Published online May 7, 2013. doi: 10.3748/wjg.v19.i17.2621
Revised: August 21, 2012
Accepted: August 25, 2012
Published online: May 7, 2013
AIM: The effects of vitamin D3 have been investigated on various tumors, including colorectal cancer (CRC). 25-hydroxyvitamin-D3-24-hydroxylase (CYP24A1), the enzyme that inactivates the active vitamin D3 metabolite 1,25-dihydroxyvitamin D3 (1,25-D3), is considered to be the main enzyme determining the biological half-life of 1,25-D3. During colorectal carcinogenesis, the expression and concentration of CYP24A1 increases significantly, suggesting that this phenomenon could be responsible for the proposed efficacy of 1,25-D3 in the treatment of CRC. The aim of this study was to investigate the anti-tumor effects of vitamin D3 on the human CRC cell line Caco-2 after inhibition of the cytochrome P450 component of CYP24A1 activity.
METHODS: We examined the expression of CYP24A1 mRNA and the effects of 1,25-D3 on the cell line Caco-2 after inhibition of CYP24A1. Cell viability and proliferation were determined by means of sulforhodamine-B staining and bromodeoxyuridine incorporation, respectively, while cytotoxicity was estimated via the lactate dehydrogenase content of the cell culture supernatant. CYP24A1 expression was measured by real-time reverse transcription polymerase chain reaction. A number of tetralone compounds were synthesized to investigate their CP24A1 inhibitory activity.
RESULTS: In response to 1,25-D3, CYP24A1 mRNA expression was enhanced significantly, in a time- and dose-dependent manner. Caco-2 cell viability and proliferation were not influenced by the administration of 1,25-D3 alone, but were markedly reduced by co-administration of 1,25-D3 and KD-35, a CYP24A1-inhibiting tetralone. Our data suggest that the mechanism of action of co-administered KD-35 and 1,25-D3 does not involve a direct cytotoxic effect, but rather the inhibition of cell proliferation.
CONCLUSION: These findings demonstrate that the selective inhibition of CYP24A1 by compounds such as KD-35 may be a new approach for enhancement of the anti-tumor effect of 1,25-D3 on CRC.
- Citation: Kósa JP, Horváth P, Wölfling J, Kovács D, Balla B, Mátyus P, Horváth E, Speer G, Takács I, Nagy Z, Horváth H, Lakatos P. CYP24A1 inhibition facilitates the anti-tumor effect of vitamin D3 on colorectal cancer cells. World J Gastroenterol 2013; 19(17): 2621-2628
- URL: https://www.wjgnet.com/1007-9327/full/v19/i17/2621.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i17.2621
Epidemiologic studies have suggested that maintenance of an adequate level of vitamin D may reduce the incidence and development of several types of tumors, including breast, prostate and colorectal cancers (CRC)[1-4]. The role of vitamin D deficiency in the development of CRC, and the potential use of vitamin D in the treatment of CRC have been the focus of a number of studies, as CRC is one of the most common cancers[5].
There is a vast array of evidence suggesting a protective effect of vitamin D against CRC[6-8]. There is an inverse association between the serum level of 25-hydroxy vitamin D3 (25-D3) and the risk of CRC[1,9]. In ulcerative colitis, low expression of the vitamin D receptor (VDR) is associated with an elevated risk of the development of CRC[10]. An inadequate dietary intake of vitamin D and a vitamin D deficiency promote the development and growth of CRC in mice[3,11]. In elderly women, higher plasma levels of 25-D3 are accompanied by a lower risk of CRC[12]. Further studies have shown that vitamin D may have a preventive role not only in CRC, but also in other cancers of the alimentary tract[13]. Nevertheless, the exact cellular pathway for the putative anti-tumor effect of vitamin D remains unclear. The action of 1,25-dihydroxyvitamin-D3 (1,25-D3) through the nuclear VDRs is delayed, but the immediate responses triggered from the cell by cytosolic VDRs acting through Ca2+ influx might also play an important role in this process[14]. However, the application of 1,25-D3 in tumor treatment is restricted due to its tendency to cause hypercalcemia[15].
The anti-tumor efficacy of vitamin D in tumor cell cultures is somewhat contradictory[16-19]. Some cancer cell lines are more susceptible to vitamin D treatment than others[19,20], and the vitamin D-sensitive cell cultures have been shown to resemble early-stage tumors[20]. During the progression of the cancer, this susceptibility is gradually lost, but the underlying pathophysiological process of this loss is not clear. Though numerous clinical studies have been conducted with vitamin D or its analogs, the anti-tumor results were largely disappointing[21]. The current evidence suggests that a relationship does exist between vitamin D and cancer, but the strength of this relationship appears to weaken on progression from the preclinical to the clinical situation[22]. Thus, further examinations are needed to identify factors influencing the anti-tumor effect of vitamin D on tumor cells.
The mitochondrial enzyme cytochrome P450 component of 25-hydroxyvitamin-D3-24-hydroxylase (CYP24A1), which is the major 1,25-D3-inactivating enzyme, is considered to be an essential factor determining the biological half-life of 1,25-D3. Previous immunohistochemical studies have shown that the level of CYP24A1 rises significantly as the course of colorectal carcinogenesis progresses[20,23]. This fact might explain why 1,25-D3 cannot exert its anti-tumor effect in many pathological situations. It has also been demonstrated that the higher the level of CYP24A1, the more malignant the CRC[24]. A concomitantly increased expression of the proliferation marker Ki-67 in human CRC samples suggests that the overexpression of CYP24A1 reduces the local availability of 1,25-D3, and hence its antiproliferative effect[24]. Other mechanisms to may be involved in the development of 1,25-D3 insensitivity such as the downregulation of the VDRs[25].
In the present study, we set out to investigate the effects of 1,25-D3 on CRC cells after the inhibition of CYP24A1 activity.
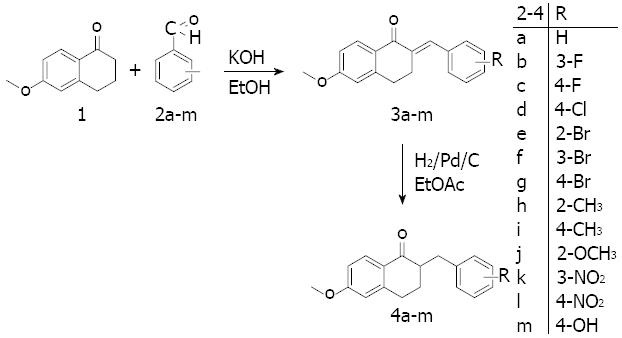
The ability of tetralones to inhibit CYP24A1 is less than that of their azole counterparts, but a greater degree of selectivity can be achieved with tetralones through the mechanism of their binding to the active site. Instead of binding to the heme iron, they interact with the active site if the enzyme through hydrogen bonds and van der Waals forces[26]. Thirteen new 2-substituted-benzyl-6-methoxy-1-tetralones synthesized in the Department of Organic Chemistry in Szeged were utilized in the present study.
The method employed for the preparation of the tetralones[27] involved the condensation of commercially available 6-methoxy-1-tetralone with benzaldehyde or a substituted benzaldehyde (Figure 1). 6-methoxy-1-tetralone (1) was dissolved in 4% ethanolic KOH solution, the appropriate benzaldehyde (2a-m) was added, and the reaction mixture was stirred at room temperature for 1-8 h until the starting material had disappeared (thin layer chromatography monitoring), and then allowed to stand overnight. The precipitate that formed was filtered off, washed with water, purified by flash chromatography on silica gel, and recrystallized from ethanol. The synthesis of the hydroxy derivative necessitated initial protection of the hydroxy group in the 4-hydroxybenzaldehyde with a tetrahydropyranyl group, which was stable under the basic ethanolic KOH condensation conditions. The protecting group was removed by heating with aqueous hydrochloric acid in a mixture of ethyl acetate and ethyl methyl ketone. In the next step, the 2-substituted-benzylidene-6-methoxy-1-tetralones (3a-m) were dissolved in ethyl acetate, and hydrogenated at 1 atm in the presence of Pd/C as catalyst for 1 h at room temperature. The catalyst was subsequently removed by filtration through a bed of silica gel, the solvent was evaporated in vacuo, and purification by flash chromatography on silica gel furnished the 2-substituted-benzyl-6-methoxy-1-tetralones (4a-m).
The resulting tetralones were dissolved individually in dimethyl sulfoxide at a concentration of 10 mmol/L and stored at 4 °C until use. In cell culture experiments, compounds (4a-m) were dissolved in sterile culture medium (GIBCO’s OPTI-MEM, Life Technologies-Invitrogen, Carlsbad, CA, United States) to the desired concentration. 1,25-D3 at 1 and 10 nmol/L and an untreated control were also applied in these experiments.
The human epithelial colorectal adenocarcinoma cell line Caco-2 obtained from ECACC was maintained in Dulbecco’s Modified Eagle Medium (D-MEM, Sigma, St. Louis, MO, United States) supplemented with 10% fetal calf serum (FCS, Sigma) and 1% antibiotic, antimycotic solution (Sigma) at 37 °C in a humidified atmosphere containing 5% CO2. Cells were cultured in 6-, 24- and 96-well plates, and all measurements were carried out in triplicate. The cell line was genotyped and identified as Caco-2 in 2011 on the basis of the results of STR analysis (DSMZ Profile Database, http://www.dsmz.de). Twenty-four hours before treatment, the medium was changed to GIBCO’s OPTI-MEM (Life Technologies-Invitrogen, Carlsbad, CA, United States). All experiments were carried out with cells from passages 5-25.
The protein dye sulforhodamine-B (SRB), was used to test various tetralone derivatives in various concentrations for various incubation times in 96-well plates to determine the effects of the compounds alone and in the presence of 1,25-D3 on the Caco-2 cell number. After removal of the culture medium, 100 μL of trichloroacetic acid was used to fix the cells during an incubation period of 30 min. The plates were then rinsed 5 times with distilled water. The cells were stained with a 0.4% solution of SRB (Sigma) in acetic acid for 30 min. After removal of the excess dye solution the plates were rinsed 4 times with 1% acetic acid solution and allowed to dry at room temperature. The bound SRB was dissolved in unbuffered Trisma-Sol and the plates were shaken for 5 min. The plates were measured in an Infinite M200 reader (Tecan AG, Männedorf, Switzerland) at 520 nm.
Levels of cytotoxicity were quantified after treatment through measurement of the lactate dehydrogenase (LDH) levels in the wells by using the Cytotoxicity Detection KitPLUS (Roche, Indianapolis, IN, United States). The greater the number of cells that die due to the cytotoxic effect, the higher the amount of LDH in the medium. The experiments were carried out in accordance with the kit manufacturer’s instructions.
Cell proliferation was quantified by measurement of the incorporation of 5-bromo-2’-deoxyuridine (BrdU) into the cellular DNA by means of Cell Proliferation enzyme-linked immunosorbent assay, BrdU (colorimetric) (Roche). The experiments were carried out in accordance with the manufacturer’s instructions.
RNA was isolated through use of the High Pure RNA Isolation Kit (Roche) as prescribed in the manufacturer’s instructions. The isolated RNA was translated by using Moloney murine leukemia virus reverse transcriptase in accordance with the manufacturer’s instructions (Promega, Madison, WI, United States). Predesigned and validated gene-specific TaqMan Gene Expression Assays from Life Technologies (Life Technologies, Foster City, CA, United States) were used in triplicate for quantitative real-time polymerase chain reaction (PCR) according to the manufacturer’s protocol. Each set contained gene-specific forward and reverse primers and fluorescence-labeled probes. The probes span an exon junction and do not detect genomic DNA [ABI Taqman assay No’s are hs00167999_m1 and hs99999905_m1, for CYP24A1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), respectively]. The PCR assays were carried out with the following protocol: denaturation for 10 min at 95 °C, and 45 cycles of denaturation for 15 s at 95 °C, annealing and extension for 1 min at 60 °C. The PCR reaction volume of 20 μL contained 2 μL cDNA, 10 μL of TaqMan 2x Universal PCR Master Mix NoAmpErase UNG (Life Technologies), 1 μL of gene-specific TaqMan Gene Expression Assay Mix and 7 μL of water. GAPDH was used as a housekeeping gene to normalize for RNA loading. Samples were analyzed using the ABI Prism 7500 real-time PCR system (Life Technologies). Relative quantification (RQ) studies were carried out on collected data (threshold cycle numbers, referred to as Ct) with the 7500 System SDS software 1.3 (Life Technologies).
Data were analyzed by using SPSS for Windows, release 18 (IBM, Armonk, NY, United States). Final data are presented as the means ± SD of at least three independent measurements. Statistical analysis was performed with the unpaired Student t-test; results with P≤ 0.05 were considered statistically significant.
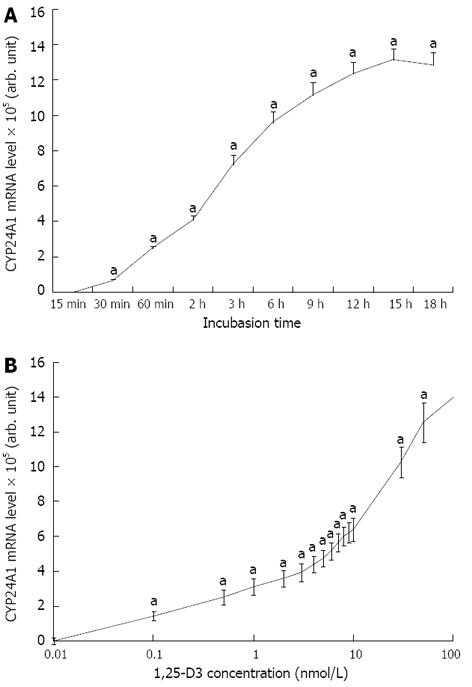
An increase in CYP24A1 mRNA level of six orders of magnitude was observed after a brief period of 1,25-D3 treatment. The increase in CYP24A1 mRNA expression was very rapid and it could be observed after 30 min of 1,25-D3 administration, and reached a maximum after 12-16 h of incubation (Figure 2A). After 4 h of incubation in the presence of 1 and 10 nmol/L 1,25-D3, the level of CYP24 mRNA was elevated to 311405-fold and 612801-fold, respectively, relative to the untreated controls (Figure 2B).
Certain of the tetralones were found to decrease the Caco-2 cell viability but only after 2-4 d of incubation with 1,25-D3. These compounds were tested at various concentrations for various periods to optimize the effect of 1,25-D3 in reducing the total Caco-2 cell count. Finally, compound KD-35 was selected for further and detailed investigations.
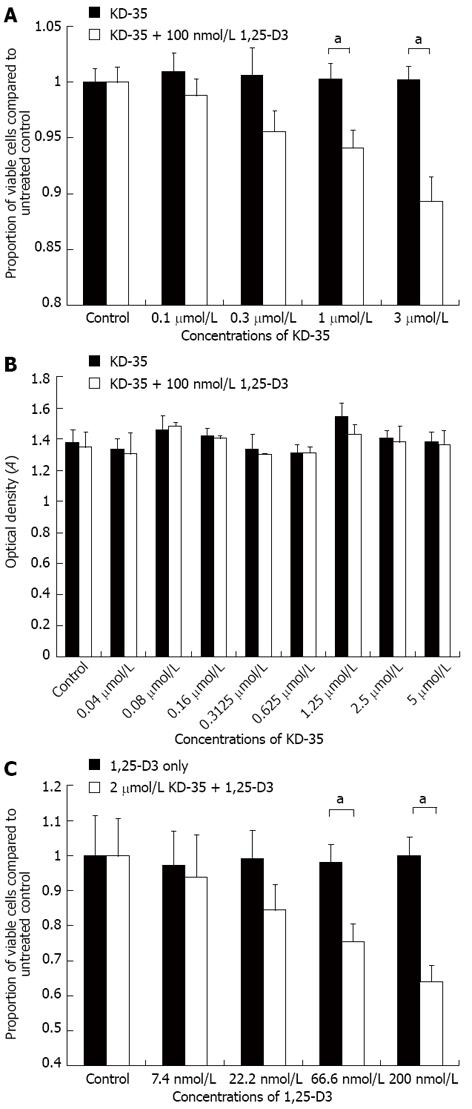
When Caco-2 cells were incubated for 4 d in the presence of 100 nmol/L 1,25-D3 with 0.1, 0.3, 1 or 3 μmol/L KD-35, the cell number was reduced by 2.17%, 5.07%, 6.18% and 10.93%, respectively, relative to the controls treated with only 100 nmol/L 1,25-D3 or 3 μmol/L KD-35 (Figure 3).
To determine the cause of the decrease in viable cell number in the presence of KD-35 and 1,25-D3, we measured LDH concentration in the cell suspension. The concentration of KD-35 ranged between 0.04 μmol/L and 5 μmol/L. Half of the wells were treated with KD-35 and 100 nmol/L 1,25-D3, the other half were treated with KD-35 only. All experiments were carried out in triplicate. Incubation lasted for 4 d. In all of the experimental setups, the LDH concentrations did not differ significantly in the presence of KD-35 alone or in combination with 1,25-D3 (Figure 3).
In the presence of 2 μmol/L KD-35, the following concentrations of 1,25-D3 were used: 7.4, 22.2, 66.6 and 200 nmol/L. Half of the wells were treated only with 2 μmol/L KD-35. Incubation lasted for 4 d. After incubation, the 5-BrdU label was added for an additional 2 h. The reduction in cell number relative to the control was 3.43%, 14.81%, 22.49% and 35.81%, respectively, compared to the wells with 1,25-D3 only (Figure 3).
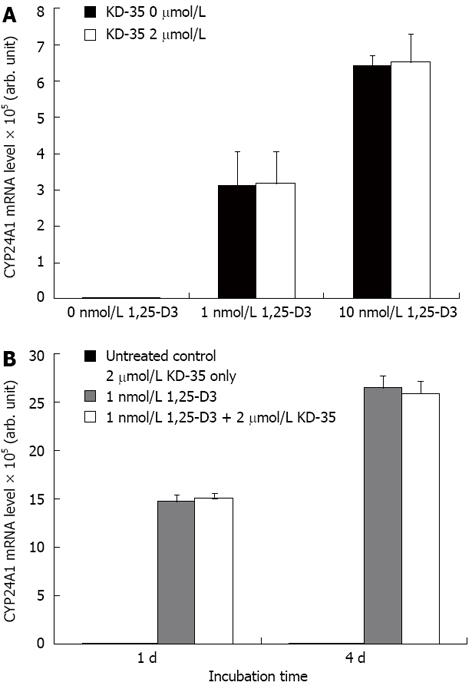
The amount of CYP24A1 mRNA expressed in the presence of various concentrations of KD-35 did not differ from that of the untreated controls. The CYP24A1 mRNA expression did not depend significantly on the duration of incubation with KD-35 (Figure 4).
We have identified a new tetralone compound, KD-35, that effectively and markedly stimulates the anti-proliferative effect of 1,25-D3 in the CRC cell line Caco-2.
CYP24A1, a member of the cytochrome P450 (CYP450) enzyme superfamily is the key enzyme in the metabolism of vitamin D neutralizing the active metabolite 1,25-D3, and thereby controlling its concentration in the tissues. The CYP450 enzymes all display an iron-containing heme domain at the active site. There are two types of enzyme blockers: azoles and non-azoles[28]. The N-heterocyclic ring of azoles is linked directly to the iron in the heme domain and, although this inhibition is very potent, it is not selective. Since the other enzymes involved in vitamin D metabolism (CYP27A1 and CYP27B1) are also members of the CYP450 superfamily, this type of nonselective inhibition is not specific for CYP24A1.
The enzyme inhibitory effect of non-azoles is mediated through hydrogen bonds and hydrophobic interactions with the active site of the enzyme. This is a more flexible mechanism which may permit significant selectivity though the inhibitory effect may be less than that of azoles[26]. We investigated 13 tetralones (non-azoles) in a search for a compound that is effective locally in the colon and is not strongly absorbed, so that the risk of adverse systemic effects is minimized.
Most of the 13 tetralones were either toxic or ineffective, even in the presence of 1,25-D3. Only in the presence of KD-35 did 1,25-D3 markedly inhibit Caco-2 cell proliferation without pronounced cytotoxicity of the tetralone alone. Such inhibition was not observed in the absence of KD-35. Unfortunately, two of the three most effective tetralones exhibited much higher cytotoxicity at higher concentrations than KD-35. The question arises as to whether KD-35 exerts its effect via CYP24A1 inhibition. We did not measure CYP24A1 enzyme activity directly since this is technically extremely difficult. It is also complicated to measure the intermediates of the CYP24A1 reaction. Moreover, a simple enzyme kinetic measurement would not reveal whether the compound enters the cell. We therefore chose an indirect approach: to prove the biological efficacy of the compound. KD-35 was found to exert an effect that allowed 1,25-D3 to reduce Caco-2 cell proliferation effectively, as reflected by an altered BrdU incorporation. Direct cytotoxicity was excluded by the LDH measurements, and no change in CYP24A1 mRNA expression was detected in response to KD-35, which ruled out alterations in protein synthesis. Obviously, no direct evidence was obtained to support direct enzyme inhibition, but an alternative mechanism is highly unlikely with this non-azole.
Two major pathways are mediated through the VDRs: the Wnt-beta-catenin pathway, which is responsible for the loss of adherent cell type, and the E-cadherin pathway, which is responsible for cell-to-cell adhesion and cell differentiation[29,30]. The administration of 1,25-D3 suppresses the Wnt-beta-catenin pathway and induces the expression of E-cadherin. The Wnt-beta-catenin pathway is constitutionally overregulated in most CRCs, due to the mutation of several members of the pathway (APC, AXIN2, etc.)[29]. There are other participants in colorectal carcinogenesis, such as estrogen receptors, which elevate the number of VDRs in the mucosal cells of the alimentary tract, or SNAIL, which inhibits the E-cadherin pathway and expression of VDRs[30-35]. Another important factor in the mucosal cell transition toward adenocarcinoma is an elevated level of CYP24A1, the intracellular concentration of which correlates with the dignity of the tumor[24].
Our results corroborate the earlier finding[36] that the presence of 1,25-D3 dramatically stimulates the expression of CYP24A1 in CRC cells[24,37]. Two vitamin D-responsive elements are present in the promoter region of CYP24A1[38]. Through this pathway, 1,25-D3 stimulates its own destruction through metabolism into inactive forms by enhancing the expression of CYP24A1[39].
Besides the genomic effects, there have also been reports of immediate nongenomic mechanisms. A possible mode of action is activation of the RhoA-ROCK-p38MAPK-MSK signaling pathway. This pathway mediates the induction of CST5, which is possibly responsible for tumor suppression and the level of CYP24A1; as a negative feedback mechanism, this eliminates 1,25-D3 from the cell[40]. VDRs found in other tumor cell membranes may bind 1,25-D3, and the complex could induce a rapid influx of Ca2+ into the cell[40], which activates RhoA-ROCK and then the p38MAPK-MSK-1 pathway. Besides the nongenomic activation of this pathway, a vitamin D-responsive element can also be identified in the -1k promoter region of the RhoA gene (http://www.cbil.upenn.edu/cgi-bin/tess/tess). RhoA plays an important role in the induction of CDH1/E-cadherin, which is crucial for the acquisition of the polarity and adhesive phenotype of cancer cells[29].
In view of these data, the elevation of CYP24A1 expression might be a self-defense mechanism of tumor cells. By inhibiting the inactivating enzyme, the amount of active vitamin D or its analogs required to elicit their marked anti-tumor effect could be reduced in vivo, thereby preventing elevation of the serum Ca2+ level and avoiding hypercalcemia[36]. The inhibition of CYP24A1 may allow 1,25-D3 to exert its anti-tumor effect, in this way leading to a new approach in the treatment of CRC in the future.
The effects of vitamin D3 have been investigated on various tumors, including colorectal cancer (CRC). The cytochrome P450 component of 25-hydroxyvitamin D3-24-hydroxylase (CYP24A1), the enzyme that inactivates the active vitamin D3 metabolite 1,25-dihydroxyvitamin-D3 (1,25-D3) is considered to be the main enzyme determining the biological half-life of 1,25-D3. During colorectal carcinogenesis, the expression and concentration of CYP24A1 increases significantly, suggesting that this phenomenon could be responsible for the controversial efficacy of 1,25-D3 in the treatment of CRC. In the present study, authors set out to investigate the effects of 1,25-D3 on CRC cells after the inhibition of CYP24A1 activity.
The anti-tumor effect of vitamin D3 has been a focus of interest during the last 10-15 years. However, vitamin D3 cannot exert this important effect in a number of tumors. The reasons for this have been investigated intensively. One possible explanation for the reduced anti-tumor efficacy of vitamin D3 is the accelerated neutralization of the active vitamin D3 compound in certain cases, e.g., CRC, liver and papillary thyroid cancers.
The authors synthesized a number of compounds potentially able to inhibit the action of CYP24A1, the enzyme neutralizing the effects of vitamin D3. One of these compounds, KD-35, had inhibitory potential without an apparent toxic effect. In the presence of KD-35, vitamin D3 markedly inhibited the growth of CRC cells.
Selective inhibition of the CYP24A1 by compounds such as KD-35 may permit a new approach to enhancement of the anti-tumor effect of 1,25-D3 on CRC.
The authors tackled an interesting topic for investigation. The manuscript is investigating the association between CYP24A1 inhibition and anti-tumor effect of 1a, 25-dihydroxyvitamin-D3 in Caco-2 CRC line. A careful assessment was considered using appropriate cell assays. A major finding of the study was that Caco-2 cell viability and proliferation were markedly reduced in response to 1,25-D3 when the CYP24A1 was inhibited (by KD-35, one of the tetralone compounds).
P- Reviewers Braet F, Lakatos PL S- Editor Gou SX L- Editor Cant MR E- Editor Zhang DN
| 1. | Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, Jansen EH, Slimani N, Byrnes G, Rinaldi S. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340:b5500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 2. | Kampman E, Slattery ML, Caan B, Potter JD. Calcium, vitamin D, sunshine exposure, dairy products and colon cancer risk (United States). Cancer Causes Control. 2000;11:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Tangpricha V, Spina C, Yao M, Chen TC, Wolfe MM, Holick MF. Vitamin D deficiency enhances the growth of MC-26 colon cancer xenografts in Balb/c mice. J Nutr. 2005;135:2350-2354. [PubMed] |
| 4. | Zeeb H, Greinert R. The role of vitamin D in cancer prevention: does UV protection conflict with the need to raise low levels of vitamin D? Dtsch Arztebl Int. 2010;107:638-643. [PubMed] |
| 5. | Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1612] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 6. | Cross HS, Nittke T, Peterlik M. Modulation of vitamin D synthesis and catabolism in colorectal mucosa: a new target for cancer prevention. Anticancer Res. 2009;29:3705-3712. [PubMed] |
| 7. | Giovannucci E. Strengths and limitations of current epidemiologic studies: vitamin D as a modifier of colon and prostate cancer risk. Nutr Rev. 2007;65:S77-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Rheem DS, Baylink DJ, Olafsson S, Jackson CS, Walter MH. Prevention of colorectal cancer with vitamin D. Scand J Gastroenterol. 2010;45:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 355] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 10. | Wada K, Tanaka H, Maeda K, Inoue T, Noda E, Amano R, Kubo N, Muguruma K, Yamada N, Yashiro M. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep. 2009;22:1021-1025. [PubMed] |
| 11. | Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, Giovannucci EL. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502-1508. [PubMed] |
| 13. | Giovannucci E. The epidemiology of vitamin D and colorectal cancer: recent findings. Curr Opin Gastroenterol. 2006;22:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Ordóñez-Morán P, Muñoz A. Nuclear receptors: genomic and non-genomic effects converge. Cell Cycle. 2009;8:1675-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Mikhail N. Clinical significance of vitamin D deficiency in primary hyperparathyroidism, and safety of vitamin D therapy. South Med J. 2011;104:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Cho YL, Christensen C, Saunders DE, Lawrence WD, Deppe G, Malviya VK, Malone JM. Combined effects of 1,25-dihydroxyvitamin D3 and platinum drugs on the growth of MCF-7 cells. Cancer Res. 1991;51:2848-2853. [PubMed] |
| 17. | Liu G, Hu X, Chakrabarty S. Vitamin D mediates its action in human colon carcinoma cells in a calcium-sensing receptor-dependent manner: downregulates malignant cell behavior and the expression of thymidylate synthase and survivin and promotes cellular sensitivity to 5-FU. Int J Cancer. 2010;126:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Tanaka H, Abe E, Miyaura C, Kuribayashi T, Konno K, Nishii Y, Suda T. 1 alpha,25-Dihydroxycholecalciferol and a human myeloid leukaemia cell line (HL-60). Biochem J. 1982;204:713-719. [PubMed] |
| 19. | Lechner D, Kállay E, Cross HS. 1alpha,25-dihydroxyvitamin D3 downregulates CYP27B1 and induces CYP24A1 in colon cells. Mol Cell Endocrinol. 2007;263:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Bareis P, Kállay E, Bischof MG, Bises G, Hofer H, Pötzi C, Manhardt T, Bland R, Cross HS. Clonal differences in expression of 25-hydroxyvitamin D(3)-1alpha-hydroxylase, of 25-hydroxyvitamin D(3)-24-hydroxylase, and of the vitamin D receptor in human colon carcinoma cells: effects of epidermal growth factor and 1alpha,25-dihydroxyvitamin D(3). Exp Cell Res. 2002;276:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Ma Y, Trump DL, Johnson CS. Vitamin D in combination cancer treatment. J Cancer. 2010;1:101-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Mocellin S. Vitamin D and cancer: deciphering the truth. Biochim Biophys Acta. 2011;1816:172-178. [PubMed] |
| 23. | Edlich R, Mason SS, Chase ME, Fisher AL, Gubler K, Long WB, Giesy JD, Foley ML. Scientific documentation of the relationship of vitamin D deficiency and the development of cancer. J Environ Pathol Toxicol Oncol. 2009;28:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Horváth HC, Lakatos P, Kósa JP, Bácsi K, Borka K, Bises G, Nittke T, Hershberger PA, Speer G, Kállay E. The candidate oncogene CYP24A1: A potential biomarker for colorectal tumorigenesis. J Histochem Cytochem. 2010;58:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Larriba MJ, Martín-Villar E, García JM, Pereira F, Peña C, de Herreros AG, Bonilla F, Muñoz A. Snail2 cooperates with Snail1 in the repression of vitamin D receptor in colon cancer. Carcinogenesis. 2009;30:1459-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Aboraia AS, Makowski B, Bahja A, Prosser D, Brancale A, Jones G, Simons C. Synthesis and CYP24A1 inhibitory activity of (E)-2-(2-substituted benzylidene)- and 2-(2-substituted benzyl)-6-methoxy-tetralones. Eur J Med Chem. 2010;45:4427-4434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Yee SW, Jarno L, Gomaa MS, Elford C, Ooi LL, Coogan MP, McClelland R, Nicholson RI, Evans BA, Brancale A. Novel tetralone-derived retinoic acid metabolism blocking agents: synthesis and in vitro evaluation with liver microsomal and MCF-7 CYP26A1 cell assays. J Med Chem. 2005;48:7123-7131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Schuster I, Egger H, Nussbaumer P, Kroemer RT. Inhibitors of vitamin D hydroxylases: structure-activity relationships. J Cell Biochem. 2003;88:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Ordóñez-Morán P, Larriba MJ, Pálmer HG, Valero RA, Barbáchano A, Duñach M, de Herreros AG, Villalobos C, Berciano MT, Lafarga M. RhoA-ROCK and p38MAPK-MSK1 mediate vitamin D effects on gene expression, phenotype, and Wnt pathway in colon cancer cells. J Cell Biol. 2008;183:697-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Peña C, García JM, Silva J, García V, Rodríguez R, Alonso I, Millán I, Salas C, de Herreros AG, Muñoz A. E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: clinicopathological correlations. Hum Mol Genet. 2005;14:3361-3370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Gilad LA, Bresler T, Gnainsky J, Smirnoff P, Schwartz B. Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK 1/2 signaling pathway in colon and breast cancer cells. J Endocrinol. 2005;185:577-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Gilad LA, Tirosh O, Schwartz B. Phytoestrogens regulate transcription and translation of vitamin D receptor in colon cancer cells. J Endocrinol. 2006;191:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Lechner D, Bajna E, Adlercreutz H, Cross HS. Genistein and 17beta-estradiol, but not equol, regulate vitamin D synthesis in human colon and breast cancer cells. Anticancer Res. 2006;26:2597-2603. [PubMed] |
| 34. | Larriba MJ, Muñoz A. SNAIL vs vitamin D receptor expression in colon cancer: therapeutics implications. Br J Cancer. 2005;92:985-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Pálmer HG, Larriba MJ, García JM, Ordóñez-Morán P, Peña C, Peiró S, Puig I, Rodríguez R, de la Fuente R, Bernad A. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10:917-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 208] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Tashiro K, Abe T, Oue N, Yasui W, Ryoji M. Characterization of vitamin D-mediated induction of the CYP 24 transcription. Mol Cell Endocrinol. 2004;226:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Meyer MB, Goetsch PD, Pike JW. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3. J Biol Chem. 2010;285:15599-15610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Chen KS, DeLuca HF. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim Biophys Acta. 1995;1263:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 203] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 591] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 40. | Ordóñez-Morán P, Alvarez-Díaz S, Valle N, Larriba MJ, Bonilla F, Muñoz A. The effects of 1,25-dihydroxyvitamin D3 on colon cancer cells depend on RhoA-ROCK-p38MAPK-MSK signaling. J Steroid Biochem Mol Biol. 2010;121:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |