Published online Mar 28, 2013. doi: 10.3748/wjg.v19.i12.1953
Revised: December 20, 2012
Accepted: January 11, 2013
Published online: March 28, 2013
Processing time: 153 Days and 19.9 Hours
AIM: To investigate the indications, resection rate, and safety of endoscopic submucosal dissection (ESD) for neoplastic lesions in the gastrointestinal tract at a European referral center.
METHODS: We carried out a retrospective analysis of the ESD procedures performed in our center for mucosal neoplastic and submucosal lesions of the gastrointestinal tract. The duration of the procedure, en bloc and complete (R0) resection rates, and complication rates were evaluated. Variables were reported as mean ± SD or simple proportions. Univariate analysis and comparisons of procedure times and resection rates were performed using Mann-Whitney U tests, or χ2 tests for dichotomous variables.
RESULTS: Between 2007 and 2011, ESD was performed in a total of 103 patients (46.7% male, mean age 64.0 ± 12.7 years). The indications for the procedure were epithelial tumor (n = 54), submucosal tumor (n = 42), or other (n = 7). The total en bloc resection rate was 90.3% (93/103) and R0 resection rate 80.6% (83/103). The median speed of the procedure was 15.0 min/cm2. The complete resection rate was lower for submucosal tumors arising from the muscle layer (68%, 15/22, P < 0.05). Resection speed was quicker for submucosal tumors localized in the submucosal layer than for lesions arising from the muscularis propria layer (8.1 min/cm2vs 17.9 min/cm2, P < 0.05). The R0 resection rate and speed were better in the last 24 mo (90.1%, 49/54 and 15.3 min/cm2) compared to the first 3 years of treatment (73.5%, 36/49, P < 0.05 and 22.0 min/cm2, P < 0.05). Complications occurred in 14.6% (n = 15) of patients, including perforation in 5.8% (n = 6), pneumoperitoneum in 3.9% (n = 4), delayed bleeding in 1.9% (n = 2), and other in 2.9% (n = 3). Only one patient with delayed perforation required surgical treatment. During the mean follow-up of 26 ± 15.3 mo, among patients with R0 resection, recurrence occurred in one patient (1.2%).
CONCLUSION: ESD is an effective and safe method for resection of neoplastic lesions with low recurrence. Speed and the R0 resection rate increased after 50 procedures.
- Citation: Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, Karpińska K, Marlicz W, Milkiewicz P, Starzyńska T. Endoscopic submucosal dissection for the treatment of neoplastic lesions in the gastrointestinal tract. World J Gastroenterol 2013; 19(12): 1953-1961
- URL: https://www.wjgnet.com/1007-9327/full/v19/i12/1953.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i12.1953
In Japan and South Korea, endoscopic submucosal dissection (ESD) is a commonly accepted method for the resection of early neoplastic lesions in the upper and lower gastrointestinal tract. Good results from and the safety of procedures performed in the last few years have resulted in an increase in the number of procedures. Publications of the results of treatment with this method include more than 1000 patients. Although the dynamic development of this method is visible in Far East countries, this method is still in development in Europe and has not gained in popularity. A few publications describe the results of treatment in more than 50 patients, and some small series of patients or case reports have been published, but there is an overall lack of new European data.
ESD involves the removal of both benign neoplastic (premalignant) and malignant non-invasive lesions, aiming for the highest R0 resection rate (83%-98%) and lowest rate of local recurrence (0%-3%) of all endoscopic techniques[1]. The removal of submucosal lesions is also possible, including those growing out of the proper muscle layer[2].
The papers from Japan and Asia focus on confirming that ESD is a safe method with few complications and a mortality rate of 0%. The time needed to perform the procedure significantly decreases with the number of procedures performed, but it is still longer than the time needed for mucosectomy.
The present paper is one of the few European reports showing the results of treating gastrointestinal malignancies with ESD in a referral center. We present the indications, results, and complications with regard to different parts of the gastrointestinal tract.
The procedures were performed between April 2007 and December 2011. Before qualifying for the procedure, patients received both oral and written explanations of the endoscopic examination and possible treatment options, and they signed an informed written consent form.
For each patient with pathology of the upper gastrointestinal tract, endoscopic ultrasound (EUS) was performed before admission for ESD. The sector-scanning echoendoscope (Olympus GF-UM130 or GF-UE160 Olympus Medical Systems Co., Tokyo, Japan) or linear echoendoscope (Olympus GF-UCT140) were used to examine lesion size, EUS layer from which it derives (submucosal tumors, SMTs) or infiltrated layers (other lesions), SMT growth type (inside or outside the walls of the gastrointestinal tract), and lymph node diameter.
Neoplastic mucosal tumors were included in the study if > 10 mm with a low risk of lymph node metastases. Lesions in the stomach were included based on the expanded criteria from the Japanese Gastric Cancer Association (JGCA)[3]: well-differentiated carcinoma without ulceration, irrespective of size; well-differentiated carcinoma with ulceration (type III) ≤ 30 mm; or well-differentiated carcinoma with submucosal invasion and no more than 500 μm in size.
Exclusion criteria were lack of consent from the patient for the endoscopic procedure, massive infiltration of the submucosal layer or infiltration of muscle layers assessed in EUS, or enlarged local lymph nodes or metastases found in imaging studies (i.e., EUS, ultrasound, and computed tomography).
Patients were qualified for ESD if any of the following were in the previous endoscopic examination: large sessile polyp (LST) (type Is) that could not be removed in one piece, granular type (LST-G) flat polyps with a dominant nodule greater than 2 cm, non-granular type (LST-NG) flat polyps of any size, or scars from previous non-therapeutic tumor resections. Exclusion criteria were a lack of consent from the patient for the endoscopic procedure or deep ulceration apparent in the lesion, suggesting massive submucosal invasion.
Resection of SMT by ESD was performed if the tumor was 1 to 8 cm in size with no growth outside the gastrointestinal tract on EUS. Patients who did not meet these criteria were referred to follow-up (submucosal lesions < 1 cm), mucosectomy (smaller or pedunculated lesions), or surgical treatment (submucosal lesions > 8 cm, epithelial tumors). All cases but one lipoma (bleeding gastric tumor) were excluded from endoscopic treatment.
Before the treatment of epithelial malignancies by ESD, indigo carmine chromoendoscopy, magnifying endoscopy, NBI, or a combination of these techniques was performed to qualify the patients for the procedure based on the classification of Paris[4] and properly assess the lateral margins of the lesion. ESD in the upper gastrointestinal tract was performed under general anesthesia, whereas analgosedation with midazolam (2.5-5 mg iv) and fentanyl (50-100 μg iv) was used for procedures in the lower gastrointestinal tract. An Olympus endoscope GIF-T140 with a transparent cap (Olympus D-201-12 704) was used, allowing a better view of the submucosal layer during the procedure. For submucosal injection, 0.9% NaCl solution with epinephrine (1 mg/250 mL NaCl) and indigo carmine dye was used. In some cases in which the elevation of the mucosa after the injection of the solution was too short, a solution of hyaluronic acid was used (Sigmavisc, Hyaltech Ltd, Livingston, United Kingdom).
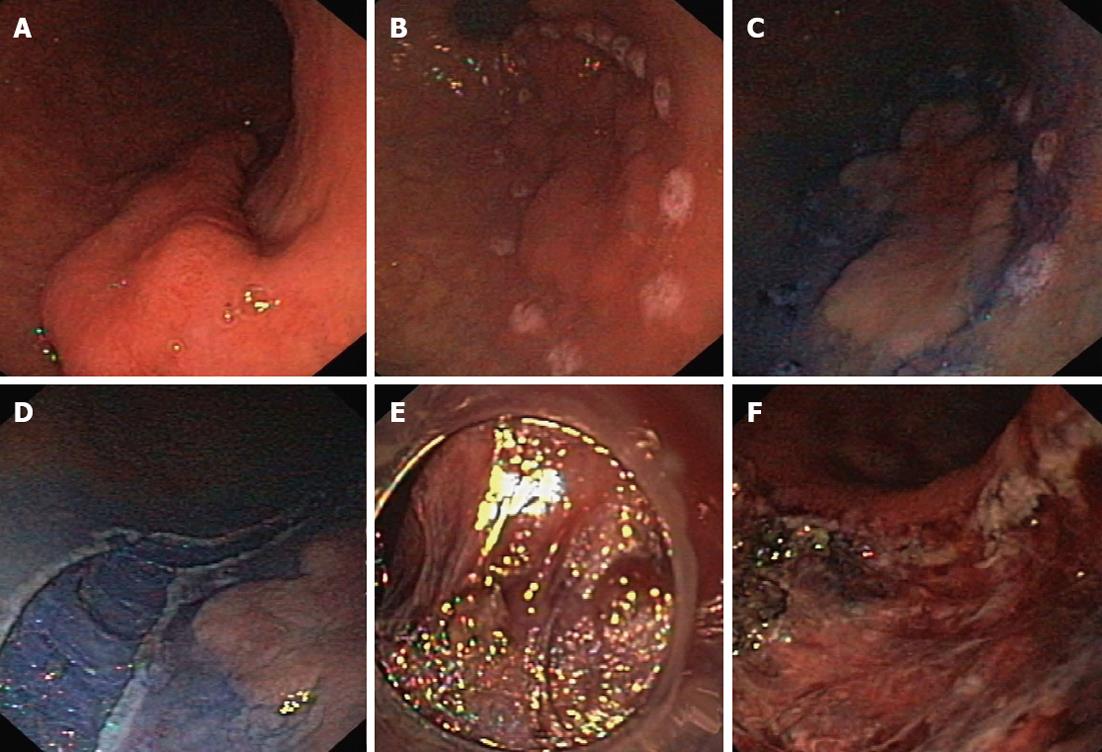
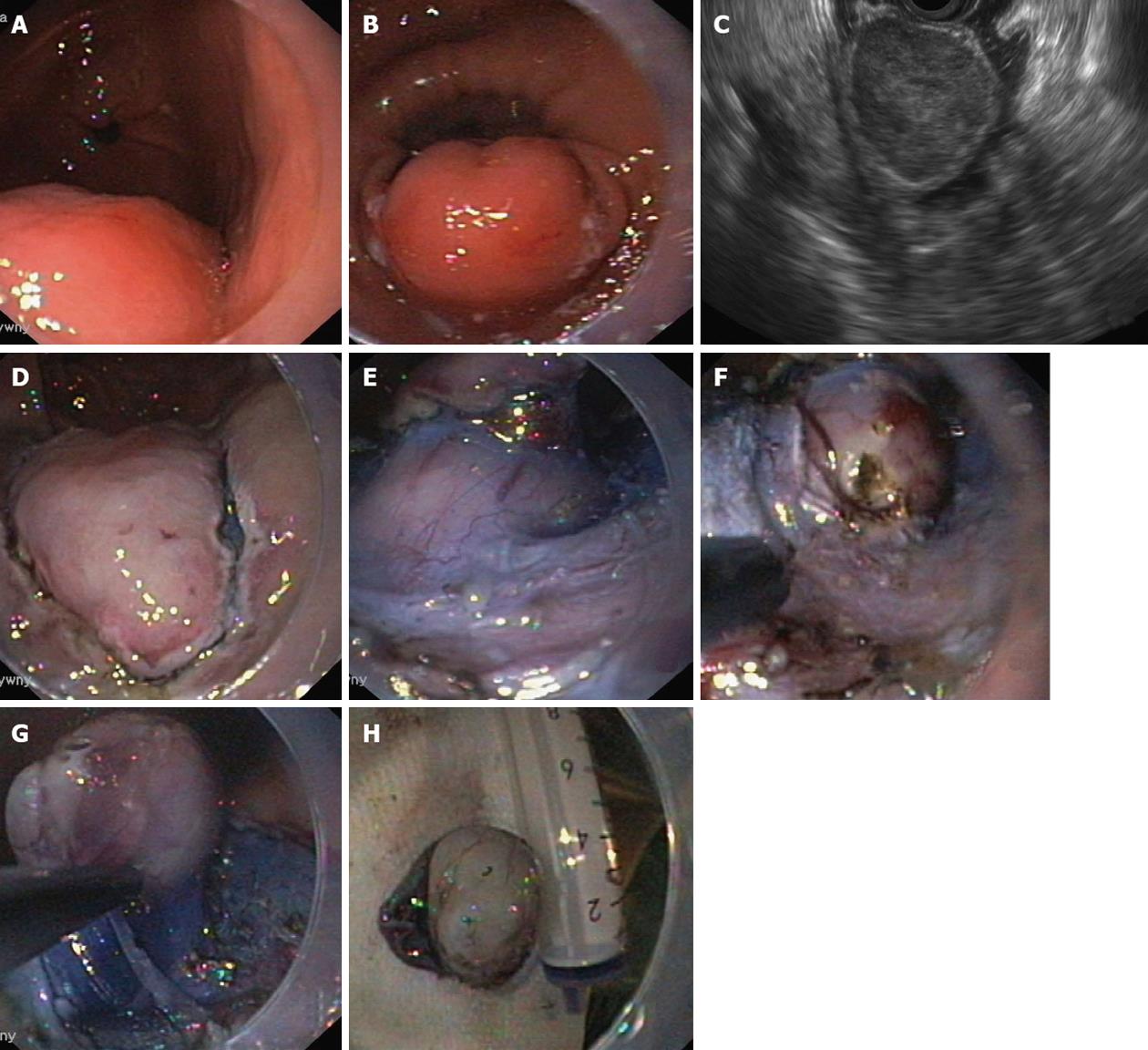
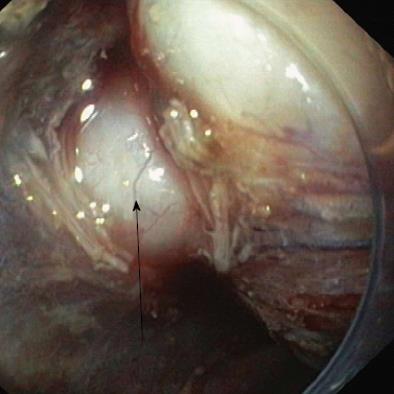
The resection was started by marking the lesion borders with the needle knife (Olympus KD-441Q) or dual knife (Olympus KD-650L) using forced coagulation of 35 W (Erbe ICC 200, Erbe Elektromedizin GmbH, Tübingen, Germany). The solution was injected submucosally next to the markers. The initial incision, approximately 3-5 mm in length, was made with a needle knife, and then a circular incision was made around the lesion using the isolated-tip knife IT or IT-2 (Olympus KD-611L, EndoCut mode, effect 3, 100 W) or a dual knife (EndoCut mode, effect 3, 35-50 W). The next step was the injection of the same solution directly into the submucosal layer under the lesion. The blue color of the indigo carmine allowed this layer to be distinguished from the proper muscle and the lesion itself. Dissection of the submucosal layer and the tumor was performed using the IT knife, hook-knife (Olympus KD-620LR, EndoCut mode, effect 2-3, 50-90 W), flex-knife (Olympus KD-630L), or dual knife (Figures 1 and 2). Muscular SMTs attached to the muscle layer by muscle fibers or muscle pedicle were cut away from the muscle layer of the wall (Figure 3). Tumors that were fused tightly with muscle and over a larger area were cut away with a loop, or most of the lesion was cut away to obtain enough material for pathological examination. Scar tissue was cut using a needle knife along the muscle layer. Lesions located in the cardia and just behind the anal canal were removed mostly in retroflexion. During the procedure and immediately after the removal of a tumor, all visible blood vessels in the submucosal and muscle layer were coagulated using a coagrasper (Olympus FD-410LR, soft coagulation 35 W) or argon coagulation (Erbe APC 300, 35 W, flow 1.6 L/min), or hemoclips (Olympus HX-610-135) applied. The removed lesion was affixed to a polystyrene substrate, fixed in formalin, and examined morphologically.
Bleeding during the procedure that did not cause hemodynamic disorders or anemia was not considered a complication. All bleeding was treated endoscopically with coagrasper hemostatic forceps, argon beamer coagulation, or hemoclip application. Perforations noticed during the procedure were treated endoscopically by closing the wall defect using hemoclips. After the procedure, antibiotics were administered intravenously (amoxicillin 2 × 1000 mg) for 5 d, as well as proton pump inhibitors if the lesion was resected in the upper gastrointestinal tract (omeprazol 8 mg/h iv for 2 d, then 2 × 20 mg orally for 8 wk). After ESD in the lower gastrointestinal tract, patients received ciprofloxacin (2 × 200 mg iv) and metronidazol (3 × 500 mg iv). On the day of the procedure (day zero) the patients fasted. The first day after the procedure the patients received neutral fluids orally, and normal diet the next day. Colon patients received normal diet from the first day after the procedure. The patients were discharged from the hospital on day 3-5 after the procedure.
The resected specimens were pinned to a mounting board with clearly marked oral and anal orientations and routine formalin fixation performed. The borders of each specimen were colored with ink and sections taken every 2 mm. The pathological reports of the resected specimens included the macroscopic appearance, size, histological type (the most important Lauren classification of gastric cancer), and extent of the tumor depth. The presence of ulceration and lymphovascular involvement, as well as the status of the vertical and lateral resection margins, were reported in detail.
In addition, the SMT preparations were labeled with DAKO antibodies (Dako Polska Sp.z o.o.). Gastrointestinal stromal tumors (GISTs) were characterized by a positive reaction to c-KIT (CD 117) or DOG-1 and CD34 antibodies. Leiomyomas were diagnosed when the mesenchymal tumors had a positive reaction for smooth muscle actin and desmin. A positive reaction for S-100 protein and negative reaction for muscle markers and CD117 indicated nerve tumors. The neoplastic potential of the stromal tumors was determined on the basis of their size and mitotic index (MI, number of mitoses counted in 50 large fields) according to the classification of Miettinen et al[5].
The criteria for curative resection of cancer lesions were: the depth of neoplasm infiltration limited to the mucosal or superficial submucosal layer (up to 500 μm in the cardia and stomach and up to 1000 μm in the large intestine as measured from the lower border of the muscle layer of the mucosa), no infiltration or congestion of carcinoma cells in blood vessels and lymph vessels (angioinvasion), lateral and bottom margins free of neoplasm, and well or medium-differentiated cancer. For GIST, the only criterion was confirmed tissue-free margins.
All side effects of the dissection were recorded according to standard procedures[6,7]. The first follow-up visit was 2-3 wk after ESD. The first follow-up endoscopy was performed 3 or 12 mo after surgery using a standard endoscope or EUS. Patients with incompletely resected neoplastic changes were referred for surgery. A control endoscopy after 3 mo was performed in the case of piecemeal resection of the tumor or uncertain histopathological confirmation of the complete tumor resection (Rx). The following cases were qualified for endoscopy after 12 mo: removal of a tumor that fulfilled the criteria for R0, resection of non-neoplastic lesions, and incomplete SMT resection of non-stromal tumors.
Variables were reported as mean ± SD or simple proportions. Univariate analysis and comparisons of procedure times and resection rates were performed using Mann-Whitney U tests, or χ2 tests for dichotomous variables. Statistica 9.1 software was used for all data analyses (StatSoft, Inc. 2010; Statistica).
Over a period of 57 mo, ESD was performed in 103 patients (46 males, 44.66%). The mean patient age was 64.0 ± 12.7 years. A total of 69 procedures were performed in the upper gastrointestinal tract, 34 in the colon. The indications for resection were epithelial tumors (n = 54), SMT (n = 42), scars from previous non-therapeutic tumor resection (n = 2), and others (n = 5). All procedures were performed by two physicians (Białek A, Pertkiewicz J).
The total en bloc and R0 resection rates were 90.3% (93/103) and 80.6% (83/103), respectively. The rate of en bloc and R0 resection for epithelial lesions reached 85.3% (52/61), for overall SMT the rates were 97.6% (41/42) and 73.8% (31/42), respectively, but did not differ significantly. The complete resection rate for SMTs arising from the muscle layer was 68% (15/22), which is significantly lower than that of epithelial lesions (P < 0.05).
The mean speed of performing the procedure was 19.0 ± 14.6 min/cm2. The overall resection speed for SMTs was 15.85 ± 10.99 and 21.17 ± 16.41 min/cm2 for mucosal lesions, but this difference was not significant. The resection speed was faster for SMTs localized in the submucosal layer compared to lesions arising from the muscularis propria layer (8.1 min/cm2vs 17.9 min/cm2, P < 0.05).
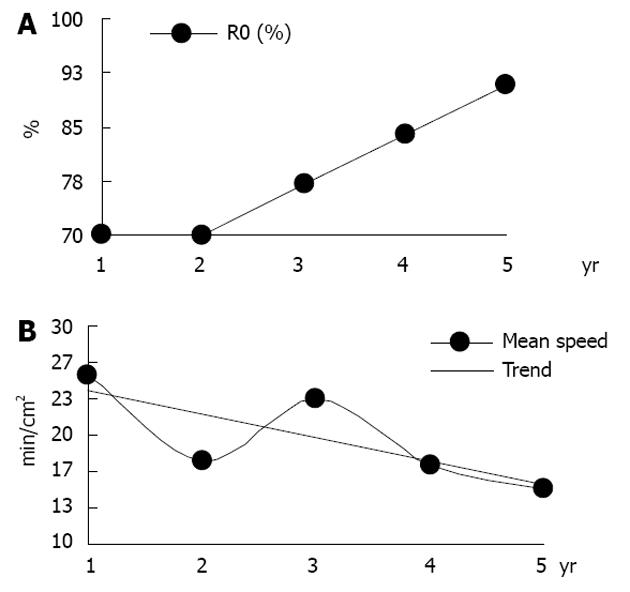
During the first 3 years following ESD, 49 procedures were performed. Thirty-six of the procedures (73.5%) were R0 resections, and the procedure speed was 22.02 ± 15.33 min/cm2. In the last 24 mo, the R0 resection rate increased to 90.1% (49/54, P < 0.05, Table 1; Figure 4) with a mean speed of 15.3 ± 13.25 min/cm2 (P < 0.05, Table 1; Figure 4). The complication rates did not differ significantly. During a mean follow-up of 26 ± 15.3 mo, recurrence was detected in only one patient who underwent R0 resection (1.2%, 1/83), a 54-year-old female with intestinal type gastric cancer (G1, M2), and recurrence occurred within 6 mo of the procedure. The patient was sent for surgery and middle grade dysplasia diagnosed in the resected specimen.
| Performing ESD (yr) | Procedures (n) | Speed (min/cm2) (mean ± SD) | R0 |
| 1 | 10 | 25.5 ± 25.54 | 70.0% |
| 2 | 17 | 17.6 ± 25.54 | 70.0% |
| 3 | 22 | 23.4 ± 11.87 | 77.0% |
| 4 | 32 | 17.4 ± 15.33 | 84.0% |
| 5 | 22 | 15.1 ± 12.94 | 90.9% |
ESD procedures were performed in the lower esophagus and cardia in 14 patients. The indications for the procedure were SMTs suspected to be GISTs in 5 patients and mucosal tumors in 9 patients.
Morphologically, tumors were type I-1, IIa-2, IIa+c-3, IIb+c-2 and IIb-1 according to the Paris classification. The mean tumor size was 2.37 ± 0.95 cm for all lesions, 2.36 ± 1.0 cm for mucosal lesions, and 2.4 ± 0.96 cm for SMTs. The en bloc and R0 resection rates as well as the histological diagnoses of resected tumors are shown in Table 2.
| Diagnosis | Patients | En bloc | R0 | |
| Esophagus | SMT | 5 | 5 (100.0) | 3 (60.0) |
| GIST | 1 | 1 (100.0) | 1 (100.0) | |
| Leiomyoma | 4 | 4 (100.0) | 2 (50.0) | |
| Non-SMT | 9 | 9 (100.0) | 6 (66.7) | |
| Early cancer/HGD | 6 | 6 (100.0) | 3 (50.0) | |
| Neoplasia grade min/med | 3 | 3 (100.0) | 3 (100.0) | |
| Stomach | SMT | 35 | 34 (97.1)a | 27 (77.1) |
| GIST | 18 | 18 (100.0) | 16 (88.9) | |
| Leiomyoma | 6 | 5 (83.3) | 3 (50.0) | |
| Other | 11 | 11 (100.0) | 8 (72.7) | |
| Non-SMT | 19 | 14 (73.7)a | 16 (84.2) | |
| Early cancer/HGD | 12 | 8 (66.7) | 10 (83.3) | |
| Neoplasia grade min/med | 4 | 3 (75.0) | 3 (75.0) | |
| Other | 3 | 3 (100.0) | 3 (100.0) | |
| Colon | SMT | 2 | 2 (100.0) | 2 (100.0) |
| Fibroepithelioma | 1 | 1 (100.0) | 1 (100.0) | |
| Leiomyoma | 1 | 1 (100.0) | 1 (100.0) | |
| Non-SMT | 32 | 28 (87.5) | 29 (90.6) | |
| Early cancer/HGD | 19 | 17 (89.5) | 17 (89.5) | |
| Neoplasia grade min/med | 11 | 10 (90.9) | 10 (90.9) | |
| Other | 2 | 1 (50.0) | 2 (100.0) |
The mean procedure duration was 99 ± 77.2 min and significantly shorter for the resection of SMTs (38 ± 13.03 min) than for mucosal lesions (128 ± 80.06 min, P < 0.05). Overall, the speed of resection was 24.96 ± 22.71 min/cm2 and significantly slower for the resection of mucosal lesions (33.29 ± 24.4 min/cm2) than for SMTs (9.96 ± 6.9 min/cm2, P < 0.05). The overall rate of en bloc and R0 resection was 100% (14/14) and 64.3% (9/14), respectively, and it did not differ significantly between mucosal lesions and SMTs.
Both of the incompletely removed SMTs were leiomyomas. The three neoplastic lesions were adenocarcinomas; cancerous infiltration was present in the lower margin (SM3) in two cases and in the lateral margin in one case. One of the patients was treated surgically; the adenocarcinoma was found in neither the cardia nor the lymph nodes of the surgical specimen. The other two patients were not qualified for surgery because of a lack of consent and high risk of surgery due to serious co-morbidities.
ESD was performed in the stomach in 54 patients, among which 35 were suspected to have GIST (mean size 2.9 ± 1.2 cm) and 19 mucosal neoplastic lesions (mean size 2.6 ± 1.1 cm). According to the Paris classification, the mucosal lesions were type Is-2, IIa+c-11, IIa-4, IIb-1 and IIb+c-1. Histological diagnoses and the rates of en bloc and R0 resection are shown in Table 2. Four lesions in the stomach qualified according to JGCA classic criteria[7] were removed completely (R0 resection rate 100%). Out of 12 lesions, only 9 (75.0%) reached R0 resection according to expanded criteria.
In two cases of incomplete resection of SMT, GIST was diagnosed. In one of these patients, the tumor was 4 cm, MI = 6 (risk of progression: moderate), and the patient referred for surgery; in the other, the tumor was 1.9 cm, MI = 3 (risk of progression: low), and the patient referred for follow-up examination. In two cases of incomplete resection of adenocarcinoma, one had a lower margin positive for cancer (sm3) and the other a lateral margin positive for cancer (piecemeal resection). The first patient qualified for surgical resection, but the other did not give consent for surgical treatment; in 33 mo of follow-up no tumor recurrence was found.
The mean duration of the endoscopic procedure was 103.8 ± 77.3 min for all lesions, 108.1 ± 88.0 min for epithelial lesions, and 101.4 ± 72.1 min for SMTs. The mean speed of dissection was 18.05 ± 12.1 min/cm2 for all lesions, 20.1 ± 13.4 min/cm2 for epithelial lesions, and 16.9 ± 11.4 min/cm2 for SMTs. The speed of the resection of SMTs connected to the muscle layer (n = 19) was 19.7 ± 8.5 min/cm2 and 14.2 ± 15.2 min/cm2 for tumors without such a connection (n = 12).
In the large intestine, ESD was performed in 34 patients. The main indications were laterally spreading tumor (LST) type IIa-11 or IIa+c-9 and polyps type Is-10. In two cases, ESD was performed for the radicalization of previously incomplete polypectomy procedures and in two cases due to symptomatic SMTs of the rectum. Low or middle grade dysplasia was diagnosed in 11 cases, and high-grade dysplasia or adenocarcinoma in 21 cases (Table 2). Two SMTs were diagnosed as fibroepithelioma and leiomyoma. The majority of lesions were located in the rectum (n = 28), followed by the sigmoid colon (n = 5) and ascending colon (n = 1). The rate of en bloc and R0 resection was 87.5% (28/32) and 90.6% (29/32), respectively. Among the lesions with incomplete resection, two adenocarcinomas with infiltration of the lower cut border (sm3) and tubular adenoma with low-grade dysplasia were diagnosed. In both cases of adenocarcinoma, the patients underwent surgical treatment, but cancerous tissue was not found in the surgical specimen. The patient with non-radically resected adenoma was administered follow-up examinations (20 mo) with no recurrence.
The average overall time and speed of treatment was 82.0 ± 56.6 min and 17.9 ± 14.2 min/cm2, respectively, 85.1 ± 56.9 min and 18.2 ± 14.5 min/cm2, respectively, for epithelial neoplastic lesions, and 32.5 ± 3.5 min/cm2 and 11.9 ± 9.3 min/cm2, respectively, for SMTs.
Severe complications occurred in two patients in the form of perforations, resulting in prolonged hospitalization for more than 10 d, including surgical treatment in one case. Mild or moderate complications occurred in 13 patients: 2 patients with delayed bleeding treated by transfusion, 1 patient with a mucosal pharyngeal sphincter tear who required prolonged hospitalization for less than 3 d, 4 with pneumoperitoneum who required prolonged hospitalization for less than 3 d, 4 patients with perforation treated conservatively and requiring prolonged hospitalization for 4-10 d, and 2 patients with pyloric stenosis requiring additional endoscopy. The complication rate according to localization is presented in Table 3.
| Patients | Delayed bleeding | Perforation | Other (n) | ||
| Cardia | 14 | 0 | 1 | Pneumoperitoneum | 2 |
| Stomach | 54 | 2 | 5 | ||
| Upper part | 21 | 1 | 4 | Mucosal tear of lower pharyngal sphincter region, pneumoperitoneum | 3 |
| Middle part | 7 | 0 | 1 | ||
| Lower part | 26 | 1 | 0 | Stenosis of the pylorus | 2 |
| Colon | 34 | 0 | 0 | 0 | |
| Duodenum | 1 | 0 | 0 | 0 | |
| All | 103 | 2 | 6 | 7 | |
In six patients with perforation, endoscopic closure of the defect was performed using hemoclips, decompression of the peritoneum by puncture, and conservative treatment with fasting, antibiotic therapy, and active suction with a nasogastric tube. In one of the patients, delayed perforation occurred on the fourth day when trying to implement oral feeding.
Bleeding in two patients was controlled by hemoclip application, and patients required the transfusion of two units of blood. In two patients, stenosis occurred within 4 wk after dissection of neoplastic lesions in the pylorus. Both patients were successfully treated with 20-mm balloon dilatation in one and two sessions. In one patient a mucosal tear in the throat sphincter occurred when removing a large, > 3 cm resected SMT. The patient had no symptoms and did not require additional treatment.
ESD is a technique aimed at resecting early neoplastic lesions in the gastrointestinal tract without compromising the integrity of the wall. The technique allows R0 resection to be achieved, even for large mucosal and submucosal lesions, by removing them in one piece (en bloc), which allows proper pathological evaluation of specimens. The method is the most effective of the endoscopic resection techniques and used by many centers in Japan and the Far East. Many papers, including the multicenter studies and those presenting the results of treatment in over 1000 patients, confirm the technique’s efficacy in both the upper and lower parts of the gastrointestinal tract[8-12]. The percentage of en bloc resection is estimated to range from 92%-100% in the upper gastrointestinal tract and 81.6%-92.7% in the lower gastrointestinal tract, and the rate of R0 resection is estimated to be 73.6%-94.7% in the upper gastrointestinal tract and 69.7%-89% in the lower gastrointestinal tract, which is significantly higher than that of mucosectomy techniques, in which R0 resection is estimated to be 33%-56%[1,9,13], especially for the resection of large tumors (> 2 cm in size). ESD also allows resection of SMTs, even those growing from the muscularis propria[2,10-18], and allows surgeons to save the organ via a minimally invasive resection of the lesion itself.
Despite such good results of treatment, the method is still rarely used in Europe[2,19-22]. To the best of our knowledge, only two European papers from Augsburg, Germany, present the results of treating more than 100 patients in one center[19,20]. One of the reasons for this low usage could be the time it takes to perform the procedure, especially at the beginning of the learning curve. In the Japanese centers, which have published the results of treating more than 1000 patients, the average time for ESD in the stomach is approximately 37 min[19]. In the present study, both time and speed were worse than in the Japanese studies due to the relatively low volume at the center. The number of ESD procedures necessary to master the method is thought to be approximately 50. In the present study, the speed of performing the procedure increased approximately 30% after the first 49 procedures over 3 years. Similarly, Probst et al[20] and Japanese authors noted a significant increase in the speed of the resection after 40-50 procedures[13,19].
The location of the lesions in the upper part of the stomach, their size, and the presence of submucosal fibrosis are associated with longer procedure duration, a lower resection rate, and a higher rate of complications[23-25]. In the present study, the percentage of resection related to lesion localization and timing in the stomach did not differ significantly, probably due to a heterogeneous patient group and a small number of procedures in different locations.
The other factor responsible for the small number of ESD procedures in Europe may be the greater number of complications compared to mucosectomy. The most serious complication of ESD is perforation, which occurs in 1.2%-9.7% of cases, and bleeding, which occurs in 0.1%-15.6% of procedures[1,8,13,20]. In the present study, the percentage of complications was similar, 4.9% for perforation and 1.9% for delayed bleeding, and surgical treatment was required in only one case. Importantly, the mortality rate after endoscopic treatment is 0% in both the present study and most published papers. Factors associated with a higher incidence of complications have been identified, including location in the upper and middle part of the stomach, lesion size, and the number of procedures performed when less than 50[11-12,25,26]. Also, in the present study, lesions located in the upper and middle third of the stomach were associated with a higher incidence of perforation, as five of six perforations occurred in these locations.
In the present study, the rate of en bloc resection and R0 resection was 90.3% and 80.6%, respectively, which is comparable with other European studies but lower than that of studies from Japan and South Korea. This difference is due to the lesser experience of authors in the first years of implementing the procedure. In the last two years, the rate of R0 resection increased from 73.5% to 90.1%, which approached the level of Japanese authors (Table 1; Figure 2). This finding confirms a long learning curve for this technique, which was nearly three years in our study, with 49 treatments completed. A similar tendency has been noticed by other authors, with the rate of total en bloc resection beginning at 50%-65.7% and increasing to 72.2%-100%[1,19]. An important factor in achieving R0 resection was a larger proportion of eligible patients fulfilling extended (n = 12), compared to classical (n = 4), indications for endoscopic resection of gastric cancer. The rate of R0 resection for classical indications in the present study was 100% (4/4), which was higher than the rate with the extended criteria (9/12, 75%). A similar tendency was observed by Probst et al[20], who reported 90% (9/10) and 68.6% (35/51), respectively, and Japanese authors, who reported 97.1% and 91.1%, respectively[19,27,28].
In the present study, the significant effect on the lower total resection rate comprised a relatively large proportion of patients with SMTs, including tumors arising from the muscularis propria layer, for which the R0 resection rate was significantly lower. Among the patients with confirmed complete R0 resection, only one recurrence occurred within 6 mo of the procedure (1.2%). In the publications from the last few years, the rate of recurrence after R0 resection was 0%-5.1%[29-31].
A limitation of this study is the relatively small number of procedures performed in the reference center and retrospective type of analysis.
In summary, ESD allows endoscopic resection of tumors with high efficacy and low complication rates, even in a low-volume center. Most complications, including perforation, are mild or moderate in severity and can be treated endoscopically or conservatively. Both speed and complete resection rate improved after approximately 50 procedures.
In Japan and South Korea, endoscopic submucosal dissection (ESD) is a commonly accepted method for the resection of early neoplastic lesions in the upper and lower gastrointestinal tract. Although the dynamic development of this method is visible in Far East countries, ESD is still in development in Europe and has not gained in popularity. A few publications have described the results of treatment in more than 50 patients, and some small series of patients or case reports have been published, but new European data is lacking overall. The present paper is one of the few European reports showing the results of treating gastrointestinal malignancies with ESD in a referral center. Authors present the indications, results, and complications with regard to different parts of the gastrointestinal tract.
ESD is a technique aimed at resecting early neoplastic lesions in the gastrointestinal tract without compromising the integrity of the wall. The technique allows R0 resection to be achieved, even for large mucosal and submucosal lesions, by removing them in one piece (en bloc), which allows for proper pathological evaluation of specimens. The method is the most effective endoscopic resection technique to date and is used by many centers in Japan and the Far East.
One of the reasons for the lack of usage of ESD could be the time required to perform the procedure, especially at the beginning of the learning curve. In the present study, the speed of performing the procedure and R0 resection rate increased approximately 30% after the first 49 procedures over 3 years. Other authors have also noted a significant increase in the speed and R0 resection rate after 40 to 50 procedures. Another factor potentially responsible for the small number of ESD procedures in Europe may be the greater number of complications associated with ESD compared to mucosectomy. The most serious complications of ESD are perforation, which occurs in 1.2%-9.7% of cases, and bleeding, which occurs in 0.1%-15.6% of cases. In this study, the percentage of complications was similar: 4.9% for perforation and 1.9% for delayed bleeding, irrespective of the center experience. Surgical treatment was required in only one case. The significant effect on the total resection rate included a relatively large proportion of patients with submucosal tumors, including tumors arising from the muscularis propria layer, for which the R0 resection rate was significantly lower. Among the patients with confirmed complete R0 resection, only one recurrence occurred within 6 mo of the procedure (1.2%). In publications from the last few years, the rate of recurrence after R0 resection has been 0%-5.1%.
ESD allows endoscopic resection of tumors with high efficacy and low complication rates, even in a low-volume center. Most complications, including perforations, are mild or moderate in severity and can be treated endoscopically or conservatively. Both speed and the complete resection rate improved after approximately 50 procedures.
R0 resection: Complete resection of the lesion confirmed by a pathologist by microscopic examination of the tumor-free borders of resected species.
The authors report European experience for ESD. Though the number of cases is small and some points need to be clear, this is valuable study as they mentioned that is European own experience.
P- Reviewer Kim JC S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Hulagu S, Senturk O, Aygun C, Kocaman O, Celebi A, Konduk T, Koc D, Sirin G, Korkmaz U, Duman AE. Endoscopic submucosal dissection for premalignant lesions and noninvasive early gastrointestinal cancers. World J Gastroenterol. 2011;17:1701-1709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpińska K, Ławniczak M, Starzyńska T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012;75:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Hermanek P. The second English edition of the Japanese Classification of Gastric Carcinoma. A Western commentary. Gastric Cancer. 1999;2:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1315] [Article Influence: 59.8] [Reference Citation Analysis (4)] |
| 5. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [PubMed] |
| 6. | Cotton PB. Outcomes of endoscopy procedures: struggling towards definitions. Gastrointest Endosc. 1994;40:514-518. [PubMed] |
| 7. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A, Petersen BT, Petrini JL. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1832] [Article Influence: 122.1] [Reference Citation Analysis (1)] |
| 8. | Sugimoto T, Okamoto M, Mitsuno Y, Kondo S, Ogura K, Ohmae T, Mizuno H, Yoshida S, Isomura Y, Yamaji Y. Endoscopic submucosal dissection is an effective and safe therapy for early gastric neoplasms: a multicenter feasible study. J Clin Gastroenterol. 2012;46:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 576] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 10. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 12. | Akasaka T, Nishida T, Tsutsui S, Michida T, Yamada T, Ogiyama H, Kitamura S, Ichiba M, Komori M, Nishiyama O. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by osaka university ESD study group. Dig Endosc. 2011;23:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] |
| 14. | Lee IL, Lin PY, Tung SY, Shen CH, Wei KL, Wu CS. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy. 2006;38:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Shi Q, Zhong YS, Yao LQ, Zhou PH, Xu MD, Wang P. Endoscopic submucosal dissection for treatment of esophageal submucosal tumors originating from the muscularis propria layer. Gastrointest Endosc. 2011;74:1194-1200. [PubMed] |
| 16. | Li QL, Yao LQ, Zhou PH, Xu MD, Chen SY, Zhong YS, Zhang YQ, Chen WF, Ma LL, Qin WZ. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc. 2012;75:1153-1158. [PubMed] |
| 17. | Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, Maselli R, Kudo S. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44:225-230. [PubMed] |
| 18. | Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy. 2012;44:231-235. [PubMed] |
| 19. | Hwang JC, Kim JH, Kim JH, Shin SJ, Cheong JY, Lee KM, Yoo BM, Lee KJ, Cho SW. Endoscopic resection for the treatment of gastric subepithelial tumors originated from the muscularis propria layer. Hepatogastroenterology. 2009;56:1281-1286. [PubMed] |
| 20. | Probst A, Pommer B, Golger D, Anthuber M, Arnholdt H, Messmann H. Endoscopic submucosal dissection in gastric neoplasia - experience from a European center. Endoscopy. 2010;42:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Probst A, Golger D, Arnholdt H, Messmann H. Endoscopic submucosal dissection of early cancers, flat adenomas, and submucosal tumors in the gastrointestinal tract. Clin Gastroenterol Hepatol. 2009;7:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa N, Lopes C, Moreira-Dias L. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim do H, Song HJ, Lee GH. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Ahn JY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim do H, Song HJ, Lee GH, Jung HY. Procedure time of endoscopic submucosal dissection according to the size and location of early gastric cancers: analysis of 916 dissections performed by 4 experts. Gastrointest Endosc. 2011;73:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 26. | Cho KB, Jeon WJ, Kim JJ. Worldwide experiences of endoscopic submucosal dissection: not just Eastern acrobatics. World J Gastroenterol. 2011;17:2611-2617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Farhat S, Chaussade S, Ponchon T, Coumaros D, Charachon A, Barrioz T, Koch S, Houcke P, Cellier C, Heresbach D. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy. 2011;43:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Kim ES, Cho KB, Park KS, Lee KI, Jang BK, Chung WJ, Hwang JS. Factors predictive of perforation during endoscopic submucosal dissection for the treatment of colorectal tumors. Endoscopy. 2011;43:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Yamaguchi N, Isomoto H, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Ohnita K, Hayashi T, Nakao K. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Lee H, Yun WK, Min BH, Lee JH, Rhee PL, Kim KM, Rhee JC, Kim JJ. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2011;25:1985-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Jang JS, Choi SR, Qureshi W, Kim MC, Kim SJ, Jeung JS, Han SY, Noh MH, Lee JH, Lee SW. Long-term outcomes of endoscopic submucosal dissection in gastric neoplastic lesions at a single institution in South Korea. Scand J Gastroenterol. 2009;44:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |