Published online Mar 28, 2013. doi: 10.3748/wjg.v19.i12.1901
Revised: September 19, 2012
Accepted: October 30, 2012
Published online: March 28, 2013
AIM: To investigate the efficacy of Magliasa, a traditional Iranian formula, on experimental colitis.
METHODS: After botanical authentication of herbal ingredients, formulation of Magliasa, quantitative determination of total glucosinolates and total phenolic content, and analysis of the thin layer chromatography profile were performed. Colitis was then induced in male rats by instillation of 2,4,6-trinitrobenzenesulfonic acid (TNBS) in all groups, aside from the Sham group. The experimental groups consisted of: the Sham group that received only normal saline; the Mag-50, Mag-100 and Mag-200 groups, which received 50, 100 and 200 mg/kg per day of Magliasa, respectively; the control group, which received vehicle water orally; the infliximab group, which received infliximab (5 mg/kg per day, subcutaneously); and the Dexa group, which received dexamethasone (1 mg/kg per day, orally). After completing the treatment period (2 wk), the rats were sacrificed, the colon was removed, its macroscopic and microscopic changes were recorded, and tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), total antioxidant capacity, myeloperoxidase (MPO), and lipid peroxidation (LPO) were assessed in colon homogenate.
RESULTS: The mean value of total glucosinolates in one gram of Magliasa was 19 ± 1 μmol. The mean value of the total phenolic content was 293.8 ± 17.6 mg gallic acid equivalents per 100 gram of Magliasa. Macroscopic scores were significantly decreased in Mag-100 (1.80 ± 0.58, P = 0.019) and Mag-200 (1.20 ± 0.20, P = 0.001) compared to the control group (3.40 ± 0.24), although some inflammation and hyperemia were evident. Treatment of rats by dexamethasone (0.33 ± 0.21, P < 0.001) and infliximab (0.83 ± 0.31, P < 0.001) remarkably attenuated scores where mild hyperemia was observed macroscopically. In comparison to the control group (4.00 ± 0.32), only Mag-200 (1.60 ± 0.40) showed a significant decrease in colonic histopathological scores (P = 0.005). Minimal mucosal inflammation was observed in the Dexa group (0.67 ± 0.21, P < 0.001). The levels of TNF-α, IL-1β and MPO were significantly lower in all groups compared to the controls (P < 0.05). A significant decrease in LPO was seen in the Mag-200 (3.27 ± 0.77, P = 0.01) and Dexa (3.44 ± 0.22, P = 0.011) groups in comparison to the control group (6.43 ± 0.61). Only dexamethasone caused a significant increase in antioxidant power in comparison to the control group (346.73 ± 9.9 vs 228.33 ± 2.75, P < 0.001). Infliximab and different doses of Magliasa did not show any remarkable increase in antioxidant capacity (P > 0.05). The effect of Magliasa in all of mentioned parameters, except antioxidant capacity, was dose dependent.
CONCLUSION: The effects of Magliasa in TNBS-induced colitis are encouraging and warrant clinical trials for further confirmation.
- Citation: Rahimi R, Baghaei A, Baeeri M, Amin G, Shams-Ardekani MR, Khanavi M, Abdollahi M. Promising effect of Magliasa, a traditional Iranian formula, on experimental colitis on the basis of biochemical and cellular findings. World J Gastroenterol 2013; 19(12): 1901-1911
- URL: https://www.wjgnet.com/1007-9327/full/v19/i12/1901.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i12.1901
Inflammatory bowel disease (IBD) includes two main types (Crohn’s disease and ulcerative colitis), and is categorized as one of the chronic disorders of the gastrointestinal tract with an unclear etiology. Related to the involvement of possible pathological factors such as immunological abnormalities[1], oxidative stress[2], gut microflora[3], abnormal epithelial barrier[4], and inflammatory factors[5-9], various drugs are used for the management of IBD, including anti-tumor necrosis factor-alpha (TNF-α) drugs[10-12], immunosuppressants[13,14], antibiotics[15,16], probiotics[17,18], corticosteroids[19], aminosalicylates[20,21], selective cyclooxygenase-2 inhibitors[9], nicotine preparations[22], potassium channel openers[23], adenosine triphosphate donors[24], and phosphodiesterase inhibitors[25-27]. It cannot be ignored that most of the conventional treatments for the management of IBD have serious adverse effects that reduce compliance in patients[28-30], and therefore has led researchers to work on complementary and alternative medicines that can induce remission in disease activity with better safety and tolerability[31-33]. As recently reviewed by Rahimi et al[32], there are many plants in traditional Iranian medicine (TIM) that were historically used for the management of IBD. Magliasa is a TIM herbal prescription that has been used to treat tenesmus and diarrhea mixed with blood and mucus for a long time[34]. It consists of 6 components: the seeds of Lepidium sativum, Linum usitatissimum, and Allium ampeloprasum cv. Porrum, the fruit of Bunium persicum and Terminalia chebula, and the gum resin of Pistacia lentiscus (Table 1). Different mechanisms have been described in TIM for the usefulness of these plants in the treatment of colitis, including anti-inflammatory, antiulcer, wound healing, and anti-diarrheal effects[35,36]. Regarding the aforementioned knowledge, the present study was planned to investigate the effect of Magliasa in an experimental model of colitis to determine the involved mechanisms.
| Scientific name | Iranian name | Part | Major constituents |
| Lepidium sativum | Taretizak | Seed | Glucosinolates, imidazole alkaloids, fatty acids, and sterols[60-63] |
| Bunium persicum | Zire kermani | Fruit | Flavonoids, essential oils, and tannins[69,70] |
| Linum usitatissimum | Katan | Seed | Mucilage, cyanogenic glycoside, lignans, fatty acids, and phenylpropan derivatives[74] |
| Allium ampeloprasum cv. Porrum | Tare | Seed | Saponins, flavonoids, carotenoids, and sulfur-containing compounds[78-80] |
| Terminalia chebula | Halile siah | Fruit | Tannins, anthraquinones, triterpene glycosides, and beta-Sitosterol[81,82] |
| Pistacia lentiscus | Mastaki | Gum resin | Triterpene acids and alcohols, and essential oils[81] |
Plant materials (seeds of Lepidium sativum, Linum usitatissimum, and Allium ampeloprasum cv. Porrum, fruit of Bunium persicum and Terminalia chebula, and gum resin of Pistacia lentiscus), were obtained from the local market at the Tehran bazaar in 2010. After confirmation by a botanist, voucher samples were deposited at the herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences (Tehran, Iran). 2,4,6-trinitrobenzenesulfonic acid (TNBS, Sigma-Aldrich), ethanol, methanol, ethyl acetate, n-hexane, thiobarbituric acid, trichloroacetic acid, n-butanol, hexadecyl-trimethyl-ammonium bromide, 2,4,6-tri(2-pyridyl)-S-triazine (TPTZ), hydrochloric acid, anisaldehyde, malondialdehyde, ethylenediaminetetraacetic acid, Folin-Ciocalteu reagent, toluene, dichloromethane, o-dianisidine hydrochloride, hydrogen peroxide, acetic acid, sodium acetate, Coomassie reagent, bovine serum albumin (BSA), FeCl3-6H2O, Na2SO4, H2SO4, H3PO4, KH2PO4, K2HPO4, H2O2, Na2CO3, NaHCO3, Na-K-tartrate, CuSO4-5H2O, Silica gel 60F254 (Merck, Germany), glucose kit (ZistChem, Iran), and rat-specific TNF-α and interleukin-1β (IL-1β) enzyme-linked immunosorbent assay (ELISA) kits (Bender Med Systems, Austria) were used in this study.
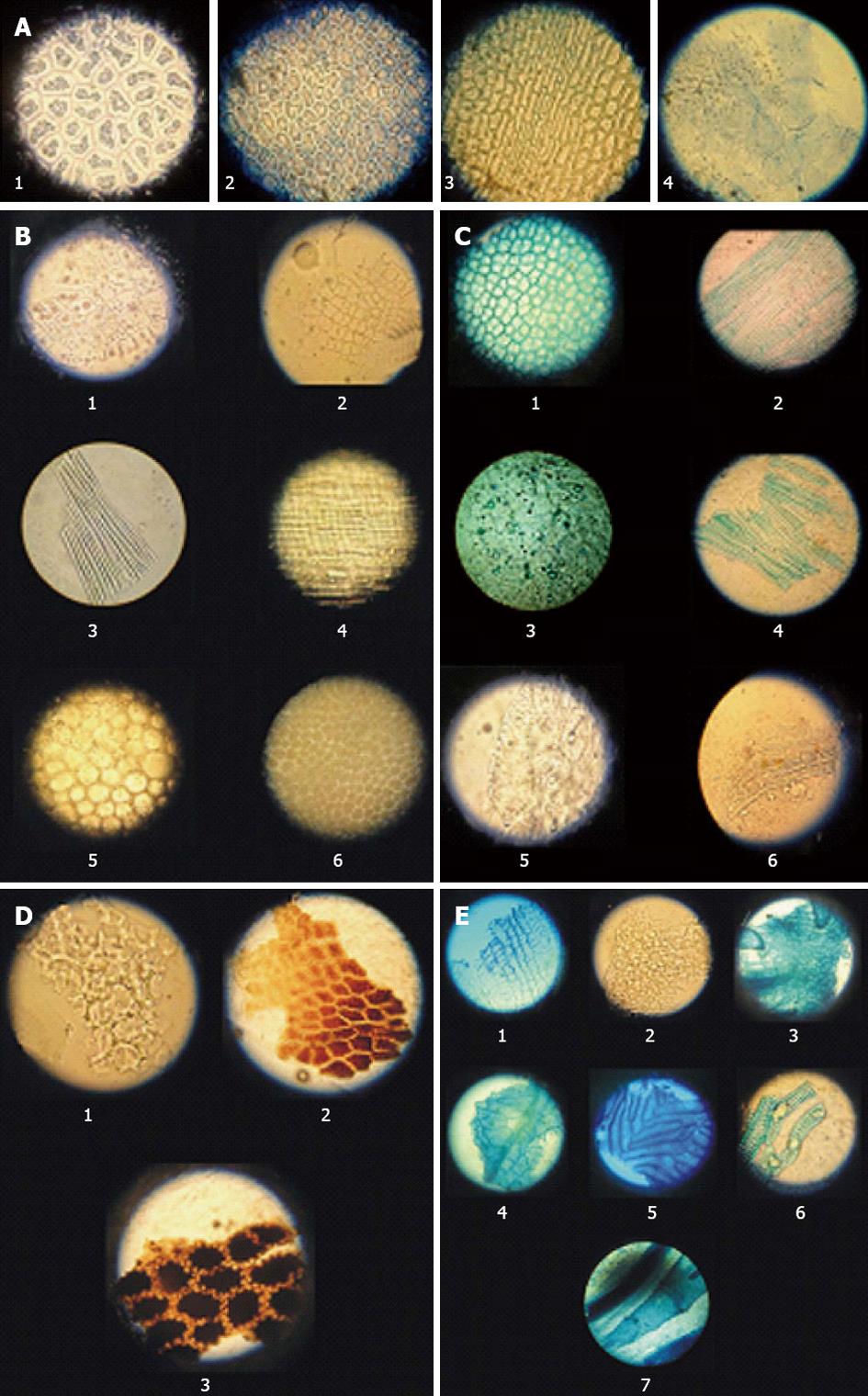
All 6 herbal ingredients were authenticated macroscopically and microscopically. Macroscopic examinations included measurements of appearance, size, shape, color, texture, odor, taste, fracture, and other characteristics according to pharmacopoeias[36,37]. Microscopic examinations determined the characteristic elements of each ingredient in powder form. For this purpose, each herbal material was mounted on a microscope slide after tissue disintegration with potassium hydroxide and cleared with sodium hypochlorite. The examination protocols followed the World Health Organization’s quality control methods for medicinal plant materials[38]. Characteristic elements were photographed via a Leitz optical microscope.
Bunium persicum fruit (22%), Linum usitatissimum seeds (8%), Allium ampeloprasum cv. Porrum seeds (8%), Terminalia chebula fruit (8%), and Pistacia lentiscus gum resin (4%) were individually powdered by milling, and then mixed. Intact non-milled seed of Lepidium sativum (50%, w/w) was added to the powdered material and again mixed.
The amount of total glucosinolates as major constituents of Lepidium sativum and the amount of total phenolic compounds as major constituents of Bunium persicum, Allium ampeloprasum cv. Porrum, and Terminalia chebula were measured in Magliasa.
Total glucosinolates were determined by the measurement of enzymatically-released glucose[39]. For this purpose, four accurately weighed 1 g samples of Magliasa were transferred into separate loaded ball-mill cups. To three cups 1 mL of water was added (samples), while the last cup had 1 mL of acidified 40% v/v methanol/water added instead (sample blank). All cups were milled side by side for 2 min, allowed to stand for 5 min, and then had 19 mL of acidified 40% v/v methanol added to each cup. After recapping and shaking vigorously, the cup contents were filtered through charcoal-coated papers. Immediately prior to colorimetric assay, each of the filtrates was diluted ten-fold with water, and then 0.2 mL was poured into separate 10 mL tubes. About 0.2 mL of water was added into a fifth tube (water blank), with 0.2 mL of standard glucose solution (1 mg/mL) (ZistChem, Iran) added into a sixth tube. Five mL of buffer/enzyme/chromogen reagent (ZistChem, Iran) was added to all tubes, mixed, and then placed in a water bath at 37 °C and read within 10-15 min. The absorbance of each solution against the water blank was measured at 610 nm.
The total phenolic contents in the medicinal plants were determined spectrophotometrically according to the Folin-Ciocalteu method[40]. Gallic acid was used to set up the standard curve. The phenolic compound content of the samples was expressed as gallic acid equivalents (GAE) in mg per 100 g of Magliasa. All the samples were analyzed in triplicate.
Thin layer chromatography (TLC) was performed to obtain preliminary data from essential oils and lipophilic substances. For this purpose, 1 g of Magliasa was extracted by shaking for 15 min in 10 mL of dichloromethane at room temperature. The suspension was filtered, and the clear filtrate evaporated to dryness. The residue was dissolved in 1 mL of toluene. Samples were then applied to the plates, which were developed at room temperature in glass chambers previously saturated for 1 h. The development distance was 5 cm. The mobile phase was n-hexane-ethyl acetate 5:4 (v/v). The spray reagent was anisaldehyde- sulfuric acid[41].
Male Wistar-albino rats, weighing between 220 and 230 g, were maintained under standard conditions of temperature (23 °C ± 1 °C), relative humidity (55% ± 10%), a 12-h dark and light period, and fed with a standard pellet diet and water ad libitum. All ethical themes of the animal studies were considered carefully, and the experimental protocol was approved by the ethical committee of Tehran University of Medical Sciences (code number of 88-04-33-10094).
Rats were randomly divided into seven groups containing six individuals in each group. Colitis was induced by the instillation of TNBS in all groups except group 1. The groups were: (1) Sham which received normal saline; (2) Mag-50 which received 50 mg/kg per day of Magliasa; (3) Mag-100 which received 10 mg/kg per day of Magliasa; (4) Mag-200 which received 200 mg/kg per day of Magliasa; (5) control which received vehicle water orally; (6) Infliximab which received infliximab (5 mg/kg per day, subcutaneously); and (7) Dexa which received dexamethasone (1 mg/kg per day, orally). Magliasa was dissolved in water and administered by gavage. The doses for Magliasa were selected after a pilot study. The effective doses of infliximab and dexamethasone were selected from our previous studies[42].
For induction of colitis, 36 h fasted rats were anesthetized with an intraperitoneal administration of 50 mg/kg of pentobarbital sodium, positioned on their right side, and then had 0.3 mL of a mixture containing six volumes of TNBS 5% w/v in H2O (equal to 15 mg TNBS) plus four volumes of ethanol (99%) instilled via the rectum using a rubber cannula (8 cm long)[43]. Following instillation of TNBS, rats were maintained in a supine Trendelenburg position in order to prevent anal leakage of TNBS. Medications were then administered to the animals for 14 d as described above. On the 15th day, the animals were sacrificed by an overdose of ether inhalation. The abdomen was rapidly dissected open and the colon was removed. The colon was cut open in an ice bath, cleansed gently using normal saline, observed normally for macroscopic changes, and scored in a manner described later. Samples were then cut into two pieces; one piece for histopathology assessment (fixed in 5 mL formalin 10%) and one piece for measuring biomarkers weighed and maintained in -20 °C for 24 h. The colonic samples were then homogenized in 10 volume ice-cold potassium phosphate buffer (50 mmol/L, pH 7.4), sonicated, and centrifuged for 30 min at 3500 ×g. The supernatants were transformed into several microtubes for separate biochemical assays, and all were kept at -80 °C until analyses[44].
The macroscopic damage was assessed and scored according to criteria as described in our previous work[45,46]. For microscopic analysis, the fixed segments in formalin 10% were embedded in paraffin and stained with hematoxylin and eosin. The scoring was performed by one who was blind to the treated groups.
Quantitive detection of TNF-α and IL-1β levels in colon tissues were performed using an ELISA kit. The absorbance of the final colored product was measured in 450 nm as the primary wavelength and 620 nm as the reference wavelength. TNF-α and IL-1β levels were expressed as pg/mg protein of tissue, as described in our previous work[44].
Total antioxidant power of the colon was evaluated by measuring the ability to reduce Fe3+ to Fe2+. Interaction of TPTZ with Fe2+ results in the formation of a blue color, with a maximum absorbance at 593 nm. Data were expressed as mmol/L ferric ions reduced to ferrous per mg of protein, as described in our previous work[47].
In this test, supernatant was combined with o-dianisidine and 0.0005% H2O2 that resulted in an absorbance at 460 nm that was measured for 3 min. One unit of myeloperoxidase (MPO) activity is described as the change in absorbance per min at room temperature in the final reaction. Details of the procedure have been described in our previous work[48].
Levels of lipid peroxidation were assessed in colon tissue using thiobarbituric acid reactive substances (TBARS) assay as described in our previous work[49]. Data were reported as μg/mg of protein.
Total protein of the tissue was measured according to the Bradford method, using BSA as the standard[50]. Results were reported as mg of protein per mL of homogenized tissue.
In order to determine the acute toxicity (LD50) of Magliasa, doses of 5, 50, 300 and 2000 mg/kg per day were gavaged to rats. The animals were observed for 1 wk and any mortality was recorded at the end of this period[51].
Data were analyzed by StatsDirect ver. 2.7.8. One-way analysis of variance followed by a Newman-Keuls post hoc test for multiple comparisons were used. P values less than 0.05 were considered significant. Results are expressed as mean ± SE.
Microscopic characteristics of different herbal components of Magliasa are shown in Figure 1.
The mean value of total glucosinolates in one gram of Magliasa was 19 ± 1 μmol. The mean value of total phenolic content was 293.8 ± 17.6 mg GAE per 100 g of Magliasa.
Table 2 summarizes the retention value of spots visible in the TLC profile of Magliasa.
| Type of extract | Solvent system | RF values | Intensity of spot |
| Dichloromethane | Hexane: ethyl acetate (5:4 v/v) | 0.048 | Moderately intense |
| 0.181 | Faint | ||
| 0.238 | Intense | ||
| 0.286 | Faint | ||
| 0.333 | Intense | ||
| 0.380 | Intense | ||
| 0.430 | Faint | ||
| 0.476 | Faint | ||
| 0.524 | Moderately intense | ||
| 0.571 | Faint | ||
| 0.619 | Faint | ||
| 0.670 | Faint | ||
| 0.714 | Faint | ||
| 0.762 | Intense | ||
| 0.838 | Faint | ||
| 0.876 | Moderately intense |
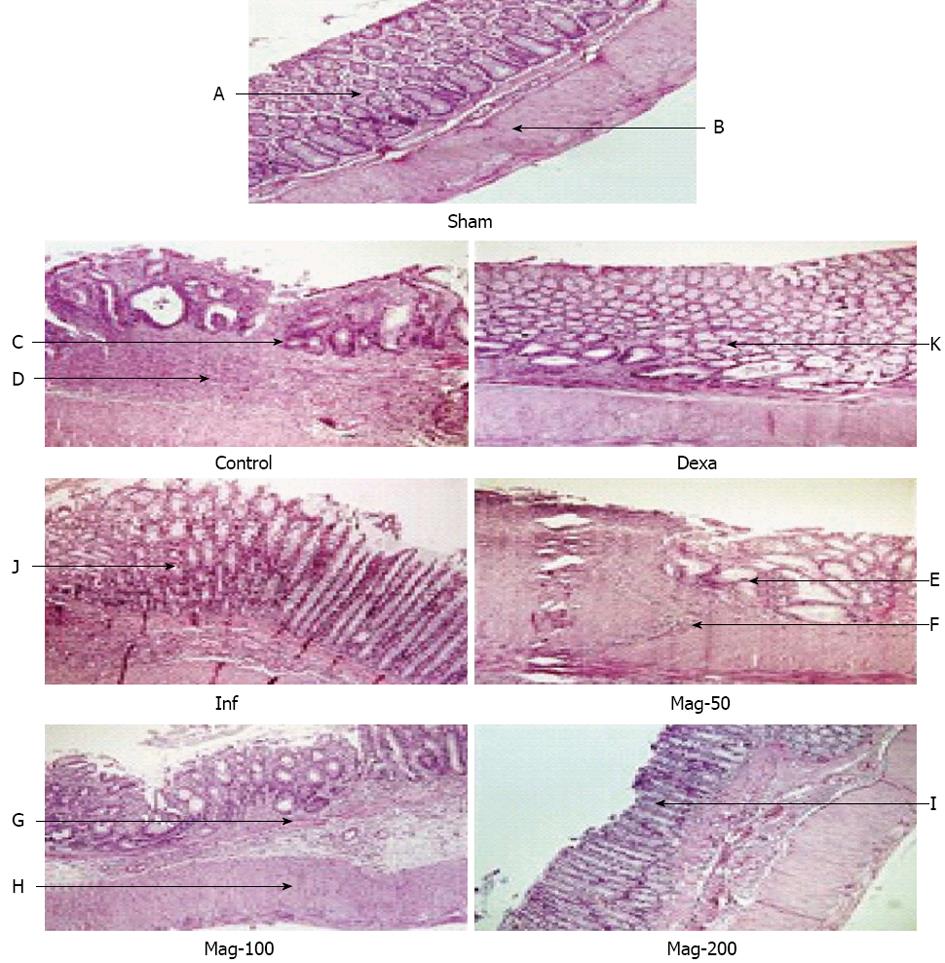
Data are shown in Table 3. The colons of the Sham group appeared normal. In contrast, intracolonic administration of TNBS led to mucosal ulceration, inflammation, adhesion, and wall thickening in the control group. Treatment with Mag-50 did not significantly reduce macroscopic scores where linear ulceration and mesenteric inflammation were observed in some samples. Administration of Maglasia reduced the macroscopic score in a dose-dependent manner, and a significant effect was observed in the Mag-100 and Mag-200 groups, although some inflammation and hyperemia were evident. The median effective dose (ED50) value was 104.78 mg/kg. Treatment of rats by dexamethasone and infliximab remarkably attenuated scores where mild hyperemia was observed macroscopically.
| Group | Macroscopic score | Microscopic score | ||
| mean ± SE | Median (range) | mean ± SE | Median (range) | |
| Sham | 0.00 ± 0.00 | 0 (0-0) | 0.00 ± 0.00 | 0 (0-0) |
| Control | 3.40 ± 0.24a | 3 (3-4) | 4.00 ± 0.32a | 4 (3-5) |
| Dexa | 0.33 ± 0.21c | 0 (0-1) | 0.67 ± 0.21c | 1 (0-1) |
| Inf | 0.83 ± 0.31c | 1 (0-2) | 1.50 ± 0.34c | 1 (1-3) |
| Mag-50 | 2.20 ± 0.37aeg | 2 (1-3) | 2.60 ± 0.51ae | 3 (1-4) |
| Mag-100 | 1.80 ± 0.58ace | 1 (1-4) | 2.40 ± 0.75a | 2 (1-5) |
| Mag-200 | 1.20 ± 0.20c | 1 (1-2) | 1.60 ± 0.40c | 1 (1-3) |
Histopathological examination of the control group showed extensive severe transmural inflammation, diffused necrosis, mucosal and submucosal polymorphonuclear (PMN) leukocyte infiltration, and crypt destruction, whereas microscopic evaluation of the Sham group showed a normal situation. In the Mag-50 group, microscopic evaluation revealed moderate mucosal and submucosal inflammation, PMN infiltration, and extensive crypt distortion. In the Mag-100 group, moderate inflammation of the mucosa and submucosa, inflammatory cell infiltration, and some crypt abscess and destruction were observed. In the Mag-200 and infliximab groups, mild focal non-hemorrhagic edema and focal submucosal PMN infiltration were observed. Minimal mucosal inflammation was observed in the Dexa group (Figure 2). Administration of Maglasia reduced the microscopic score in a dose-dependent manner, and a significant effect was observed in the Mag-200 group, with an ED50 of 132.29 mg/kg.
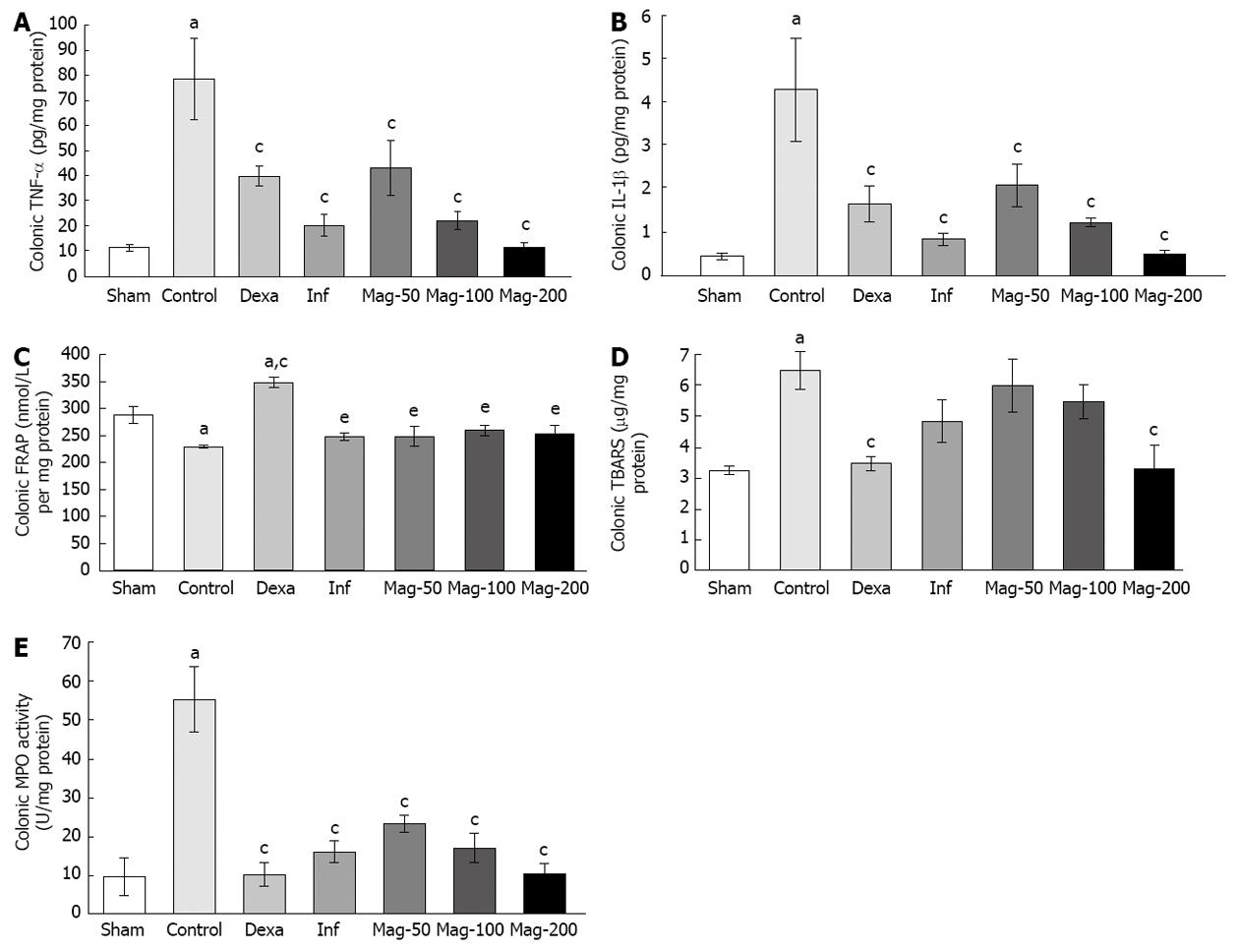
A significant difference was seen in TNF-α between the control and Sham groups (P = 0.000). TNF-α was significantly lower in all groups compared to the control, with an ED50 of 55.36 mg/kg. The level of TNF-α in the Mag-100 group (21.99 ± 3.54) was near to that of the infliximab group (20.18 ± 4.29), and both were lower than that of the Mag-50 (42.85 ± 10.87) and Dexa (39.72 ± 3.97) groups. TNF-α in the Mag-200 group (11.60 ± 1.83) was lower than that of the infliximab group, but the difference was not significant (P = 0.98, Figure 3A).
IL-1β was higher in the control group compared to the Sham (P = 0.000). IL-1β in all groups was lower than that of the control, with an ED50 of 48.78 mg/kg. IL-1β in the Mag-100 group (1.21 ± 0.10) was near to that of the Dexa group (1.64 ± 0.42), and both were higher than that of the infliximab (0.83 ± 0.14) and Mag-200 (0.48 ± 0.09) groups. IL-1β in the Mag-200 group was near to that of the Sham (0.44 ± 0.07), and was lower than infliximab, but the difference was not significant (P = 0.998, Figure 3B).
The ferric reducing/antioxidant power (FRAP) value was significantly lower in the control compared to the Sham (P = 0.008). Among interventions, only dexamethasone caused a significant increase in FRAP when compared to the control (P = 0.000). None of the Mag-50, Mag-100, Mag-200 or infliximab groups showed a significant difference to the control in FRAP (Figure 3C). The effect of Magliasa in FRAP was not dose dependent.
The TBARS value was significantly higher in the control compared to the Sham (P = 0.005), while TBARS in the Mag-200 (3.27 ± 0.77, P = 0.010) and Dexa (3.44 ± 0.22, P = 0.011) groups was significantly lower than that of the control (6.43 ± 0.61). Other groups did not show a significant difference from the control in TBARS (Figure 3D). Administration of Maglasia reduced TBARS in a dose-dependent manner, with an ED50 value of 216.4 mg/kg.
MPO in the control was significantly higher than that of the Sham (P < 0.002). Treatment with Magliasa in all groups significantly decreased MPO activity compared to the control. MPO in the Mag-200 group (10.65 ± 2.53) was lowest amongst the Mag groups, and close to the Dexa group (10.42 ± 3.18). MPO in the Mag-200 group was lower than that of the infliximab group (16.41 ± 2.89) (Figure 3E). The ED50 value was 34.38 mg/kg.
The acute toxicity test (LD50) demonstrated that Magliasa is not lethal up to a dose of 2000 mg/kg after oral administration. In the treated groups, no sign of toxicity was observed. It can therefore be considered as practically non-toxic.
There is a strong potential in the traditional and folkloric medicines of various countries, including Iran, for developing new and efficacious drugs for diseases that have a challenging treatment. One such disease is IBD. In this paper, Magliasa, one of the remedies recommended for colitis in TIM, was prepared, and its efficacy and possible mechanisms of action in different doses were evaluated in TNBS-induced colitis and compared with standard drugs. Macroscopic and microscopic scores, as criteria for colonic damage, improved by doses of 100 and 200 mg/kg per day with Magliasa. The microscopic score reduced only in the Mag-200 group, while the Mag-50 group showed no significant benefit against colonic damage. Colonic TNF-α, IL-1β and MPO activities were significantly decreased by all doses of Magliasa. TNF-α and IL-1β have been described as important mediators that contribute to intestinal inflammation in IBD patients[52-54]. Increased TNF-α has been found in the serum and mucosa of patients with IBD[55,56]. Moreover, inhibition of TNF-α by anti-TNF-α drugs, such as infliximab, has been an efficacious strategy in the management of IBD[10,57]. MPO is located in the granules of neutrophils and released upon stimulation by free radicals. The activity of MPO has been known as a marker of neutrophil penetration to the site of inflammation[58,59]. Magliasa did not affect oxidative stress as a factor involved in the pathophysiology of IBD[2]. Lipid peroxidation in the colon decreased only with a high dose of Magliasa (Mag-200). The effects of Magliasa in all investigated parameters were dose-dependent, except in total antioxidant power.
The total phenolic content of Magliasa was determined because phenolic compounds have pharmacological activities (antioxidant, anti-inflammatory, anti-diarrheal, and antimicrobial) that are all useful for the management of IBD, considering its pathogenesis. There is concern about the content uniformity of Magliasa, as intact non-milled seed of Lepidium sativum comprises 50% of the product. In addition to a reference marker for the standardization of Magliasa, total glucosinolates can be used for evaluating the content uniformity of the product.
There are some reports on the herbal ingredients of Magliasa that confirm their efficacy in IBD[32]. These reports are summarized in Table 1. Anti-inflammatory, antioxidant, analgesic, spasmolytic, antiulcer, ulcer healing, immunomodulatory, antibacterial, and anti-diarrheal activity are among the pharmacological properties of these ingredients that make them useful for IBD. It seems that the efficacy of Magliasa in IBD is due to the combination of the mentioned activities.
Overall, the results obtained from the efficacy of Magliasa on TNBS-induced colitis of rats are encouraging, although clinical trials are required for confirmation of these results.
Conventional treatments for the management of inflammatory bowel disease (IBD) have serious adverse effects that reduce patient compliance, and therefore investigators are trying to find useful compounds from complementary and alternative medicines with better safety and tolerability. There are many herbal preparations in traditional Iranian medicine (TIM) that were used for the management of IBD. Magliasa is one of them, and contains 6 components: seeds of Lepidium sativum, Linum usitatissimum, and Allium ampeloprasum cv. Porrum, fruit of Bunium persicum and Terminalia chebula, and gum resin of Pistacia lentiscus. Although, the efficacy of some herbal components of Magliasa in IBD have been confirmed by previous studies, no other study to date has investigated the beneficial effects of this preparation.
In the present study, after formulation and explanation of the quality control methods of Magliasa, its effects were investigated in trinitrobenzenesulfonic acid-induced colitis of rats to determine the involved mechanisms.
Magliasa demonstrated a significant reduction in macroscopic colonic damage, tumor necrosis factor-alpha, interleukin-1 beta, and neutrophil infiltration. Determination of total glucosinolates and total phenolic contents, as well as performing thin layer chromatography, can be used successfully for quality control of this herbal preparation.
Since the effects of Magliasa in the experimental model of colitis were encouraging, it could potentially be used as an effective medicine for IBD after confirmation of obtained results by clinical trials. Moreover, this study is a step toward strengthening TIM evidence.
Hopefully reviewers are positive to this article and believe that this TIM formula has enough support to go forward future clinical trials.
P- Reviewer Bonaz B S- Editor Gou SX L- Editor Rutherford A E- Editor Li JY
| 1. | Brown SJ, Mayer L. The immune response in inflammatory bowel disease. Am J Gastroenterol. 2007;102:2058-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci. 2007;52:2015-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 438] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 3. | Reiff C, Kelly D. Inflammatory bowel disease, gut bacteria and probiotic therapy. Int J Med Microbiol. 2010;300:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Kucharzik T, Maaser C, Lügering A, Kagnoff M, Mayer L, Targan S, Domschke W. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068-1083. [PubMed] |
| 5. | Martín MC, Martinez A, Mendoza JL, Taxonera C, Díaz-Rubio M, Fernández-Arquero M, de la Concha EG, Urcelay E. Influence of the inducible nitric oxide synthase gene (NOS2A) on inflammatory bowel disease susceptibility. Immunogenetics. 2007;59:833-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Rezaie A, Khalaj S, Shabihkhani M, Nikfar S, Zamani MJ, Mohammadirad A, Daryani NE, Abdollahi M. Study on the correlations among disease activity index and salivary transforming growth factor-beta 1 and nitric oxide in ulcerative colitis patients. Ann N Y Acad Sci. 2007;1095:305-314. [PubMed] |
| 7. | Rezaie A, Ghorbani F, Eshghtork A, Zamani MJ, Dehghan G, Taghavi B, Nikfar S, Mohammadirad A, Daryani NE, Abdollahi M. Alterations in salivary antioxidants, nitric oxide, and transforming growth factor-beta 1 in relation to disease activity in Crohn’s disease patients. Ann N Y Acad Sci. 2006;1091:110-122. [PubMed] |
| 8. | Jahanshahi G, Motavasel V, Rezaie A, Hashtroudi AA, Daryani NE, Abdollahi M. Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Dig Dis Sci. 2004;49:1752-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | El Miedany Y, Youssef S, Ahmed I, El Gaafary M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol. 2006;101:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Rahimi R, Nikfar S, Abdollahi M. Meta-analysis technique confirms the effectiveness of anti-TNF-alpha in the management of active ulcerative colitis when administered in combination with corticosteroids. Med Sci Monit. 2007;13:PI13-PI18. [PubMed] |
| 11. | Rahimi R, Nikfar S, Abdollahi M. Do anti-tumor necrosis factors induce response and remission in patients with acute refractory Crohn’s disease? A systematic meta-analysis of controlled clinical trials. Biomed Pharmacother. 2007;61:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Nikfar S, Ehteshami-Afshar S, Abdollahi M. A systematic review and meta-analysis of the efficacy and adverse events of infliximab in comparison to corticosteroids and placebo in active ulcerative colitis. Int J Pharmacol. 2011;7:325-332. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:630-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Nikfar S, Mirfazaelian H, Abdollahi M. Efficacy and tolerability of immunoregulators and antibiotics in fistulizing Crohn’s disease: a systematic review and meta-analysis of placebo-controlled trials. Curr Pharm Des. 2010;16:3684-3698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Rahimi R, Nikfar S, Rezaie A, Abdollahi M. A meta-analysis of antibiotic therapy for active ulcerative colitis. Dig Dis Sci. 2007;52:2920-2925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Rahimi R, Nikfar S, Rezaie A, Abdollahi M. A meta-analysis of broad-spectrum antibiotic therapy in patients with active Crohn’s disease. Clin Ther. 2006;28:1983-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Rahimi R, Nikfar S, Abdollahi M. A meta-analysis of the benefit of probiotics in maintaining remission of human ulcerative colitis: evidence for prevention of disease relapse and maintenance of remission. Arch Med Sci. 2008;4:185-190. |
| 18. | Rahimi R, Nikfar S, Rahimi F, Elahi B, Derakhshani S, Vafaie M, Abdollahi M. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn’s disease. Dig Dis Sci. 2008;53:2524-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185-210. [PubMed] |
| 20. | Nikfar S, Rahimi R, Rezaie A, Abdollahi M. A meta-analysis of the efficacy of sulfasalazine in comparison with 5-aminosalicylates in the induction of improvement and maintenance of remission in patients with ulcerative colitis. Dig Dis Sci. 2009;54:1157-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Comparison of mesalazine and balsalazide in induction and maintenance of remission in patients with ulcerative colitis: a meta-analysis. Dig Dis Sci. 2009;54:712-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Nikfar S, Ehteshami-Ashar S, Rahimi R, Abdollahi M. Systematic review and meta-analysis of the efficacy and tolerability of nicotine preparations in active ulcerative colitis. Clin Ther. 2010;32:2304-2315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Hosseini-Tabatabaei A, Abdollahi M. Potassium channel openers and improvement of toxic stress: do they have role in the management of inflammatory bowel disease? Inflamm Allergy Drug Targets. 2008;7:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Salari P, Abdollahi M. Current opinion in the pharmaceutical management of irritable and inflammatory bowel diseases: Role of ATP. Recent Pat Endocr Metab Immune Drug Discov. 2009;3:69-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Khoshakhlagh P, Bahrololoumi-Shapourabadi M, Mohammadirad A, Ashtaral-Nakhai L, Minaie B, Abdollahi M. Beneficial effect of phosphodiesterase-5 inhibitor in experimental inflammatory bowel disease; molecular evidence for involvement of oxidative stress. Toxicol Mech Methods. 2007;17:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Salari-Sharif P, Abdollahi M. Phosphodiesterase 4 inhibitors in inflammatory bowel disease: a comprehensive review. Curr Pharm Des. 2010;16:3661-3667. [PubMed] |
| 27. | Rahimi R, Ghiasi S, Azimi H, Fakhari S, Abdollahi M. A review of the herbal phosphodiesterase inhibitors; future perspective of new drugs. Cytokine. 2010;49:123-129. [PubMed] |
| 28. | Stein RB, Hanauer SB. Comparative tolerability of treatments for inflammatory bowel disease. Drug Saf. 2000;23:429-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Stallmach A, Hagel S, Bruns T. Adverse effects of biologics used for treating IBD. Best Pract Res Clin Gastroenterol. 2010;24:167-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 30. | Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod Toxicol. 2008;25:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Rahimi R, Mozaffari S, Abdollahi M. On the use of herbal medicines in management of inflammatory bowel diseases: a systematic review of animal and human studies. Dig Dis Sci. 2009;54:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Rahimi R, Shams-Ardekani MR, Abdollahi M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J Gastroenterol. 2010;16:4504-4514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 137] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 33. | Ardekani MR, Rahimi R, Javadi B, Abdi L, Khanavi M. Relationship between temperaments of medicinal plants and their major chemical compounds. J Tradit Chin Med. 2011;31:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Arzani MA. Tebb-e-akbari [in Persian]. Institute of Revival of Natural Medicine. 2007;778-781. |
| 35. | Tonkaboni MM. Tohfeh al- Momenin [in Persian]. Shahid Beheshti University of Medical Sciences. 2007;356-542. |
| 36. | Aghili MH. Makhzan-al-Advia [in Persian]. Tehran University of Medical Sciences. 2009;165-742. |
| 37. | Amin G. Popular medicinal plants of Iran. Tehran University of Medical Sciences. 2008;104-264. |
| 38. | World Health Organization. Quality control methods for medicinal plant materials. Geneva: World Health Organization 1998; 5. |
| 39. | Smith CA, Dacombe C. Rapid method for determining total glucosinolates in rapeseed by measurement of enzymatically released glucose. J Sci Food Agric. 1987;38:141-150. [DOI] [Full Text] |
| 40. | Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152-178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8745] [Cited by in RCA: 7830] [Article Influence: 301.2] [Reference Citation Analysis (0)] |
| 41. | Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas. 2nd ed. Springer. 200;149-152. |
| 42. | Baghaei A, Esmaily H, Abdolghaffari AH, Baeeri M, Gharibdoost F, Abdollahi M. Efficacy of Setarud (IMod), a novel drug with potent anti-toxic stress potential in rat inflammatory bowel disease and comparison with dexamethasone and infliximab. Indian J Biochem Biophys. 2010;47:219-226. [PubMed] |
| 43. | Abdolghaffari AH, Baghaei A, Moayer F, Esmaily H, Baeeri M, Monsef-Esfahani HR, Hajiaghaee R, Abdollahi M. On the benefit of Teucrium in murine colitis through improvement of toxic inflammatory mediators. Hum Exp Toxicol. 2010;29:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Ebrahimi F, Esmaily H, Baeeri M, Mohammadirad A, Fallah S, Abdollahi M. Molecular evidences on the benefit of N-acetylcysteine in experimental colitis. Cent Eur J Biol. 2008;3:135-132. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Ghafari H, Yasa N, Mohammadirad A, Dehghan G, Zamani MJ, Nikfar S, Khorasani R, Minaie B, Abdollahi M. Protection by Ziziphora clinopoides of acetic acid-induced toxic bowel inflammation through reduction of cellular lipid peroxidation and myeloperoxidase activity. Hum Exp Toxicol. 2006;25:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Nakhai LA, Mohammadirad A, Yasa N, Minaie B, Nikfar S, Ghazanfari G, Zamani MJ, Dehghan G, Jamshidi H, Boushehri VS. Benefits of Zataria multiflora Boiss in Experimental Model of Mouse Inflammatory Bowel Disease. Evid Based Complement Alternat Med. 2007;4:43-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Hasani P, Yasa N, Vosough-Ghanbari S, Mohammadirad A, Dehghan G, Abdollahi M. In vivo antioxidant potential of Teucrium polium, as compared to alpha-tocopherol. Acta Pharm. 2007;57:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Ghazanfari G, Minaie B, Yasa N, Nakhai LA, Mohammadirad A, Nikfar S, Dehghan G, Boushehri VS, Jamshidi H, Khorasani R. Biochemical and histopathological evidences for beneficial effects of satureja khuzestanica jamzad essential oil on the mouse model of inflammatory bowel diseases. Toxicol Mech Methods. 2006;16:365-372. [PubMed] |
| 49. | Amini-Shirazi N, Hoseini A, Ranjbar A, Mohammadirad A, Khoshakhlagh P, Yasa N, Abdollahi M. Inhibition of tumor necrosis factor and nitrosative/oxidative stresses by Ziziphora clinopoides (Kahlioti); a molecular mechanism of protection against dextran sodium sulfate-induced colitis in mice. Toxicol Mech Methods. 2009;19:183-189. [PubMed] |
| 50. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [PubMed] |
| 51. | Hodgson E. A textbook of modern toxicology. 3rd ed. New Jersey: John Wily and Sons 2004; . [DOI] [Full Text] |
| 52. | Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 887] [Cited by in RCA: 851] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 53. | Cominelli F, Pizarro TT. Interleukin-1 and interleukin-1 receptor antagonist in inflammatory bowel disease. Aliment Pharmacol Ther. 1996;10 Suppl 2:49-53; discussion 54. [PubMed] |
| 54. | Esmaily H, Vaziri-Bami A, Miroliaee AE, Baeeri M, Abdollahi M. The correlation between NF-κB inhibition and disease activity by coadministration of silibinin and ursodeoxycholic acid in experimental colitis. Fundam Clin Pharmacol. 2011;25:723-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Akazawa A, Sakaida I, Higaki S, Kubo Y, Uchida K, Okita K. Increased expression of tumor necrosis factor-alpha messenger RNA in the intestinal mucosa of inflammatory bowel disease, particularly in patients with disease in the inactive phase. J Gastroenterol. 2002;37:345-353. [PubMed] |
| 56. | Komatsu M, Kobayashi D, Saito K, Furuya D, Yagihashi A, Araake H, Tsuji N, Sakamaki S, Niitsu Y, Watanabe N. Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR. Clin Chem. 2001;47:1297-1301. [PubMed] |
| 57. | Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644-659, quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 459] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 58. | Grulke S, Franck T, Gangl M, Péters F, Salciccia A, Deby-Dupont G, Serteyn D. Myeloperoxidase assay in plasma and peritoneal fluid of horses with gastrointestinal disease. Can J Vet Res. 2008;72:37-42. [PubMed] |
| 59. | Miroliaee AE, Esmaily H, Vaziri-Bami A, Baeeri M, Shahverdi AR, Abdollahi M. Amelioration of experimental colitis by a novel nanoselenium-silymarin mixture. Toxicol Mech Methods. 2011;21:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Kassie F, Rabot S, Uhl M, Huber W, Qin HM, Helma C, Schulte-Hermann R, Knasmüller S. Chemoprotective effects of garden cress (Lepidium sativum) and its constituents towards 2-amino-3-methyl-imidazo[4,5-f]quinoline (IQ)-induced genotoxic effects and colonic preneoplastic lesions. Carcinogenesis. 2002;23:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Datta PK, Diwakar BT, Viswanatha S, Murthy KN, Naidu KA. Safety evaluation studies on Garden cress (Lepidium sativum L.) seeds in Wistar rats. Int J Appl Res Nat Prod. 2011;4:37-43. |
| 62. | Bafeel SO, Ali SS. The Potential Liver Toxicity of Lepidium sativum Seeds in Albino Rats. Res J Biol Sci. 2009;4:1250-1258. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Moser BR, Shah SN, Moser JK, Vaughn SF, Evangelista RL. Composition and Physical Properties of Cress (Lepidium sativum L.) and Field Pennycress (Thlaspi arvense L.) Oils. Indust Crops Prod. 2009;30:199-205. [DOI] [Full Text] |
| 64. | Diwakar BT, Lokesh BR, Naidu KA. Modulatory effect of α-linolenic acid-rich garden cress (Lepidium sativum L.) seed oil on inflammatory mediators in adult albino rats. Br J Nutr. 2011;106:530-539. [PubMed] |
| 65. | Al-Yahya MA, Mossa JS, Ageel AM, Rafatullah S. Pharmacological and safety evaluation studies on Lepidium sativum L., seeds. Phytomedicine. 1994;1:155-159. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Najeeb-Ur-Rehman MH, Alkharfy KM, Gilani AH. Prokinetic and laxative activities of Lepidium sativum seed extract with species and tissue selective gut stimulatory actions. J Ethnopharmacol. 2011;134:878-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Rehman NU, Mehmood MH, Alkharfy KM, Gilani AH. Studies on antidiarrheal and antispasmodic activities of Lepidium sativum crude extract in rats. Phytother Res. 2012;26:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Rehman NU, Khan AU, Alkharfy KM, Gilani AH. Pharmacological Basis for the Medicinal Use of Lepidium sativum in Airways Disorders. Evid Based Complement Alternat Med. 2012;2012:596524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Azizi M, Davareenejad G. Essential Oil Content and Constituents of Black Zira (Bunium persicum [Boiss.] B. Fedtsch.) from Iran During Field Cultivation (Domestication). J Essent Oil Res. 2009;21:78-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Sharififar F, Yassa N, Mozaffarian V. Bioactivity of major components from the seeds of Bunium persicum (Boiss.) Fedtch. Pak J Pharm Sci. 2010;23:300-304. [PubMed] |
| 71. | Nickavar B, Abolhasani FA. Screening of antioxidant properties of seven Umbelliferae fruits from Iran. Pak J Pharm Sci. 2009;22:30-35. [PubMed] |
| 72. | Shahsavari N, Barzegar M, Sahari MA, Naghdibadi H. Antioxidant activity and chemical characterization of essential oil of Bunium persicum. Plant Foods Hum Nutr. 2008;63:183-188. [PubMed] |
| 73. | Hajhashemi V, Sajjadi SE, Zomorodkia M. Antinociceptive and anti-inflammatory activities of Bunium persicum essential oil, hydroalcoholic and polyphenolic extracts in animal models. Pharm Biol. 2011;49:146-151. [PubMed] |
| 74. | Singh KK, Mridula D, Rehal J, Barnwal P. Flaxseed: a potential source of food, feed and fiber. Crit Rev Food Sci Nutr. 2011;51:210-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 75. | Kaithwas G, Majumdar DK. Evaluation of antiulcer and antisecretory potential of Linum usitatissimum fixed oil and possible mechanism of action. Inflammopharmacology. 2010;18:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Tülüce Y, Ozkol H, Koyuncu I. Photoprotective effect of flax seed oil (Linum usitatissimum L.) against ultraviolet C-induced apoptosis and oxidative stress in rats. Toxicol Ind Health. 2012;28:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Bommareddy A, Zhang X, Schrader D, Kaushik RS, Zeman D, Matthees DP, Dwivedi C. Effects of dietary flaxseed on intestinal tumorigenesis in Apc(Min) mouse. Nutr Cancer. 2009;61:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Fattorusso E, Lanzotti V, Taglialatela-Scafati O, Cicala C. The flavonoids of leek, Allium porrum. Phytochemistry. 2001;57:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Fattorusso E, Lanzotti V, Taglialatela-Scafati O, Di Rosa M, Ianaro A. Cytotoxic saponins from bulbs of Allium porrum L. J Agric Food Chem. 2000;48:3455-3462. [PubMed] |
| 80. | Heinonen MI, Ollilainen V, Linkola EK, Varo PT, Koivistoinen PE. Carotenoids in Finnish foods, vegetables, fruits and berries. J Agric Food Chem. 1989;37:655-659. [DOI] [Full Text] |
| 81. | Evans WC. Trease and Evans pharmacognosy. 15th Ed. WB Saunders. 2002;. |
| 82. | Manosroi A, Jantrawut P, Akazawa H, Akihisa T, Manosroi J. Biological activities of phenolic compounds isolated from galls of Terminalia chebula Retz. (Combretaceae). Nat Prod Res. 2010;24:1915-1926. [PubMed] |
| 83. | Chang CL, Lin CS. Phytochemical Composition, Antioxidant Activity, and Neuroprotective Effect of Terminalia chebula Retzius Extracts. Evid Based Complement Alternat Med. 2012;2012:125247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Das ND, Jung KH, Park JH, Mondol MA, Shin HJ, Lee HS, Park KS, Choi MR, Kim KS, Kim MS. Terminalia chebula extract acts as a potential NF-κB inhibitor in human lymphoblastic T cells. Phytother Res. 2011;25:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Sharma P, Prakash T, Kotresha D, Ansari MA, Sahrm UR, Kumar B, Debnath J, Goli D. Antiulcerogenic activity of Terminalia chebula fruit in experimentally induced ulcer in rats. Pharm Biol. 2011;49:262-268. [PubMed] |
| 86. | Nair V, Singh S, Gupta YK. Anti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental models. J Pharm Pharmacol. 2010;62:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Kim HG, Cho JH, Jeong EY, Lim JH, Lee SH, Lee HS. Growth-inhibiting activity of active component isolated from Terminalia chebula fruits against intestinal bacteria. J Food Prot. 2006;69:2205-2209. [PubMed] |
| 88. | Zhou L, Satoh K, Takahashi K, Watanabe S, Nakamura W, Maki J, Hatano H, Takekawa F, Shimada C, Sakagami H. Re-evaluation of anti-inflammatory activity of mastic using activated macrophages. In Vivo. 2009;23:583-589. [PubMed] |
| 89. | Kim HJ, Neophytou C. Natural anti-inflammatory compounds for the management and adjuvant therapy of inflammatory bowel disease and its drug delivery system. Arch Pharm Res. 2009;32:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Al-Said MS, Ageel AM, Parmar NS, Tariq M. Evaluation of mastic, a crude drug obtained from Pistacia lentiscus for gastric and duodenal anti-ulcer activity. J Ethnopharmacol. 1986;15:271-278. [PubMed] [DOI] [Full Text] |
| 91. | Kaliora AC, Stathopoulou MG, Triantafillidis JK, Dedoussis GV, Andrikopoulos NK. Alterations in the function of circulating mononuclear cells derived from patients with Crohn’s disease treated with mastic. World J Gastroenterol. 2007;13:6031-6036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 92. | Kaliora AC, Stathopoulou MG, Triantafillidis JK, Dedoussis GV, Andrikopoulos NK. Chios mastic treatment of patients with active Crohn’s disease. World J Gastroenterol. 2007;13:748-753. [PubMed] |
| 93. | Al-Habbal MJ, Al-Habbal Z, Huwez FU. A double-blind controlled clinical trial of mastic and placebo in the treatment of duodenal ulcer. Clin Exp Pharmacol Physiol. 1984;11:541-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 1.6] [Reference Citation Analysis (0)] |