Published online Apr 28, 2013. doi: 10.3748/wjg.v19.i16.2550
Revised: March 22, 2013
Accepted: March 28, 2013
Published online: April 28, 2013
Processing time: 239 Days and 12.1 Hours
AIM: To evaluate the feasibility and safety of a new style of laparoscopic and endoscopic cooperative surgery (LECS), an improved method of laparoscopic intragastric surgery (LIGS) for the treatment of gastric stromal tumors (GSTs).
METHODS: Six patients were treated with the new-style LECS. Surgery was performed according to the following procedures: (1) Exposing and confirming the location of the tumor with gastroscopy; (2) A laparoscopy light was placed in the cavity using the trocar at the navel, and the other two trocars penetrated both the abdominal and stomach walls; (3) With gastroscopy monitoring, the operation was carried out in the gastric lumen using laparoscopic instruments and the tumor was resected; and (4) The tumor tissue was removed orally using a gastroscopy basket, and puncture holes and perforations were sutured using titanium clips.
RESULTS: Tumor size ranged from 2.0 to 4.5 cm (average 3.50 ± 0.84 cm). The operative time ranged from 60 to 130 min (average 83.33 ± 26.58 min). Blood loss was less than 20 mL and hospital stay ranged from 6 to 8 d (average 6.67 ± 0.82 d). The patients were allowed out of bed 12 h later. A stomach tube was inserted for 72 h after surgery, and a liquid diet was then taken. All cases had single tumors which were completely resected using the new-style LECS. No postoperative complications occurred. Pathology of all resected specimens showed GST: no cases of implantation or metastasis were found.
CONCLUSION: New-style LECS for GSTs is a quick, optimized, fast recovery, safe and effective therapy.
Core tip: A new style of laparoscopic and endoscopic cooperative surgery (LECS) was used to treat gastric stromal tumors (GSTs) originating from the muscularis propria in this study. The operation was carried out in the gastric cavity, and the GST was completely removed using a gastroscopic light source and laparoscopic instruments. This method is minimally invasive and avoids many complications such as bleeding and intra-abdominal infection. Furthermore, the integrity of stomach structure and function is preserved. New-style LECS is a safe and effective method for the treatment of GSTs.
- Citation: Dong HY, Wang YL, Li J, Pang QP, Li GD, Jia XY. New-style laparoscopic and endoscopic cooperative surgery for gastric stromal tumors. World J Gastroenterol 2013; 19(16): 2550-2554
- URL: https://www.wjgnet.com/1007-9327/full/v19/i16/2550.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i16.2550
Gastric stromal tumors (GSTs) are potentially malignant tumors, accounting for approximately 60%-70% of gastrointestinal stromal tumors (GISTs)[1]. GSTs diffuse mainly by hematogenous metastasis and direct violation, are less likely to occur in lymph metastasis, and are not sensitive to chemotherapy or radiotherapy. The main treatment option is complete tumor resection[2-5]. Laparoscopic and endoscopic cooperative surgery (LECS) is an important therapy in the treatment of GSTs[6-8]. In this study, we used this new improved method of laparoscopic intragastric surgery (LIGS) to completely resect GSTs, and achieved good results.
From January 2011 to May 2012, 6 cases of GSTs originating from the muscularis propria were confirmed by endoscopic ultrasound (EUS) and gastroscopy. The patients were 2 males and 4 females, aged 42-63 years, with a mean age of 53.83 ± 7.94 years. All cases had a single tumor with an average size of 3.50 ± 0.84 cm. The tumors were located in the fundus fornix in 3 cases, in the posterior wall of the gastric body in 1 case and in the gastric cardia in 2 cases (Table 1).
| Patient | Sex | Age (yr) | Tumor location | Tumor size (cm) | Operation time (min) | Hospital stay (d) | Complications |
| 1 | M | 48 | Fundus fornix | 3.5 | 80 | 7 | None |
| 2 | F | 42 | Posterior wall of body | 4.5 | 50 | 7 | None |
| 3 | F | 56 | Cardia | 2.5 | 130 | 7 | None |
| 4 | F | 63 | Fundus fornix | 2.0 | 70 | 5 | None |
| 5 | F | 53 | Cardia | 3.0 | 90 | 7 | None |
| 6 | M | 61 | Fundus fornix | 3.5 | 80 | 7 | None |
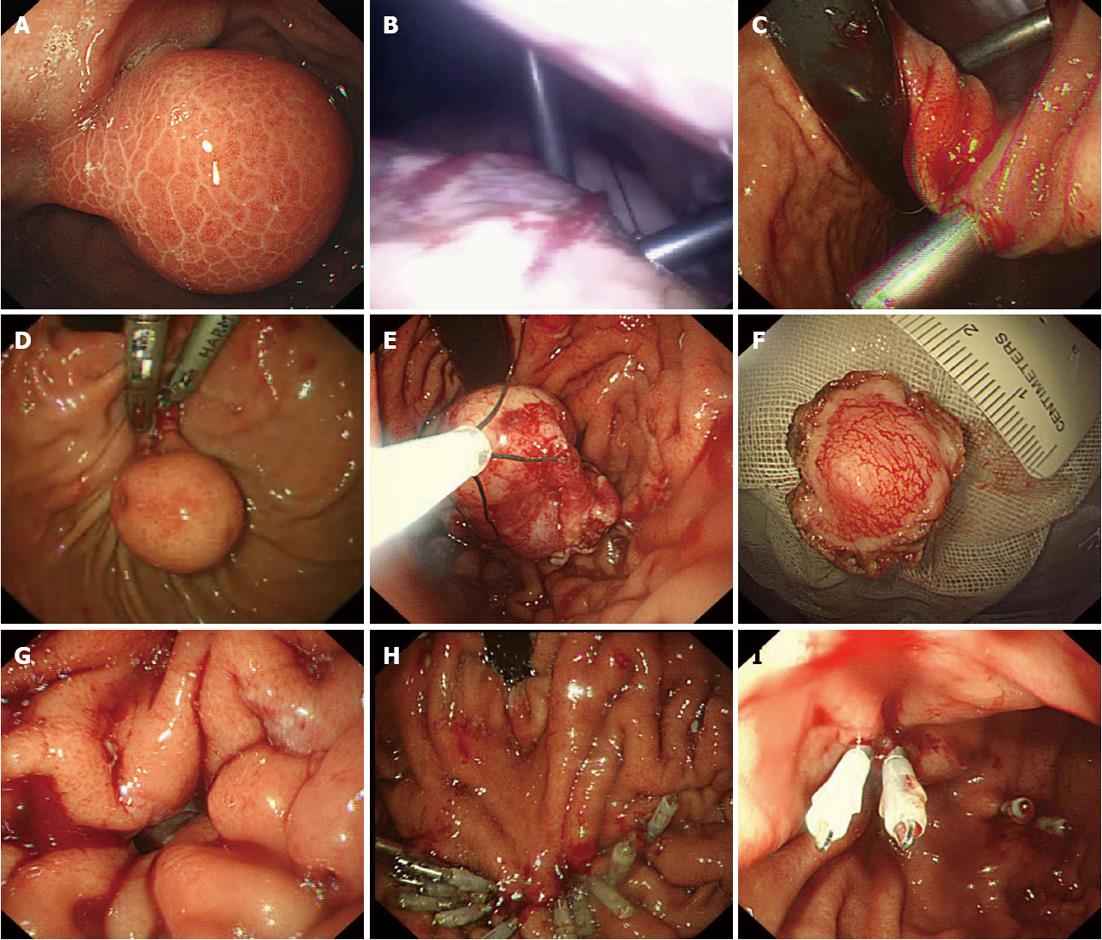
Using tracheal intubation with general anesthesia, the patients were placed in the supine position with their heads slightly to the left. Both the surgeon and the endoscopist stood on the patient’s left side. The location of the tumor was determined by the endoscopist (Figure 1A). The surgeon made a curved incision of approximately 0.5 cm at the superior border of the umbilicus, a CO2 pneumoperitoneum with pressure of 12 mmHg was established, and a 0.5 cm laparoscope was inserted for observation. Two punctures of about 0.5 cm at the left upper quadrant of the abdomen were then established. Two sutures were placed in the avascular area of the anterior wall of the stomach and exported from one of the puncture holes, in order to pull the anterior stomach wall close to the abdominal wall. The stomach was inflated during gastroscopy. Under laparoscopic monitoring, the surgeon inserted 2 ordinary 0.5 cm puncture cannulas into the stomach from the anterior wall, at a distance of more than 3 cm (Figure 1B and C). Under gastroscopic guidance, the surgeon used an ultrasound knife to completely resect the tumors (Figure 1D). Specimens were removed via the mouth using grasping forceps (Figure 1E and F). If there was no perforation, the two puncture cannulas in the gastric lumen were pulled out one by one, and the puncture holes were clipped using titanium clips via the endoscope. Otherwise, the perforation was clipped from the edge to the center using clips, the puncture cannulas were then pulled out, and the puncture holes closed by clips (Figure 1H and I). If the stomach was repeatedly inflated without leak, the gas was exhausted and a gastric tube was inserted. The pneumoperitoneum was reestablished and the hanging line in the stomach wall was removed. Intra-abdominal exudate and bleeding was fully suctioned. The pneumoperitoneum was removed when the surgeon pulled the puncture cannula out of the abdominal wall and sutured the puncture subcutaneously.
The average operative time was 83.33 ± 26.58 min and blood loss was less than 20 mL. A stomach tube was inserted for 72 h after surgery, and a liquid diet was then taken. After surgery, there was no significant abdominal pain or pneumoperitoneum. Patients were allowed out of bed 12 h later. The average length of hospital stay was 6.67 ± 0.82 d. Pathological results were GSTs in all patients, and the structure of the basal and cutting edge was normal. On the 3rd and 6th mo after surgery, the patients were reassessed by endoscopy and EUS, and all patients had healed well without metastasis.
With the development of endoscopic technology, LECS and endoscopic submucosal dissection (ESD) have been used more and more widely in the treatment of GSTs[6-13]. Conventional LECS, according to the different locations of GSTs, is mainly divided into 2 types: the laparoscope-assisted endoscopic technique (LAET) and endoscope-assisted laparoscopic technique (EALT). In addition, EALT can be divided into endoscope-assisted wedge resection (EAWR) and LIGS[6,8,14,15].
LAET mainly refers to the procedure where laparoscopy is used to closely monitor the endoscopic resection of tumors throughout the surgical process, and the timely treatment of perforation, bleeding and other complications[16]. However, when a tumor is too large or located in the posterior wall or gastric fundus, gastroscopy is very difficult to achieve. In EAWR, the tumor is resected by laparoscopy, while endoscopy plays an important role in the location of tumors, and is usually used for GSTs at the lesser curvature and the anterior wall of the stomach[17]. When the lesions are near the pylorus or cardia, a wedge resection may lead to stenosis[18].
Laparoscopic instruments are used in LIGS, including a laparoscopic light source, to puncture the gastric cavity directly and to carry out the surgery within it. The tumor is then removed from the abdominal wall, and the gastric wall and puncture holes are sutured using laparoscopic instruments[19-21].
Compared with the traditional LIGS, the advantages of the new-type LECS are as follows: (1) Using gastroscopic light, doctors can reduce the laparoscopic light holes in the gastric wall, and close laparoscopic operational holes with clips via the gastroscope. Compared with normal laparoscopic suturing, this technique is minimally invasive; (2) Tumors less than 4.5 cm in size can be removed via gastroscopy from the mouth to reduce trauma to the abdominal wall; and (3) Both the exterior and interior of the gastric cavity can be observed by laparoscopy and gastroscopy, respectively, in order to reduce the incidence of postoperative complications.
ESD is a technique which uses gastroscopy alone to resect tumors[22-24]. However, for tumors greater than 2 cm or exogenic or mixed type tumors, or if the tumor is located in an area where it is difficult to operate, ESD can result in problems, such as the risk of bleeding or perforation. For large perforations, it is more difficult to suture using clips during gastroscopy. Furthermore, longer operative time, and no peritoneal lavage and suction, can lead to pneumothorax, pneumoperitoneum, abdominal cavity cysts, peritoneal abscesses and other complications[25,26]. Compared to ESD, the advantages of new-type LECS are as follows: (1) Shortens the operative time; (2) Reduces the risk of bleeding; (3) In the event of perforation, the use of laparoscopy to suction exudate and bleeding and to execute peritoneal washing, will reduce the chance of intra-abdominal infection and other complications; and (4) Avoids the situation where observation and the operative field are unclear after perforation.
In conclusion, new-type LECS can be used to resect GSTs greater than 2 cm in size which originate from the muscularis propria, mixed tumors or where difficulty in resection is encountered by laparoscopy or gastroscopy.
Although this new type of LECS has unique advantages in the treatment of certain GSTs, this surgery has been used for less than one year. It is still at the stage of exploration and practice, and requires more cases to verify its usefulness. In addition, it requires good laparoscopic physicians, endoscopists and endoscopy nurses to help each other to accomplish the safe and effective use of this technique.
Gastric stromal tumors (GSTs) are potentially malignant tumors, and the main outcome of treatment is complete tumor resection. Laparoscopic and endoscopic cooperative surgery (LECS) is now being used more widely in the treatment of GSTs. In order to decrease complications and minimize trauma, a new type of LECS was used for GSTs in the present study.
Conventional LECS is used for the treatment of GSTs originating from the muscularis propria. However, use of the new type of LECS has rarely been reported. The authors investigated the clinical safety and efficacy of the new type of LECS for GSTs.
This is the first report on the new type of LECS for GSTs to date. This new surgery is similar to laparoscopic intragastric surgery. The operation is carried out in the stomach and the GST is completely removed using a gastroscopic light source and laparoscopic instruments. The integrity of the stomach structure and function is preserved. It is an innovative and effective operation for GSTs, and has unique advantages in the treatment of certain GSTs.
A new type of LECS was used for GSTs greater than 2 cm in size which originated from the muscularis propria, in mixed tumors and in tumors which were difficult to resect using only laparoscopy or gastroscopy.
LECS is an operation for resecting gastrointestinal tumors using laparoscopy and endoscopy. This method includes a laparoscope-assisted endoscopic technique and an endoscope-assisted laparoscopic technique.
The new-type of LECS described in this study is relatively new, and the procedures are feasible and may have some localizing advantages in selected patients.
P- Reviewer Plummer JM S- Editor Song XX L- Editor A E- Editor Xiong L
| 1. | Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Singer S, Rubin BP, Lux ML, Chen CJ, Demetri GD, Fletcher CD, Fletcher JA. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol. 2002;20:3898-3905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 310] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Ponsaing LG, Hansen MB. Therapeutic procedures for submucosal tumors in the gastrointestinal tract. World J Gastroenterol. 2007;13:3316-3322. [PubMed] |
| 4. | Judson I. Gastrointestinal stromal tumours (GIST): biology and treatment. Ann Oncol. 2002;13 Suppl 4:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Bertolini V, Chiaravalli AM, Klersy C, Placidi C, Marchet S, Boni L, Capella C. Gastrointestinal stromal tumors--frequency, malignancy, and new prognostic factors: the experience of a single institution. Pathol Res Pract. 2008;204:219-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Wilhelm D, von Delius S, Burian M, Schneider A, Frimberger E, Meining A, Feussner H. Simultaneous use of laparoscopy and endoscopy for minimally invasive resection of gastric subepithelial masses - analysis of 93 interventions. World J Surg. 2008;32:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (2)] |
| 8. | Walsh RM, Ponsky J, Brody F, Matthews BD, Heniford BT. Combined endoscopic/laparoscopic intragastric resection of gastric stromal tumors. J Gastrointest Surg. 2003;7:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Davila RE, Faigel DO. GI stromal tumors. Gastrointest Endosc. 2003;58:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Waterman AL, Grobmyer SR, Cance WG, Hochwald SN. Is endoscopic resection of gastric gastrointestinal stromal tumors safe? Am Surg. 2008;74:1186-1189. [PubMed] |
| 11. | Piccinni G, Marzullo A, Angrisano A, Iacobone D, Nacchiero M. Endoscopic resection of benign very low-risk gastric gastrointestinal stromal tumors. Is it enough? Eur J Gastroenterol Hepatol. 2007;19:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Bai J, Wang Y, Guo H, Zhang P, Ling X, Zhao X. Endoscopic resection of small gastrointestinal stromal tumors. Dig Dis Sci. 2010;55:1950-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | von Renteln D, Riecken B, Walz B, Muehleisen H, Caca K. Endoscopic GIST resection using FlushKnife ESD and subsequent perforation closure by means of endoscopic full-thickness suturing. Endoscopy. 2008;40 Suppl 2:E224-E225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Privette A, McCahill L, Borrazzo E, Single RM, Zubarik R. Laparoscopic approaches to resection of suspected gastric gastrointestinal stromal tumors based on tumor location. Surg Endosc. 2008;22:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 15. | Wolfsohn DM, Savides TJ, Easter DW, Lyche KD. Laparoscopy-assisted endoscopic removal of a stromal-cell tumor of the stomach. Endoscopy. 1997;29:679-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Kitano S, Shiraishi N. Minimally invasive surgery for gastric tumors. Surg Clin North Am. 2005;85:151-164, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Tarcoveanu E, Bradea C, Dimofte G, Ferariu D, Vasilescu A. Laparoscopic wedge resection of gastric leiomyoma. JSLS. 2006;10:368-374. [PubMed] |
| 18. | Song KY, Kim SN, Park CH. Tailored-approach of laparoscopic wedge resection for treatment of submucosal tumor near the esophagogastric junction. Surg Endosc. 2007;21:2272-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 19. | Ohashi S. Laparoscopic intraluminal (intragastric) surgery for early gastric cancer. A new concept in laparoscopic surgery. Surg Endosc. 1995;9:169-171. [PubMed] |
| 20. | Sekimoto M, Tamura S, Hasuike Y, Yano M, Murata A, Inoue M, Shiozaki H, Monden M. A new technique for laparoscopic resection of a submucosal tumor on the posterior wall of the gastric fundus. Surg Endosc. 1999;13:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Li VK, Hung WK, Chung CK, Ying MW, Lam BY, Kan DM, Chan MC. Laparoscopic intragastric approach for stromal tumours located at the posterior gastric wall. Asian J Surg. 2008;31:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567-579. [PubMed] |
| 23. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 495] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 24. | Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 233] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 26. | Yoshida N, Yagi N, Naito Y, Yoshikawa T. Safe procedure in endoscopic submucosal dissection for colorectal tumors focused on preventing complications. World J Gastroenterol. 2010;16:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |