Published online Apr 28, 2013. doi: 10.3748/wjg.v19.i16.2466
Revised: January 16, 2013
Accepted: January 29, 2013
Published online: April 28, 2013
Processing time: 201 Days and 12 Hours
AIM: To investigate outcomes of hepatocellular carcinomas (HCCs) in patients with chronic kidney disease (CKD).
METHODS: Four hundred and forty patients referred between 2000 and 2002 for management of HCCs were categorized according to their CKD stage, i.e., estimated glomerular filtration rate (eGFR) > 90 (stage 1), 60-90 (stage 2), 30-60 (stage 3), 15-30 (stage 4), and < 15 (stage 5) mL/min per 1.73 m2, respectively. Demographic, clinical and laboratory data were collected and mortality rates and cause of mortality were analyzed. The mortality data were examined with Kaplan-meier method and the significance was tested using a log-rank test. An initial univariate Cox regression analysis was performed to compare the frequency of possible risk factors associated with mortality. To control for possible confounding factors, a multivariate Cox regression analysis (stepwise backward approach) was performed to analyze those factors that were significant in univariate models (P < 0.05) and met the assumptions of a proportional hazard model.
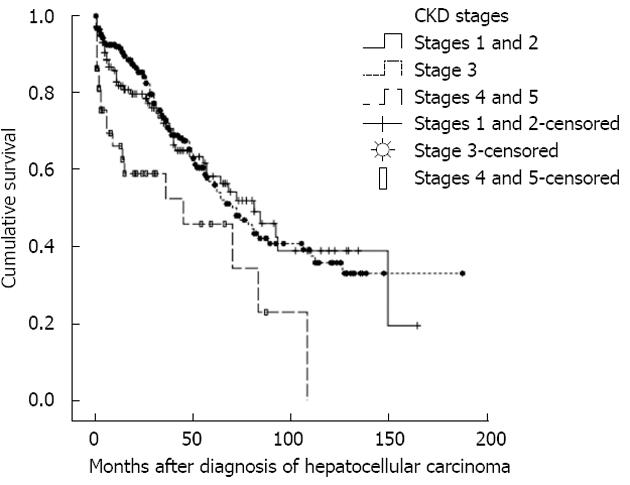
RESULTS: Most HCC patients with CKD were elderly, with mean age of diagnosis of 60.6 ± 11.9 years, and mostly male (74.8%). Hepatitis B, C and B and C co-infection virus were positive in 61.6%, 45.7% and 14.1% of the patients, respectively. It was found that patients with stages 4 and 5 CKD were not only older (P = 0.001), but also had higher hepatitis C virus carrier rate (P = 0.001), lower serum albumin level (P = 0.001), lower platelet count (P = 0.037), longer prothrombin time (P = 0.001) as well as higher proportions of advanced cirrhosis (P = 0.002) and HCCs (P = 0.001) than patients with stages 1 and 2 CKD. At the end of analysis, 162 (36.9%) patients had died. Kaplan-Meier analysis revealed that patients with stages 4 and 5 CKD suffered lower cumulative survival than stages 1 and 2 CKD (log-rank test, χ2 = 11.764, P = 0.003). In a multivariate Cox-regression model, it was confirmed that CKD stage [odds ratio (OR) = 1.988, 95%CI: 1.012-3.906, P = 0.046)], liver cirrhosis stage (OR = 3.571, 95%CI: 1.590-8.000, P = 0.002) and serum albumin level (OR = 0.657, 95%CI: 0.491-0.878, P = 0.005) were significant predictors for mortality in this population.
CONCLUSION: HCC patients with stages 4 and 5 CKD had inferior survival than stages 1 and 2 CKD. This warrants further studies.
Core tip: There is a paucity of data regarding outcomes of hepatocellular carcinoma (HCC) in patients with chronic kidney disease (CKD), even though both hepatitis B virus and CKD are endemic in Taiwan. In a large-scale study, a total of 440 patients with HCC were categorized according to their CKD stage. At the end of analysis, it was found that HCC patients with stages 4 and 5 CKD suffered poorer survival than stages 1 and 2 CKD, which might be explained by inferior liver reserve. Interestingly, tumor stage was not a significant predictor for mortality according to a multivariate Cox regression model.
- Citation: Lee CH, Hsieh SY, Lin JL, Liu MS, Yen TH. Hepatocellular carcinoma in patients with chronic kidney disease. World J Gastroenterol 2013; 19(16): 2466-2472
- URL: https://www.wjgnet.com/1007-9327/full/v19/i16/2466.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i16.2466
Taiwan has the highest prevalence and second highest incidence of end-stage renal disease (ESRD) in the world[1,2]. According to the 2012 Renal Data System of the United States[3], the United States, Taiwan and Japan continue to have some of the highest rates of incident ESRD, at 369, 361 and 288 per million population in 2010. In Taiwan, the prevalence of ESRD reached 2584 per million in 2010, while rates of 2260 and 1870 were reported in Japan and the United States. Similarly, hepatitis B virus (HBV) and hepatocellular carcinoma (HCC) are endemic in Taiwan[4-6]. The carrier rate of hepatitis B surface antigen (HBsAg) in the general population is 15%-20%[4]. Consequently, many Taiwanese patients with CKD or ESRD are also chronic HBV carriers[7].
There is strong evidence of increased cancer risk in patients with CKD[8], in patients with ESRD being treated with chronic dialysis[9-11], and in recipients of renal transplant[12]. The CKD was associated with malignancy and worse prognosis[13-16]. Moderately reduced kidney function may be an independent risk factor for cancer in older men. The risk begins when estimated glomerular filtration rate (eGFR) falls to approximately 55 mL/min and progressively increases as eGFR declines to 40 mL/min, similar to the risk of patients on dialysis or of transplant recipients[8]. Because survival time after the confirmation of recurrence was significantly shorter in the CKD group, their cumulative survival rate was significantly lower compared to that in the non-CKD group, although the difference in disease-free survival was not significant[17].
Almost all cancers in the renal parenchyma are renal cell carcinoma, whereas cancers in the urinary tract are urothelial carcinoma. These two cancers differ markedly in terms of carcinogenesis and basic biology. Renal cell carcinoma is the most common urologic cancer in Western patients on dialysis, whereas urothelial carcinoma is the most common urologic cancer in Asian patients on chronic dialysis[11]. This is a unique and distinguishing epidemiologic characteristic of the Taiwanese population[11]. On the other hand, very few data[17-22] are available regarding the outcome of patients with HCC and CKD, even though both are endemic in Taiwan.
Therefore, this study analyzed the registry of the Chang Gung Memorial Hospital to examine the epidemiology of HCC in CKD populations in Taiwan.
This retrospective observational study complied with the guidelines of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Chang Gung Memorial Hospital, a tertiary referral center in northern Taiwan. Because of the retrospective nature of this study, Institutional Review Board approval was obtained and the informed consent of risk of HCC and all treatment modalities of all patients on their initial admission was used. Moreover, all individual information was securely protected (by delinking identifying information from the main dataset) and available only to the investigators. All the data were analyzed anonymously and all primary data were collected according to epidemiologic guidelines. This policy was based on previous publications[23,24].
Four hundred and forty patients referred between 2000 and 2002 for management of HCCs were categorized according to their CKD stage, i.e., eGFR > 90 (stage 1), 60-90 (stage 2), 30-60 (stage 3), 15-30 (stage 4), and < 15 (stage 5) mL/min per 1.73 m2, respectively. Demographic, clinical, laboratory and mortality data were obtained for analysis.
HCC was diagnosed by alpha-fetoprotein, imaging studies such as ultrasonography, radio-contrast enhanced tri-phasic dynamic computed tomography, magnetic resonance imaging, angiography, and/or documented tissue histopathology[25].
Cirrhosis was diagnosed by histopathology or by laboratory tests, hepatic ultrasonography, and clinical manifestations of chronic hepatitis with portal hypertension (i.e., varices, thrombocytopenia, or splenomegaly), and/or hepatic decompensation (i.e., jaundice, prolonged prothrombin time, and ascites)[26].
The eGFR was computed using the four-variable modification of diet in renal disease (MDRD) study equation[27]. The CKD stage was defined as 1, 2, 3, 4 and 5 according to eGFR; > 90, 60-90, 30-60, 15-30, and < 15 mL/min per 1.73 m2, respectively[27].
The HCC patients in the stages 1 and 2 groups underwent tumor resection, liver transplantation, or percutaneous interventional therapies for local tumor ablation, including radio-frequency ablation, pure ethanol injection therapy, pure acetic acid injection therapy, and trans-catheter arterial chemo-embolization[28]. Patients in the stage 3 group received trans-catheter arterial chemo-embolization and radio-frequency ablation[28]. Those in stage 4 received palliative chemotherapy, trans-catheter arterial chemo-embolization, or radiotherapy, and medical care[28].
Continuous variables are expressed as means and standard deviations and categorical variables as numbers with percentages in brackets. All data were tested for normality of distribution and equality of standard deviations before analysis. For comparisons between patient groups, we used General Linear Model (Least Significant Difference test) for quantitative variables and Cross-tabulation (χ2 or Fisher’s exact tests) for categorical variables. Mortality data were compared using the Kaplan-Meier method and the significance was tested using a log-rank test. An initial univariate Cox regression analysis was performed to compare the frequency of possible risk factors associated with mortality. To control for possible confounding factors, a multivariate Cox regression analysis (stepwise backward approach) was performed to analyze those factors that were significant in univariate models (P < 0.05) and met the assumptions of a proportional hazard model. We considered results that rejected the null hypothesis with 95% confidence to be significant. All analyses were performed using IBM SPSS Statistics Version 20.
Most HCC patients with CKD were elderly, with mean age of diagnosis of 60.6 ± 11.9 years (Table 1), and mostly male (74.8%). Hepatitis B, C and B and C co-infection virus were positive in 61.6%, 45.7% and 14.1% of the patients, respectively. It was found that patients with stages 4 and 5 CKD were older (P = 0.001) and had higher hepatitis C virus (HCV) carrier rates (P = 0.001) than patients with stages 1 and 2 CKD. On the other hand, there were more male (P = 0.000) and HBV carrier rate (P = 0.002) in stages 1 and 2 than stages 4 and 5 CKD.
| Variable | Total (n = 440) | Stages 1 and 2 (n = 132) | Stage 3 (n = 263) | Stages 4 and 5 (n = 45) | P value |
| eGFR1 | 53.7 ± 27.1 | 73.5 ± 13.5 | 50.0 ± 26.4 | 17.6 ± 8.7 | 0.000 |
| Age1 (yr) | 60.6 ± 11.9 | 54.4 ± 12.3 | 63.1 ± 10.5 | 64.0 ± 11.7 | 0.000 |
| Male | 329 (74.8) | 115 (87.1) | 182 (69.2) | 32 (71.1) | 0.000 |
| HBV carrier | 271 (61.6) | 97 (73.5) | 152 (57.8) | 22 (48.9) | 0.002 |
| HCV carrier | 201 (45.7) | 42 (31.8) | 131 (49.8) | 28 (62.2) | 0.000 |
| HBV and HCV carrier | 62 (14.1) | 15 (11.4) | 39 (14.8) | 8 (17.8) | 0.488 |
| Follow-up duration1 (mo) | 37.6 ± 37.1 | 37.0 ± 37.6 | 40.7 ± 37.6 | 20.9 ± 27.4 | 0.013 |
Patients with stages 4 and 5 CKD not only had lower serum albumin level (P = 0.001) and platelet count (P = 0.037), but also had longer prothrombin time (P = 0.001) than stages 1 and 2 CKD (Table 2).
| Variable | Total (n = 440) | Stage 1 and 2 (n = 132) | Stage 3 (n = 263) | Stages 4 and 5 (n = 45) | P value |
| AFP (ng/mL) | 6202.1 ± 83392.3 | 3564.6 ± 11906.0 | 8176.6 ± 106579.9 | 1866.3 ± 4866.0 | 0.911 |
| AST (U/L ) | 84.8 ± 77.9 | 94.3 ± 76.1 | 77.4 ± 68.6 | 104.3 ± 126.5 | 0.495 |
| ALT (U/L) | 73.0 ± 90.4 | 79.4 ± 79.1 | 64.8 ± 49.2 | 103.0 ± 218.9 | 0.150 |
| T-bilirubin (mg/dL) | 1.8 ± 3.7 | 1.5 ± 1.6 | 1.8 ± 4.3 | 2.8 ± 4.4 | 0.198 |
| ALP (U/L) | 122.4 ± 92.1 | 124.0 ± 76.6 | 114.6 ± 79.7 | 164.5 ± 170.2 | 0.072 |
| Albumin (g/dL ) | 3.5 ± 0.7 | 3.6 ± 0.6 | 3.5 ± 0.7 | 3.2 ± 0.7 | 0.001 |
| Prolonged PT (s) | 1.9 ± 4.3 | 1.4 ± 1.2 | 1.6 ± 2.1 | 5.1 ± 12.4 | 0.000 |
| Platelet count (x 103/μL) | 138.2 ± 90.7 | 163.7 ± 109.7 | 126.5 ± 80.1 | 131.4 ± 73.0 | 0.037 |
Patients with stages 4 and 5 CKD had higher incidences of advanced cirrhosis than stages 1 and 2 CKD (Table 3, P = 0.002).
| Variable | Total (n = 440) | Stage 1 and 2 (n = 132) | Stage 3 (n = 263) | Stages 4 and 5 (n = 45) | P value |
| Liver cirrhosis classification(Child-Pugh score) | 0.002 | ||||
| No | 53 (12.0) | 27 (20.5) | 23 (8.7) | 3 (6.7) | |
| Class A | 236 (53.6) | 67 (50.8) | 151 (57.4) | 18 (40.0) | |
| Class B | 110 (25.0) | 30 (22.7) | 64 (24.3) | 16 (35.6) | |
| Class C | 41 (9.3) | 8 (6.1) | 25 (9.5) | 8 (17.8) | |
| Tumor stage | 0.001 | ||||
| Stage 1 | 213 (48.4) | 60 (45.5) | 133 (50.6) | 20 (44.4) | |
| Stage 2 | 113 (25.7) | 27 (20.5) | 76 (28.9) | 10 (22.2) | |
| Stage 3 | 84 (19.1) | 40 (30.3) | 36 (13.7) | 8 (17.8) | |
| Stage 4 | 30 (6.8) | 5 (3.8) | 18 (6.8) | 7 (15.6) | |
| Mortality | 162 (36.9) | 47 (35.6) | 94 (35.9) | 21 (46.7) | |
| Cause of mortality | 0.050 | ||||
| Liver failure | 27 (6.1) | 10 (7.6) | 16 (6.1) | 1 (2.2) | |
| Tumor death | 35 (8.0) | 8 (6.1) | 22 (8.4) | 5 (11.1) | |
| Gastrointestinal bleeding | 27 (6.1) | 11 (8.3) | 16 (6.1) | 0 (0) | |
| Sepsis | 69 (15.7) | 18 (13.6) | 36 (13.7) | 15 (33.3) | |
| Others | 5 (1.1) | 1 (0.8) | 4 (1.5) | 0 (0) |
Most of the HCCs were diagnosed in the early stages (Table 3). Patients with stages 4 and 5 CKD had higher proportions of advanced HCCs than stages 1 and 2 CKD (P = 0.001).
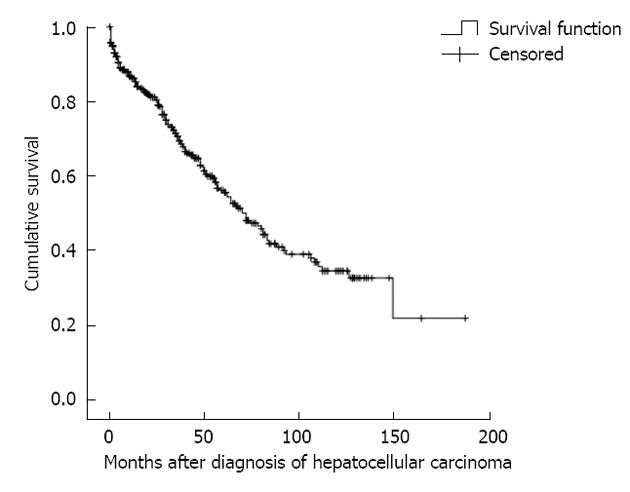
At the end of analysis, 162 (36.9%) patients had died (Table 3). It was revealed that patients with stages 4 and 5 CKD suffered higher fatal septic complications than stages 1 and 2 CKD (P = 0.050). The one-, three-, and five-year survival rates were 86.2%, 71.3% and 55.9%, respectively (Figure 1). In addition, patients with stages 4 and 5 CKD suffered lower cumulative survival than stages 1 and 2 CKD (Figure 2, log-rank test, χ2 = 11.764, P = 0.003).
In a multivariate Cox regression model (Table 4), it was confirmed that CKD stage [odds ratio (OR) = 1.988, 95%CI: 1.012-3.906, P = 0.046], liver cirrhosis stage (OR = 3.571, 95%CI: 1.590-8.000, P = 0.002) and serum albumin level (OR = 0.657, 95%CI: 0.491-0.878, P = 0.005) were significant predictors for mortality.
| Variable | Univariate analysis | Multivariate analysis | ||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |
| Chronic kidney disease stage | 2.237 (1.376-3.636) | 0.001 | 1.988 (1.012-3.906) | 0.046 |
| Liver cirrhosis stage | 4.566 (2.331-8.929) | 0.000 | 3.571 (1.590-8.000) | 0.002 |
| Tumor stage | 3.509 (1.789-6.849) | 0.000 | 2.169 (0.997-4.717) | 0.051 |
| Albumin, each increase of 1 mg/dL | 0.503 (0.399-0.633) | 0.000 | 0.657 (0.491-0.878) | 0.005 |
| Prolonged prothrombin time, each increase of 1 s | 1.062 (1.036-1.089) | 0.000 | 0.977 (0.942-1.013) | 0.215 |
The analytical data demonstrated that HCC patients with stages 4 and 5 CKD had inferior survival than stages 1 and 2 CKD. The reason is unclear but inferior liver reserve in this subgroup should be considered. In a large-scale study in Taiwan[16], there was a higher risk for overall cancer mortality in CKD patients compared to non-CKD patients (adjusted hazard ratio 1.2). Moreover, CKD was associated with increased mortality from liver, kidney, and urinary tract cancers, with adjusted hazard ratios of 1.74, 3.3, and 7.3, respectively. Most importantly, patients with stage 4 CKD had poorer prognosis than the other groups[16], but the reason was also unclear.
In the present study, patients with HCC were stratified according to the stage of CKD. Kaplan-Meier analysis revealed that patients with stages 4 and 5 CKD suffered lower cumulative survival than stages 1 and 2 CKD (P = 0.003). A multivariate Cox regression model confirmed that CKD stage (P = 0.046), liver cirrhosis stage (P = 0.002) and serum albumin level (P = 0.005) were significant predictors of mortality. Tumor stage was a significant predictor for mortality in the univariate model (P = 0.000), but not after multivariate analysis (P = 0.051). Previous study[29] also reported that eGFR, as determined by MDRD equation, might provide better prognostic accuracy than the CKD-epidemiology collaboration equations independent of liver functional reserve and tumor staging, and is a more feasible renal surrogate for outcome prediction in patients with HCC and CKD stages 1-3 receiving TACE. Notably, the TNM classification did not accurately predict survival in HCC patients with CKD.
In this study, most HCC patients with CKD are male (74.8%). Moreover, there were more male in stages 1 and 2 than stages 4 and 5 CKD (P = 0.000). In 1981, Zevin et al[30] reported a hemodialysis patient who developed HCC after long-term therapy with androgenic anabolic steroids. The tumor progressed very rapidly, with no evidence of regression despite discontinuation of the drug. The increased risk of malignancy in patients with chronic uremia and hemodialysis and the higher frequency of HCC associated with the use of anabolic steroids may explain the male predominance in CKD populations[30]. Since CKD is associated with hypogonadism, the protective effect of estrogen on liver cancer may be reduced[16]. Nevertheless, male predominance in HCC has been reported due to other than androgenic anabolic steroid use[31].
In this study, hepatitis B, C and B and C co-infection virus were positive in 61.6%, 45.7% and 14.1% of the patients, respectively. Notably, there were more HCV (P = 0.001) in stages 4 and 5 CKD than stages 1 and 2 CKD, but more HBV (P = 0.002) in stages 1 and 2 than stages 4 and 5 CKD. Hence, the incidence of HBV (61.6%) is only a little higher than that of HCV (45.7%) in this study, although the national prevalence rates among adults in Taiwan is 1%-3% and 15%-20% for HCV and HBV, respectively[32]. Notably, after implementation of national hepatitis B vaccination in Taiwan, the prevalence of HBsAg among persons younger than 15 years of age has decreased from 9.8% in 1984 to 0.7% in 1999[33]. Higher incidences of HBV and HCV infection were noted in uremic patients, possibly because of cross-infection during hemodialysis. It was found that compared to those who were negative for both markers, patients with both HBsAg and anti-HCV had increased incidence of chronicity[34]. Earlier study[35] also found that HCV infection, but not HBV infection, is significantly associated with prevalence and disease severity of CKD in chronic dialysis patients. However, another population-based study[36] found no association between HCV and risk of development of CKD.
Very few data are available regarding the outcome of HCC in CKD populations. Nevertheless, previous evidences[17-22] suggested that we should be more aggressive on the principle for management for HCC in CKD patients, because the prognosis was not different between patients with and without CKD. For example, Huo et al[18] compared the survival of 172 HCC patients with and without CKD who underwent percutaneous injection therapy. After a mean follow-up period of 24 ± 9 mo, there was no significant survival difference in patients with and without CKD [18]. Kondo et al[19] also reported that radiofrequency ablation was a safe and effective option for small HCCs in ESRD patients undergoing chronic hemodialysis. Orii et al[17] compared the outcomes of 17 patients with CKD who had undergone hepatectomy for HCC with 51 non-CKD patients subjected to hepatectomy for HCC. The operative and pathologic findings were comparable between the two groups. Post-operative circulatory insufficiency occurred more frequently in the CKD group (P = 0.013). Although the disease-free survival rates were comparable between the two groups, the overall survival rates were significantly lower in the CKD group than in the non-CKD group (P = 0.031). Orii et al[17] therefore concluded that hepatectomy for HCC should be considered even for CKD patients if careful peri-operative management and suitable multi-disciplinary treatment for recurrent disease are provided. In the study by Yeh et al[20], the outcomes of 26 ESRD-HCC patients who underwent hepatic resection were reviewed and compared to 1198 HCC patients without ESRD who underwent hepatic resection. There were more associated disease, more physical signs of anemia and post-operative complications, lower hemoglobin, platelet, and alpha-fetoprotein levels, elevated blood urea nitrogen and creatinine levels, smaller tumors, lower HBsAg positivity, higher HCV positivity, and longer hospital stays in the ESRD-HCC group compared to the HCC group. Nonetheless, the overall and disease-free survival rates were similar between the two groups[20]. Cheng et al[21] also demonstrated that the operative morbidity and mortality between ESRD and non-ESRD groups were similar. The five-year disease-free survival rates for ESRD and non-ESRD groups were 35.0% and 34.2%, respectively (P = 0.31), while the five-year actual survival rates were 67.8% and 53.3%, respectively (P = 0.54). The study suggested that liver resection for HCC was justified in selected patients with ESRD [21]. In the study by Sawada et al[22], 91 patients who underwent hepatectomy were retrospectively divided into two groups based on their creatinine clearance (Ccr) values: a group with Ccr values ≥ 50 to < 100 mL/min (n = 77) and a group with Ccr values of ≥ 20 but < 50 mL/min (n = 14). There were no statistically significant differences between the two groups in terms of intra-operative blood loss or intra-operative urine volume. The difference between the two groups in post-operative complications was not statistically significant. Thus, the team concluded that adequate indications, appropriate operative procedures, and peri-operative management might allow hepatectomy to be performed safely in patients with non-uremic minimal renal failure[22].
In conclusion, our results showed that HCC patients with stages 4 and 5 CKD had inferior survival than stages 1 and 2 CKD, which might be explained by poorer liver reserve. Nevertheless, the retrospective nature of the study, the small patient population, and the short follow-up duration are limitations that warrant further investigations to validate the conclusion drawn here.
There is a paucity of data regarding outcomes of hepatocellular carcinomas (HCCs) in patients with chronic kidney disease (CKD), even though both hepatitis B virus and CKD are endemic in Taiwan.
Previous reports found a strong evidence of increased cancer risk in patients with CKD, and the CKD was associated with malignancy and worse prognosis.
The authors analyzed the database of Chang Gung Memorial Hospital to examine the epidemiology of HCC in CKD populations in Taiwan. It was found that HCC patients with stages 4 and 5 CKD had inferior survival than stages 1 and 2 CKD. On the other hand, the TNM classification (or tumor stage) did not accurately predict survival in HCC patients with CKD.
The data is important to understand the outcome of HCC in CKD population in Taiwan, an area with highest prevalence and second highest incidence of end-stage renal disease in the world.
HCC is the most common type of liver cancer. Most cases of HCC are secondary to either a viral hepatitis infection or cirrhosis. CKD is the slow loss of kidney function over time. There are five stages of CKD, but kidney function is normal in stage 1, and minimally reduced in stage 2, moderately reduced in stage 3, severely reduced in stage 4, and very severely reduced (or called end-stage renal disease) in stage 5.
The epidemiology of HCC in CKD population has not been extensively investigated. Therefore, this data is particularly important.
P- Reviewers Rampoldi L, Iwasaki Y, Woo KT S- Editor Song XX L- Editor A E- Editor Xiong L
| 1. | Yen TH, Lin JL, Lin-Tan DT, Hsu CW. Association between body mass and mortality in maintenance hemodialysis patients. Ther Apher Dial. 2010;14:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Yen TH, Lin JL, Lin-Tan DT, Hsu CW, Chen KH, Hsu HH. Blood cadmium level’s association with 18-month mortality in diabetic patients with maintenance haemodialysis. Nephrol Dial Transplant. 2011;26:998-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012. Available from: http://www.usrds.org/adr.aspx. |
| 4. | Yen TH, Huang CC, Lin HH, Huang JY, Tian YC, Yang CW, Wu MS, Fang JT, Yu CC, Chiang YJ. Does hepatitis C virus affect the reactivation of hepatitis B virus following renal transplantation? Nephrol Dial Transplant. 2006;21:1046-1052. [PubMed] |
| 5. | Lee CH, Chang CJ, Lin YJ, Yeh CN, Chen MF, Hsieh SY. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer. 2009;100:1765-1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Wang SH, Chen LM, Yang WK, Lee JD. Increased extrinsic apoptotic pathway activity in patients with hepatocellular carcinoma following transarterial embolization. World J Gastroenterol. 2011;17:4675-4681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Johnson DW, Dent H, Yao Q, Tranaeus A, Huang CC, Han DS, Jha V, Wang T, Kawaguchi Y, Qian J. Frequencies of hepatitis B and C infections among haemodialysis and peritoneal dialysis patients in Asia-Pacific countries: analysis of registry data. Nephrol Dial Transplant. 2009;24:1598-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Wong G, Hayen A, Chapman JR, Webster AC, Wang JJ, Mitchell P, Craig JC. Association of CKD and cancer risk in older people. J Am Soc Nephrol. 2009;20:1341-1350. [PubMed] |
| 9. | Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney AP, Briggs D. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93-99. [PubMed] |
| 10. | Stewart JH, Buccianti G, Agodoa L, Gellert R, McCredie MR, Lowenfels AB, Disney AP, Wolfe RA, Boyle P, Maisonneuve P. Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol. 2003;14:197-207. [PubMed] |
| 11. | Wang TY, Hu CJ, Kuo CW, Chen Y, Lin JL, Yang CW, Yen TH. High incidence and recurrence of transitional cell carcinoma in Taiwanese patients with end-stage renal disease. Nephrology (Carlton). 2011;16:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Wang HB, Hsieh HH, Chen YT, Chiang CY, Cheng YT. The outcome of post-transplant transitional cell carcinoma in 10 renal transplant recipients. Clin Transplant. 2002;16:410-413. [PubMed] |
| 13. | Stengel B. Chronic kidney disease and cancer: a troubling connection. J Nephrol. 2010;23:253-262. [PubMed] |
| 14. | Russo P. End stage and chronic kidney disease: associations with renal cancer. Front Oncol. 2012;2:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Na SY, Sung JY, Chang JH, Kim S, Lee HH, Park YH, Chung W, Oh KH, Jung JY. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol. 2011;33:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Weng PH, Hung KY, Huang HL, Chen JH, Sung PK, Huang KC. Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol. 2011;6:1121-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Orii T, Takayama T, Haga I, Fukumori T, Amada N. Efficacy of a liver resection for hepatocellular carcinoma in patients with chronic renal failure. Surg Today. 2008;38:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Huo TI, Huang YH, Wu JC, Lee PC, Chang FY, Lee SD. Percutaneous injection therapy for hepatocellular carcinoma in patients with chronic renal insufficiency. Eur J Gastroenterol Hepatol. 2004;16:325-331. [PubMed] |
| 19. | Kondo Y, Yoshida H, Tomizawa Y, Tateishi R, Shiina S, Tagawa K, Omata M. Percutaneous radiofrequency ablation of hepatocellular carcinoma in 14 patients undergoing regular hemodialysis for end-stage renal disease. AJR Am J Roentgenol. 2009;193:964-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Yeh CN, Lee WC, Chen MF. Hepatic resection for hepatocellular carcinoma in end-stage renal disease patients: two decades of experience at Chang Gung Memorial Hospital. World J Gastroenterol. 2005;11:2067-2071. [PubMed] |
| 21. | Cheng SB, Wu CC, Shu KH, Ho WL, Chen JT, Yeh DC, Liu TJ, P’eng FK. Liver resection for hepatocellular carcinoma in patients with end-stage renal failure. J Surg Oncol. 2001;78:241-246; discussion 241-246. [PubMed] |
| 22. | Sawada T, Kita J, Rokkaku K, Kato M, Shimoda M, Kubota K. Hepatectomy in patients with nonuremic minimal renal failure. J Gastrointest Surg. 2006;10:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Liu SH, Lin JL, Weng CH, Yang HY, Hsu CW, Chen KH, Huang WH, Yen TH. Heart rate-corrected QT interval helps predict mortality after intentional organophosphate poisoning. PLoS One. 2012;7:e36576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Yang CJ, Lin JL, Lin-Tan DT, Weng CH, Hsu CW, Lee SY, Lee SH, Chang CM, Lin WR, Yen TH. Spectrum of toxic hepatitis following intentional paraquat ingestion: analysis of 187 cases. Liver Int. 2012;32:1400-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Shiina S, Tateishi R, Imamura M, Teratani T, Koike Y, Sato S, Obi S, Kanai F, Kato N, Yoshida H. Percutaneous ethanol injection for hepatocellular carcinoma: 20-year outcome and prognostic factors. Liver Int. 2012;32:1434-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Grattagliano I, Ubaldi E, Bonfrate L, Portincasa P. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 27. | Mula-Abed WA, Al Rasadi K, Al-Riyami D. Estimated Glomerular Filtration Rate (eGFR): A Serum Creatinine-Based Test for the Detection of Chronic Kidney Disease and its Impact on Clinical Practice. Oman Med J. 2012;27:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6567] [Article Influence: 469.1] [Reference Citation Analysis (1)] |
| 29. | Lee YH, Hsu CY, Huang YH, Su CW, Lin HC, Lee RC, Chiou YY, Huo TI, Lee SD. Selecting a prognostic renal surrogate for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Gastroenterol Hepatol. 2012;27:1581-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Zevin D, Turani H, Cohen A, Levi J. Androgen-associated hepatoma in a hemodialysis patient. Nephron. 1981;29:274-276. [PubMed] |
| 31. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1488] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 32. | Wang CS, Chang TT, Yao WJ, Chou P. Comparison of hepatitis B virus and hepatitis C virus prevalence and risk factors in a community-based study. Am J Trop Med Hyg. 2002;66:389-393. [PubMed] |
| 33. | Ni YH, Chang MH, Huang LM, Chen HL, Hsu HY, Chiu TY, Tsai KS, Chen DS. Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann Intern Med. 2001;135:796-800. [PubMed] |
| 34. | Chen KS, Lo SK, Lee N, Leu ML, Huang CC, Fang KM. Superinfection with hepatitis C virus in hemodialysis patients with hepatitis B surface antigenemia: its prevalence and clinical significance in Taiwan. Nephron. 1996;73:158-164. [PubMed] |
| 35. | Lee JJ, Lin MY, Yang YH, Lu SN, Chen HC, Hwang SJ. Association of hepatitis C and B virus infection with CKD in an endemic area in Taiwan: a cross-sectional study. Am J Kidney Dis. 2010;56:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Asrani SK, Buchanan P, Pinsky B, Rey LR, Schnitzler M, Kanwal F. Lack of association between hepatitis C infection and chronic kidney disease. Clin Gastroenterol Hepatol. 2010;8:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |