenetic alterations are involved in the course of carcinogenesis and progression of gastric cancer[5-8]. However, the molecular mechanism involved in the development of gastric cancer remains unclear.
The distal-less homeobox (DLX) gene family, a homolog of Drosophila distal-less, comprises six DLX genes in humans, of which three exist as bigene clusters: DLX-1/DLX-2, DLX-3/DLX-4, DLX-5/DLX-6[9]. The DLX gene family has crucial roles in regulating embryonic development, tissue homeostasis, lymphocyte development, cell cycle and apoptosis[10-14]. However, the role of the DLX gene family in tumor development has only recently been explored. As a member of DLX gene family, the abnormal expression of DLX2 has been reported in many human hematological malignancies and solid tumors, including acute lymphoblastic leukemia, acute myeloid leukemia, melanoma, glioma, breast, lung, prostate, ovarian and colon cancer[9,14-17]. A recent study showed that the expression of DLX2 plays a critical role in shifting transforming growth factor β (TGF-β) from its tumor suppressive to its tumor-promoting functions[13]. Moreover, abnormal TGF-β expression is involved in tumor progression, metastasis, angiogenesis and poor survival of gastric cancer[18,19]. These studies led us to investigate the possible role of DLX2 in the development of gastric adenocarcinoma.
In the present study, we assessed the expression of DLX2 in gastric adenocarcinoma tissues and adjacent normal tissues by immunohistochemistry. Correlations of DLX2 expression with clinicopathological features and survival of gastric adenocarcinoma patients were then analyzed.
Gastric adenocarcinoma tissues were obtained from gastrectomy specimens of 129 patients from the Department of Surgery and Pathology, the Second Affiliated Hospital of Kunming Medical University. Sixty samples of normal gastric tissues were collected from gastrectomy specimens of adjacent gastric cancer margins greater than 5 cm and served as controls. All operations were performed between January 2001 and June 2007. Patient diagnosis was established pathologically, and no patient had received chemotherapy or radiotherapy prior to surgery. All tissue specimens were formalin-fixed and paraffin-embedded. There were 87 males and 42 females (mean age, 57.6 years; range, 26-84 years). The age and gender of patients, tumor size, tumor location, histological differentiation, depth of invasion, status of lymph node metastasis and distant metastasis were obtained from histopathology records. The stage was determined according to the 7th edition of the AJCC Tumor Staging Manual and Japanese Classification 2011 in gastric cancer[20,21]. Forty-three cases were categorized as stage I , 43 were stage II, 34 were stage III and nine were stage IV. All patients had follow-up records for more than 5 years. The follow-up deadline was July 2012. The survival time was determined from the date of surgery to the follow-up deadline or date of death, which was mostly caused by recurrence or metastasis. The hospital’s ethics committee approved this study.
Immunohistochemical analysis was used to investigate DLX2 expression in 129 cases of gastric adenocarcinoma tissues and 60 cases of adjacent normal tissues. According to protocol[22,23] for immunohistochemistry on paraffin-embedded tissue sections, paraffin-embedded blocks were sectioned at about 4 μm thickness. Slides were baked at 60 °C for 2 h, deparaffinized with xylene and rehydrated using an alcohol gradient (100% alcohol, 95% alcohol, 80% alcohol, and 70% alcohol). After microwave pretreatment in citrate buffer (pH 6.0) for antigen retrieval, sections were treated with 3% hydrogen peroxide in methanol to block endogenous peroxidase activity. Sections were incubated with 1% bovine serum albumin to block nonspecific binding, and then incubated overnight at 4 °C with the polyclonal antibody against DLX2 (Epitomics, Inc., California, United States) at a dilution of 1:100. Phosphate buffer solution (PBS) was used as a negative control. After rinsing 3 × 3 min with PBS, tissue sections were treated with peroxidase-linked secondary antibody (Maixin-Bio, Inc., Fuzhou, China) for 30 min at room temperature. Staining was carried out with diaminobenzidine chromogen (Maixin-Bio, Inc., Fuzhou, China) and counterstained with hematoxylin. All slides were then dehydrated using an alcohol gradient, and mounted with a coverslip.
The results of immunostaining were reviewed and scored independently by two observers in a blinded fashion without knowledge of clinical and pathological information. To avoid artifactual effects, the cells on the margins of sections and areas with poorly presented morphology were not counted. Five fields (× 400 magnification) per tissue section, chosen at random, were counted. The immunostaining reaction was semiquantitatively evaluated based on the proportion of positive cells and the median staining intensity in normal gastric epithelial cells or tumor cells. The proportion of positive cells was scored as follows: 0, ≤ 5%; 1, 6%-25%; 2, 26%-50% and 3, ≥ 51%. The sections were considered to be positively stained when there were more than 5% of observed cells with immunostaining. Staining intensity was graded according to the following criteria: 0, no staining; 1, weak staining, light yellow; 2, moderate staining, yellow brown; and 3, strong staining, brown. The immunoreactive score was calculated based on the proportion score multiplied by the staining intensity score. All immunoreactive scores were less than 4 in adjacent normal tissues; therefore, the results of immunostaining in tumor tissues were divided into two groups, low expression (immunoreactive score ≤ 3) and high expression (immunoreactive score ≥ 4).
All statistical analyses were performed using the SPSS 17.0 software. Correlation of DLX2 expression with clinicopathological parameters was calculated by Pearson χ2 test, χ2 test with continuity correction and Spearman’s rank correlation test, respectively. Univariate survival analysis was assessed by the Kaplan-Meier method and the difference in survival curves was analyzed by the log-rank test. The Cox proportional hazards regression model was used to analyze independent prognostic factors. All reported P values were two-sided and P < 0.05 was considered statistically significant.
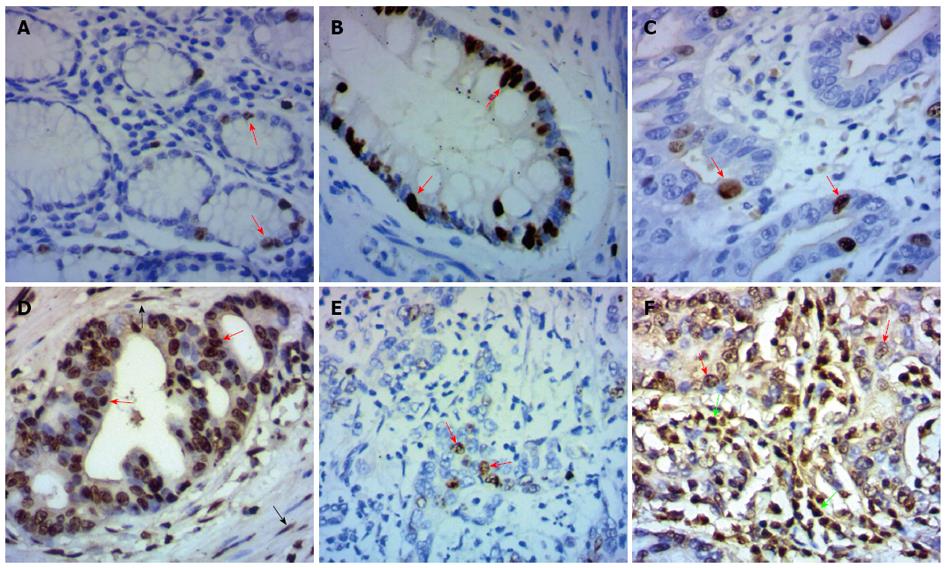
In the present study, immunohistochemical analysis was carried out to investigate the DLX2 expression in 129 gastric adenocarcinoma tissues and 60 adjacent normal tissues. Positive expression of DLX2 was detected in 68 (52.7%) cases of 129 gastric cancer tissues and in 14 (23.3%) cases of 60 adjacent normal tissues. The difference of DLX2 expression between gastric cancer tissues and adjacent normal tissues was statistically significant (χ2 = 14.391, P < 0.001). Moreover, high expression of DLX2 was detected in 48 (37.2%) cases of 129 human gastric cancer tissues, but not in adjacent normal tissues. DLX2 staining was detected mainly in the nuclei of normal gastric epithelial cells (Figure 1A) or tumor cells (Figure 1C-F) . In addition, increased expression of DLX2 was detected in intestinal metaplasia cells (Figure 1B), fibroblasts (Figure 1D) and inflammatory cells (Figure 1F) around tumor cells.
The expression of DLX2 in gastric adenocarcinoma was significantly correlated with tumor size (P = 0.001), depth of invasion (P = 0.008), lymph node metastasis (P = 0.023) and TNM stages (P = 0.020), but was not correlated with age, gender, histological differentiation and distant metastasis (P > 0.05, Table 1). Spearman’s rank correlation test showed that DLX2 expression was positively related to tumor size (P < 0.001), depth of invasion (P = 0.010), lymph node metastasis (P = 0.013) and TNM stages (P = 0.004).
| Clinicopathological features | Cases | DLX2 expression | χ2test | P value | |
| Low | High | ||||
| Age (yr) | 0.954 | 0.329 | |||
| < 60 | 69 | 46 (66.7) | 23 (33.3) | ||
| ≥ 60 | 60 | 35 (58.3) | 25 (41.7) | ||
| Gender | 1.989 | 0.158 | |||
| Female | 42 | 30 (71.4) | 12 (28.6) | ||
| Male | 87 | 51 (58.6) | 36 (41.4) | ||
| Tumor size (cm) | 11.518 | 0.001 | |||
| < 5 | 68 | 52 (76.5) | 16 (23.5) | ||
| ≥ 5 | 61 | 29 (47.5) | 32 (52.5) | ||
| Tumor location | 2.335 | 0.506 | |||
| Upper | 14 | 10 (71.4) | 4 (28.6) | ||
| Middle | 20 | 14 (70.0) | 6 (30.0) | ||
| Lower | 84 | 52 (61.9) | 32 (38.1) | ||
| Diffuse | 11 | 5 (45.5) | 6 (54.5) | ||
| Histologic differentiation | 3.186 | 0.364 | |||
| Well | 16 | 11 (68.8) | 5 (31.3) | ||
| Moderately | 28 | 21 (75.0) | 7 (25.0) | ||
| Poorly | 74 | 42 (56.8) | 32 (43.2) | ||
| Other | 11 | 7 (63.6) | 4 (36.4) | ||
| Depth of invasion | 11.940 | 0.008 | |||
| T1 | 28 | 24 (85.7) | 4 (14.3) | ||
| T2 | 24 | 16 (66.7) | 8 (33.3) | ||
| T3 | 58 | 28 (48.3) | 30 (51.7) | ||
| T4 | 19 | 13 (68.4) | 6 (31.6) | ||
| Lymph node metastasis | 9.577 | 0.023 | |||
| N0 | 69 | 50 (72.5) | 19 (27.5) | ||
| N1 | 17 | 12 (70.6) | 5 (29.4) | ||
| N2 | 25 | 11 (44.0) | 14 (56.0) | ||
| N3 | 18 | 8 (44.4) | 10 (55.6) | ||
| Distant metastasis | 0.012 | 0.914 | |||
| M0 | 120 | 76 (63.3) | 44 (36.7) | ||
| M1 | 9 | 5 (55.6) | 4 (44.4) | ||
| TNM stages | 9.849 | 0.020 | |||
| I | 43 | 35 (81.4) | 8 (18.6) | ||
| II | 43 | 24 (55.8) | 19 (44.2) | ||
| III | 34 | 17 (50.0) | 17 (50.0) | ||
| IV | 9 | 5 (55.6) | 4 (44.4) | ||
To investigate whether the ranks of percentage or staining intensity of DLX2 expression was more prominent for the immunoreactive assessment, the statistical analysis of the correlation between clinicopathological parameters and the proportion score or staining intensity score of DLX2 expression was calculated separately. As shown in Table 2, the proportion score of DLX2 expression was significantly correlated with tumor size (P = 0.002), depth of invasion (P = 0.016) and lymph node metastasis (P = 0.002). The staining intensity score was significantly correlated with tumor size (P = 0.029) and lymph node metastasis (P = 0.044). These results suggest that the ranks of percentage of DLX2 expression in gastric cancer tissues may be more prominent than staining intensity for the immunoreactive assessment.
| Clinicopathological features | Cases | Proportion score | χ2test | P value | Staining intensity | χ2test | P value | ||||
| 0 | 1 | 2, 3 | 0 | 1, 2 | 3 | ||||||
| Age (yr) | 5.637 | 0.060 | 3.924 | 0.141 | |||||||
| < 60 | 69 | 39 | 7 | 23 | 29 | 26 | 14 | ||||
| ≥ 60 | 60 | 22 | 12 | 26 | 16 | 25 | 19 | ||||
| Gender | 2.583 | 0.275 | 1.785 | 0.410 | |||||||
| Female | 42 | 22 | 8 | 12 | 18 | 15 | 9 | ||||
| Male | 87 | 39 | 11 | 37 | 27 | 36 | 24 | ||||
| Tumor size (cm) | 12.975 | 0.002 | 7.115 | 0.029 | |||||||
| < 5 | 68 | 42 | 9 | 17 | 30 | 26 | 12 | ||||
| ≥ 5 | 61 | 19 | 10 | 32 | 15 | 25 | 21 | ||||
| Histological differentiation | 3.302 | 0.192 | 0.286 | 0.867 | |||||||
| Well and moderately | 44 | 24 | 8 | 12 | 16 | 16 | 12 | ||||
| Poorly and other | 85 | 37 | 11 | 37 | 29 | 35 | 21 | ||||
| Invasion depth | 8.291 | 0.016 | 3.724 | 0.155 | |||||||
| T1, T2 | 52 | 31 | 9 | 12 | 22 | 21 | 9 | ||||
| T3, T4 | 77 | 30 | 10 | 37 | 23 | 30 | 24 | ||||
| Lymph node metastasis | 17.511 | 0.002 | 9.815 | 0.044 | |||||||
| N0 | 69 | 39 | 11 | 19 | 30 | 20 | 19 | ||||
| N1 | 17 | 6 | 6 | 5 | 4 | 11 | 2 | ||||
| N2, N3 | 43 | 16 | 2 | 25 | 11 | 20 | 12 | ||||
| TNM stages | 5.982 | 0.050 | 0.753 | 0.686 | |||||||
| I, II | 86 | 43 | 16 | 27 | 32 | 32 | 22 | ||||
| III, IV | 43 | 18 | 3 | 22 | 13 | 19 | 11 | ||||
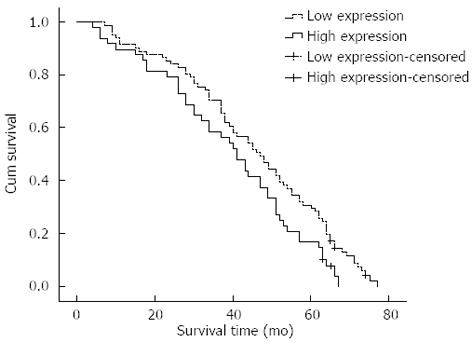
The Kaplan-Meier survival analysis revealed that survival time of patients with high DLX2 expression was significantly shorter than for those with low DLX2 expression (χ2 = 4.986, P = 0.026; Figure 2). The mean survival time of the former was only 39.216 mo (95%CI: 34.030-44.402), whereas the mean survival time of latter was 45.669 mo (95%CI: 41.426-49.912). For patients with high DLX2 expression, the cumulative 3- and 5-year survival rates were 58.3% and 16.7%, respectively, which was significantly lower than those for patients with low DLX2 expression (70.4% and 29.6%, respectively).
Additionally, the clinicopathological features for possible prognostic effects in gastric cancer were analyzed by Cox regression analysis. The following six clinicopathological features were selected for evaluation: tumor size, tumor location, depth of invasion, lymph nodes metastasis, distant metastasis and DLX2 expression (all P < 0.05 in univariate survival analysis). The multivariate analysis showed that invasion depth (P < 0.001), lymph nodes metastasis (P = 0.001) and distant metastasis (P < 0.001) were independent prognostic factors for patients with gastric adenocarcinoma, but DLX2 expression, tumor location and tumor size were not independent prognostic factors (Table 3).
| Variables | B | P value | Exp (B) | 95%CI for Exp (B) |
| Tumor location | 0.145 | 0.261 | 1.156 | 0.898-1.488 |
| Tumor size (< 5 cm vs≥ 5 cm) | 0.176 | 0.421 | 1.193 | 0.777-1.832 |
| Depth of invasion | 0.726 | < 0.001 | 2.067 | 1.570-2.722 |
| Lymph node metastasis | 0.303 | 0.001 | 1.354 | 1.126-1.629 |
| Distant metastasis (no vs yes) | 2.415 | < 0.001 | 11.185 | 4.187-29.878 |
| Distal-less homeobox 2 expression (low vs high) | -0.214 | 0.308 | 0.808 | 0.535-1.218 |
In the present study, DLX2 expression levels were investigated in 129 gastric adenocarcinoma tissues and 60 adjacent normal tissues by immunohistochemistry. We showed that DLX2 expression was more frequent in gastric cancer tissues than in adjacent normal tissues. The expression of DLX2 in gastric cancer tissues was significantly associated with the size of the tumor, the depth of invasion, lymph node metastasis and TNM stages. Based on these results, we suggest that increased expression of DLX2 may correlate with the advanced stage of gastric adenocarcinoma.
In several investigations, it has been shown that the abnormal expression of DLX2 in cancer cells is associated with tumor progression. However, the mechanism of DLX2’s involvement in tumor progression is not clear. Yilmaz et al[14] showed that expression of DLX2 correlated significantly with advanced tumor progression and with the metastatic potential of melanoma, glioma, lung, and prostate cancers. In their research, they found that DLX2 counteracted TGF-β-induced cell-cycle arrest and apoptosis in mammary epithelial cells, and DLX2 expression supported experimental tumor growth and metastasis of B16 melanoma cells. These results established that DLX2 has an important role in shifting TGF-β from its tumor suppressive to its tumor-promoting functions. Additionally, Lee et al[16] found that DLX2 expression was higher in breast and ovarian cancer tissues compared with the adjacent normal tissues. Furthermore, DLX2 expression was related to poor differentiation grade of ovarian cancer. DLX2 short hairpin RNA inhibited the metabolic stress-induced increase in propidium iodide-positive cell population and high mobility group box 1 and lactate dehydrogenase release. They concluded that DLX2 might be involved in tumor progression via the regulation of metabolic stress-induced necrosis.
In our research, high expression of DLX2 was detected in intestinal metaplasia, which is a risk factor for development of gastric cancer[24,25], indicating that increased expression of DLX2 might contribute to an early event of gastric cancer development. In addition, we found that increased expression of DLX2 was detected in inflammatory cells around tumor cells. Recent studies have expanded the concept that inflammation is a critical component of tumor progression[26]. Moreover, the mediators and cellular effectors of inflammation are important constituents of the local environment of tumors[27]. These results further support the hypothesis that DLX2 is involved in the development of gastric adenocarcinoma.
In 2010, Morini et al[9] found that expression of DLX2 was detected in 21.6% of the patients with breast cancer, and was significantly correlated with prolonged disease-free survival and reduced incidence of relapse. DLX5 expression was detected in 2.2% of all cases, displaying reduced disease-free survival and high incidence of relapse. In all cases, they found mutually exclusive expression of DLX2 and DLX5. Their study suggested that DLX genes were involved in human breast cancer progression, and that DLX2 and DLX5 genes might serve as prognostic markers. In our research, the Kaplan-Meier survival analysis revealed that the survival times of gastric adenocarcinoma patients with high DLX2 expression were significantly shorter than those with low DLX2 expression. However, the multivariate analysis showed that DLX2 expression was not an independent prognostic factor in gastric adenocarcinoma. The multivariate analysis might mask DLX2’s contribution to survival rate. Therefore, DLX2 expression might not be related with poor prognosis in patients with gastric adenocarcinoma.
In conclusion, our study demonstrates that increased expression of DLX2 may correlate with the advanced stage of gastric adenocarcinoma, and it may contribute to tumor development. These findings further support the hypothesis that, as a key regulator of embryogenesis, DLX2 may also play a critical role in tumor development. Consequently, further investigation is necessary to clarify the role of DLX2 in the development of gastric adenocarcinoma.
Gastric cancer is one of the leading causes of cancer-related death worldwide because of its frequency, poor prognosis and limited treatment options. Although the incidence of gastric cancer has been declining for several decades in most Western countries, it remains a crucial public health problem in developing countries. Several studies have demonstrated that various genetic and epigenetic alterations are involved in the course of carcinogenesis and progression of gastric cancer.
The distal-less homeobox (DLX) gene family exerts an important role in regulating embryonic development, tissue homeostasis, lymphocyte development, cell cycle and apoptosis. However, the role of the DLX gene family in tumor development has only recently been explored. As a member of DLX gene family, the abnormal expression of distal-less homeobox 2 (DLX2) has also been reported in many human solid tumors and hematological malignancies.
This is the first study attempt to explore the expression of DLX2 in gastric adenocarcinoma and its clinicopathological significance. This study suggests that increased expression of DLX2 may correlate with the advanced stage of gastric adenocarcinoma, and it may contribute to tumor development.
By understanding the expression of DLX2 and its correlation with clinicopathological features of gastric adenocarcinoma, this study will form the basis for further research to explore the mechanism of tumor development, and may represent a potential therapeutic target of gastric adenocarcinoma.
Homeobox genes encode transcription factors that play essential roles in controlling cell growth and differentiation during embryonic development. These genes are characterized by a highly conserved 61-amino acid homeodomain that binds DNA elements containing a TAAT core motif. Many homeobox genes are aberrantly expressed in a wide variety of solid tumors and hematological malignancies.
The authors investigated the expression of DLX2 in gastric adenocarcinoma tissues and adjacent normal tissues by immunohistochemistry. Correlations of DLX2 expression with clinicopathological features and prognosis of patients with gastric adenocarcinoma were then analyzed. This study has shown that increased expression of DLX2 may correlate with the advanced stage of gastric adenocarcinoma, and it may contribute to tumor development. These results are interesting and may represent a novel molecular mechanism of gastric adenocarcinoma development.
P- Reviewers Nagahara H, Singh SR S- Editor Wen LL L- Editor Stewart GJ E- Editor Lu YJ
| 1. | Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci. 2010;14:302-308. [PubMed] |
| 2. | Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 3. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 4. | Chen W, Zeng H, Zheng R, Zhang S, He J. Cancer incidence and mortality in China, 2007. Zhongguo Aizheng Yanjiu. 2012;24:1-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Nobili S, Bruno L, Landini I, Napoli C, Bechi P, Tonelli F, Rubio CA, Mini E, Nesi G. Genomic and genetic alterations influence the progression of gastric cancer. World J Gastroenterol. 2011;17:290-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Yasui W, Sentani K, Sakamoto N, Anami K, Naito Y, Oue N. Molecular pathology of gastric cancer: research and practice. Pathol Res Pract. 2011;207:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Bornschein J, Rokkas T, Selgrad M, Malfertheiner P. Gastric cancer: clinical aspects, epidemiology and molecular background. Helicobacter. 2011;16 Suppl 1:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Tamura G. Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World J Gastroenterol. 2006;12:192-198. [PubMed] |
| 9. | Morini M, Astigiano S, Gitton Y, Emionite L, Mirisola V, Levi G, Barbieri O. Mutually exclusive expression of DLX2 and DLX5/6 is associated with the metastatic potential of the human breast cancer cell line MDA-MB-231. BMC Cancer. 2010;10:649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Suh Y, Obernier K, Hölzl-Wenig G, Mandl C, Herrmann A, Wörner K, Eckstein V, Ciccolini F. Interaction between DLX2 and EGFR regulates proliferation and neurogenesis of SVZ precursors. Mol Cell Neurosci. 2009;42:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Panganiban G, Rubenstein JL. Developmental functions of the Distal-less/Dlx homeobox genes. Development. 2002;129:4371-4386. [PubMed] |
| 12. | Kraus P, Lufkin T. Dlx homeobox gene control of mammalian limb and craniofacial development. Am J Med Genet A. 2006;140:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Sunwoo JB, Kim S, Yang L, Naik T, Higuchi DA, Rubenstein JL, Yokoyama WM. Distal-less homeobox transcription factors regulate development and maturation of natural killer cells. Proc Natl Acad Sci USA. 2008;105:10877-10882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Yilmaz M, Maass D, Tiwari N, Waldmeier L, Schmidt P, Lehembre F, Christofori G. Transcription factor Dlx2 protects from TGFβ-induced cell-cycle arrest and apoptosis. EMBO J. 2011;30:4489-4499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Ferrari N, Palmisano GL, Paleari L, Basso G, Mangioni M, Fidanza V, Albini A, Croce CM, Levi G, Brigati C. DLX genes as targets of ALL-1: DLX 2,3,4 down-regulation in t(4; 11) acute lymphoblastic leukemias. J Leukoc Biol. 2003;74:302-305. [PubMed] |
| 16. | Lee SY, Jeon HM, Kim CH, Ju MK, Bae HS, Park HG, Lim SC, Han SI, Kang HS. Homeobox gene Dlx-2 is implicated in metabolic stress-induced necrosis. Mol Cancer. 2011;10:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Starkova J, Gadgil S, Qiu YH, Zhang N, Hermanova I, Kornblau SM, Drabkin HA. Up-regulation of homeodomain genes, DLX1 and DLX2, by FLT3 signaling. Haematologica. 2011;96:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Hong S, Lee HJ, Kim SJ, Hahm KB. Connection between inflammation and carcinogenesis in gastrointestinal tract: focus on TGF-beta signaling. World J Gastroenterol. 2010;16:2080-2093. [PubMed] |
| 19. | Hawinkels LJ, Verspaget HW, van Duijn W, van der Zon JM, Zuidwijk K, Kubben FJ, Verheijen JH, Hommes DW, Lamers CB, Sier CF. Tissue level, activation and cellular localisation of TGF-beta1 and association with survival in gastric cancer patients. Br J Cancer. 2007;97:398-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 21. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 22. | Hwang TL, Lee LY, Wang CC, Liang Y, Huang SF, Wu CM. CCL7 and CCL21 overexpression in gastric cancer is associated with lymph node metastasis and poor prognosis. World J Gastroenterol. 2012;18:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Luo D, Lu ML, Zhao GF, Huang H, Zheng MY, Chang J, Lv L, Luo JB. Reduced Popdc3 expression correlates with high risk and poor survival in patients with gastric cancer. World J Gastroenterol. 2012;18:2423-2429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Sakitani K, Hirata Y, Watabe H, Yamada A, Sugimoto T, Yamaji Y, Yoshida H, Maeda S, Omata M, Koike K. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J Gastroenterol Hepatol. 2011;26:1570-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Busuttil RA, Boussioutas A. Intestinal metaplasia: a premalignant lesion involved in gastric carcinogenesis. J Gastroenterol Hepatol. 2009;24:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11277] [Article Influence: 490.3] [Reference Citation Analysis (2)] |
| 27. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8323] [Article Influence: 489.6] [Reference Citation Analysis (0)] |