Published online Mar 14, 2013. doi: 10.3748/wjg.v19.i10.1551
Revised: October 15, 2012
Accepted: October 30, 2012
Published online: March 14, 2013
Processing time: 182 Days and 3.1 Hours
AIM: To evaluate the inhibitory effects of Scolopendra subspinipes mutilans (SSM) on cerulein-induced acute pancreatitis (AP) in a mouse model.
METHODS: SSM water extract (0.1, 0.5, or 1 g/kg) was administrated intraperitoneally 1 h prior to the first injection of cerulein. Once AP developed, the stable cholecystokinin analogue, cerulein was injected hourly, over a 6 h period. Blood samples were taken 6 h later to determine serum amylase, lipase, and cytokine levels. The pancreas and lungs were rapidly removed for morphological examination, myeloperoxidase assay, and real-time reverse transcription polymerase chain reaction. To specify the role of SSM in pancreatitis, the pancreatic acinar cells were isolated using collagenase method. Then the cells were pre-treated with SSM, then stimulated with cerulein. The cell viability, cytokine productions and high-mobility group box protein-1 (HMGB-1) were measured. Furthermore, the regulating mechanisms of SSM action were evaluated.
RESULTS: The administration of SSM significantly attenuated the severity of pancreatitis and pancreatitis associated lung injury, as was shown by the reduction in pancreatic edema, neutrophil infiltration, vacuolization and necrosis. SSM treatment also reduced pancreatic weight/body weight ratio, serum amylase, lipase and cytokine levels, and mRNA expression of multiple inflammatory mediators such as tumor necrosis factor-α and interleukin-1β. In addition, treatment with SSM inhibited HMGB-1 expression in the pancreas during AP. In accordance with in vivo data, SSM inhibited the cerulein-induced acinar cell death, cytokine, and HMGB-1 release. SSM also inhibited the activation of c-Jun NH2-terminal kinase, p38 and nuclear factor (NF)-κB.
CONCLUSION: These results suggest that SSM plays a protective role during the development of AP and pancreatitis associated lung injury via deactivating c-Jun NH2-terminal kinase, p38 and NF-κB.
-
Citation: Jo IJ, Bae GS, Park KC, Choi SB, Jung WS, Jung SY, Cho JH, Choi MO, Song HJ, Park SJ.
Scolopendra subspinipes mutilans protected the cerulein-induced acute pancreatitis by inhibiting high-mobility group box protein-1. World J Gastroenterol 2013; 19(10): 1551-1562 - URL: https://www.wjgnet.com/1007-9327/full/v19/i10/1551.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i10.1551
The annual incidence of acute pancreatitis (AP) is in the range of 300 or more patients per million[1,2]. The mortality rate for severe AP is approximately 30%, whereas that for moderate pancreatitis is about 3%. The main causes of death are circulatory shock renal, respiratory, and hepatic failure. Thus, many patients with AP develop multiple organ failure (MOF)[3]. Generally, AP is characterized by activation of pancreatic digestive enzyme production, widespread inflammatory cell infiltration, leukocyte activation, and release of various pro-inflammatory mediators such as tumor necrosis factor (TNF)-α and interleukin (IL)[4-6]. Although numerous approaches have attempted to identify the pathogenesis of AP[7-9], the detailed mechanism remains unclear.
Recent studies have shown that high-mobility group box protein-1 (HMGB-1) is a late activator in the inflammatory cascade, when released into the extracellular space[10]. Neutralization of HMGB-1 has been shown to protect against systemic inflammatory responses such as in sepsis and MOF as HMGB-1 acts as a downstream cytokine of early inflammatory factors such as TNF and ILs[11-13]. In addition, HMGB-1 has been speculated to be a target for treating AP[14].
Scolopendra subspinipes mutilans (SSM) is a venomous arthropod, which can be found throughout the world. SSM and its venom have been reported to exhibit many biochemical and physiological effects[15,16]. The water soluble fractions from SSM have antimicrobial and anti-inflammatory activity and hemolytic action of the toxins[17,18]. In addition, SSM has been prescribed for the treatment of cardiovascular diseases in South Korea, China, and other Far Eastern Asian countries for several hundred years[16]. However, the protective activities of SSM in cerulein-induced AP have not been examined to date. Our study was designed to assess the protective effect of SSM in cerulein-induced AP.
Here, we investigated the in vivo and in vitro activities of SSM using a murine model of experimental pancreatitis. To examine the role of SSM in AP, we examined pancreatic and lung histology, myeloperoxidase (MPO) activity, pancreatic weight (PW)/body weight (BW) ratio, levels of serum amylase, lipase, and cytokines such as TNF-α and IL-1β as well as expression levels of HMGB-1. Furthermore, we examined mitogen activated protein kinases (MAPKs) and nuclear factor (NF)-κB to find out the inhibitory mechanisms of SSM in AP.
Avidin-peroxidase, cerulein, hexadecyltrimethylammonium bromide, Triton X-100, and tetramethylbenzidine were purchased from Sigma-Aldrich (St. Louis, MO, United States). Anti-mouse TNF-α and IL-1β antibodies, and recombinant TNF-α and IL-1 were purchased from R-D Systems (Minneapolis, MN, United States).
SSM was purchased from a standard commercial source (Omni Herb, Seoul, South Korea). The identity of the SSM was confirmed by Professor Seung-Heon Hong from Wonkwang University. SSM was prepared by decocting the dried prescription of SSM (100 g) with boiling distilled water (1 L). The decoction time was about 2 h. The water extract was frozen at -80 °C and then freeze-dried to produce a powder form (20.4 g). The yield of extract was 20.4%. The powder was extracted with distilled water and filtered. The filtrates were stored at 4 °C until use.
All experiments were performed according to protocols approved by the Animal Care Committee of Wonkwang University. C57BL/6 mice (age 6-8 wk; weight 15-20 g) were purchased from Orient Bio (Sungnam, KyungKiDo, South Korea). All animals were bred and housed in standard shoebox cages in a climate-controlled environment with an ambient temperature of 23 ± 2 °C and a 12-h light-dark cycle for 7 d. The animals were fed standard laboratory chow, given water, and were randomly assigned to the control or experimental groups. The mice were fasted for 18 h before the induction of AP. Six mice were included in each experimental group.
AP was induced by intraperitoneal injection of supramaximal concentrations of the stable cholecystokinin analog cerulein (50 μg/kg) or saline; injections were performed hourly for 6 h. To verify the prophylactic effects of SSM, SSM (0.1, 0.5, or 1 g/kg) was injected 1 h before the first cerulein injection. Mice were sacrificed 6 h after the last cerulein injection. Blood samples were taken to determine serum amylase, lipase, and cytokine levels. For histological examination and scoring, the entire pancreas and lungs were rapidly removed from each mouse and fixed in formalin. To measure tissue MPO activity, as an indicator of neutrophil sequestration, and to perform real-time reverse transcriptase-polymerase chain reaction (RT-PCR) examinations, 3 portions of both pancreas and lungs were stored at -80 °C.
The entire pancreas of at least 6 mice from each treatment group were examined and semi-quantitatively assessed for levels of necrosis, vacuolization, inflammation, and edema. The entire section, representing a minimum of 100 fields, was examined for each sample and scored on a scale of 0-3 (0 being normal and 3 being severe) on the basis of the number of necrotic acinar cells and the presence of vacuolization, interstitial edema, and inflammatory cells infiltration. These characteristics include the presence of acinar-cell ghosts, vacuolization and swelling of the acinar cells, and/or the destruction of the histoarchitecture of whole or parts of the acini. For scoring the lungs, the sections were examined for the presence of interstitial inflammation and edema.
Blood samples, for the determination of serum amylase and lipase levels, were obtained 6 h after induction of pancreatitis. Mice were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (4 mg/kg). After anesthetization, blood was withdrawn from the heart of each mouse into a syringe. The levels of serum amylase and lipase were measured using an assay kit (BioAssay Systems, Hayward, CA, United States).
RT-PCR was performed to measure mRNA transcript levels in the mouse pancreatic tissues and pancreatic acinar cells. Total RNA was isolated from the mouse pancreas using TRIzol (Invitrogen, Carlsbad, CA, United States) and was subjected to reverse transcription using SuperScript II RT (Invitrogen, Garlsbad, CA, United States). TaqMan quantitative RT-PCR using the LightCycler 2.0 detection system was performed according to the instructions of the manufacturer (Roche, Basel, Switzerland). For each sample, triplicate test reactions and a control reaction without reverse transcription were analyzed for expression of the gene of interest, and the results were normalized to those of the “housekeeping” hypoxanthine-guanine phosphoribosyl transferase (HPRT) mRNA. Arbitrary expression units were calculated by dividing the expression level for the gene of interest by the ribosomal protein HPRT mRNA expression level. The sequences of forward, reverse, and probe oligonucleotide primers for multiplex real-time TaqMan PCR were as follows: for mouse TNF-α (forward, 5’-TCTCTTCAAGGGACAAGGCTG-3’; reverse, 5’-ATAGCAAATCGGCTGACGGT-3’; probe, 5’-CCCGACTACGTGCTCCTCACCCA-3’), for mouse IL-1β (forward, 5’-TTGACGGACCCCAAAAGAT-3’; reverse, 5’-GAAGCTGGATGCTCTCATCTG-3’; universal probe, M15131.1-Roche Applied Science).
Enzyme-linked immunosorbent assays (ELISAs) for TNF-α and IL-1β were carried out in duplicate in 96-well plates (Nunc, Roskilde, Denmark), which had been incubated with 100 L aliquots of either anti-mouse TNF-α or anti-mouse IL-1β monoclonal antibodies (1.0 μg/mL in phosphate-buffered saline (PBS) at pH 7.4) overnight. The plates were washed in PBS containing 0.05% Tween-20 and blocked with PBS containing 10% fetal bovine serum for 2 h. After additional washes, the standards and the serum, pancreatic homogenates and pancreatic acinar cell supernants were added to the plates and incubated at room temperature for 3 h. To obtain pancreatic homogenates, the pancreas were thawed and then homogenized in PBS. After washing the wells, 0.2 μg/mL of biotinylated anti-mouse TNF-α or IL-1β were added to each well. Incubation was continued at room temperature for 1 h. The wells were washed, avidin-peroxidase was added, and plates were incubated for 30 min at room temperature. Wells were washed again, and 3, 3’, 5, 5’-tetramethylbenzidine substrate was added. Color development was measured at 450 nm using an automated microplate ELISA reader. Standard curves were obtained for each sample by using serial dilutions of recombinant TNF-α and IL-1β.
Neutrophil sequestration in the pancreas was quantified by measuring the tissue MPO activity. Tissue samples were thawed, homogenized in 20 mmol/L phosphate buffer (pH 7.4), and centrifuged (15 000 revolution/min, 10 min), and the resulting pellet was resuspended in 50 mmol/L phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide. The sample was then centrifuged (15 000 revolution/min, 5 min), and the supernatant used for the MPO assay. The reaction mixture consisted of the supernatant, 1.6 mmol/L tetramethylbenzidine, 80 mmol/L sodium phosphate buffer (pH 5.4), and 0.3 mmol/L hydrogen peroxide. The mixture was incubated at 37 °C for 110 s, the reaction was terminated with 2 mol/L of H2SO4, and the absorbance was measured at 450 nm. This absorbance was then corrected for the DNA content of the tissue sample.
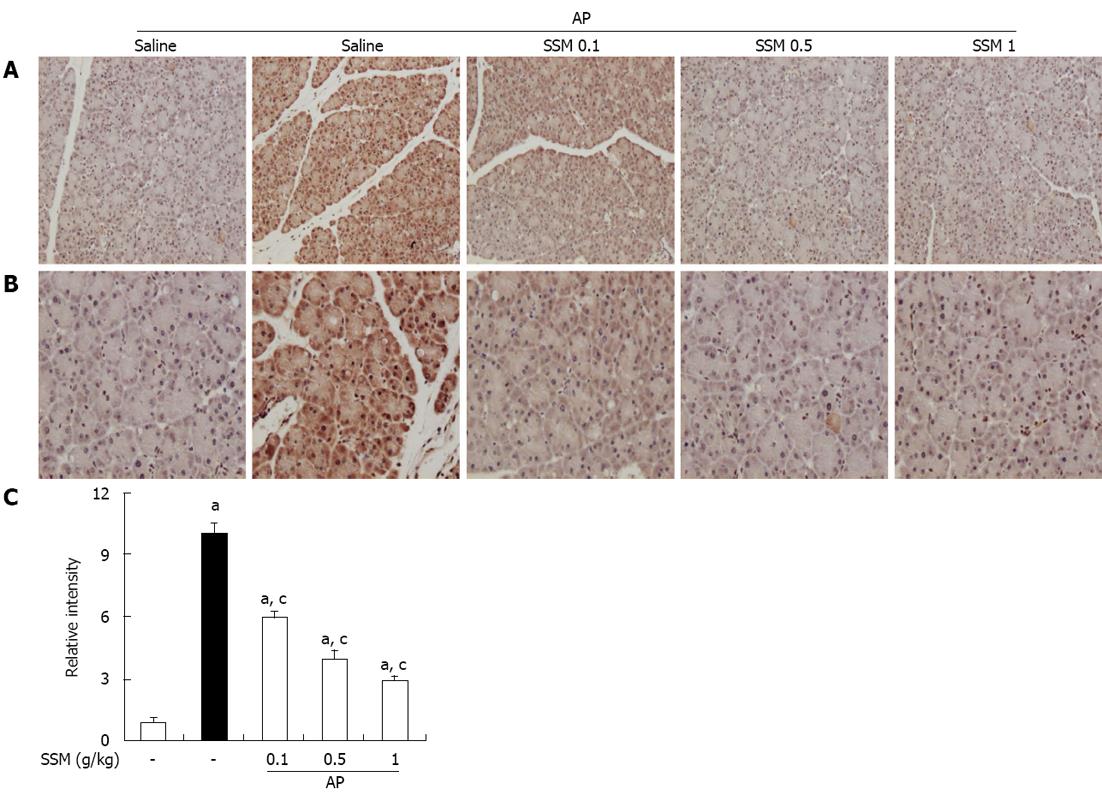
Fixed pancreatic tissues were embedded in paraffin, cut into 4-mm sections, and stained with hematoxylin-eosin for standard histological examination. Immunohistochemical (IHC) staining for HMGB-1 was performed using a DAB IHC kit (DAKO, Cytomation, Denmark). The relative intensity was measured using the Leica microscopy software (Wetzlar, Germany).
Pancreatic acini were isolated from C57BL/6 mice using collagenase digestion. All experiments were performed according to protocols approved by the Animal Care Committee of Wonkwang University. Briefly, pancreatic tissue was minced with scissors and digested for 15 min in solution Q (120 mmol NaCl, 20 mmol HEPES, 5 mmol KCl, 1 mmol MgCl2, 1 mmol CaCl2, 10 mmol sodium pyruvate, 10 mmol ascorbate, 10 mmol glucose, 0.1% bovine serum albumin, 0.01% soybean trypsinogen inhibitor, and 150 units of collagenase/mL). Cells were continuously shaken and gassed with 100% O2 in a 37 °C water bath and subsequently washed in fresh isolation medium. After collagenase digestion, the tissue was gently pipetted. Dispersed acini were filtered through a 150-μm nylon mesh, centrifuged 3 times (each for 90 s at 720 rpm), resuspended in Waymouth medium (Invitrogen, Gibco, CA) and incubated with 95% O2 and 5% CO2 for 4 h.
Cell viability was assayed using a modified colorimetric technique that is based on the ability of live cells to convert the tetrazolium compound 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) into purple formazan crystals. MTT (5 mg/mL) was dissolved in Kreb’s-Henseleit buffer (115 mmol NaCl, 3.6 mmol KCl, 1.3 mmol KH2PO4, 25 mmol NaHCO3, 1 mol CaCl2, and 1 mol MgCl2), and 50 μL was added to each well. After incubating for 30 min at 37 °C, the suspension was removed, and the formazan crystals formed were dissolved in 200 μL dimethyl sulfoxide. Aliquots from each well were seeded in the wells of a 96-well plate in duplicate and assayed at 540 nm using a microplate ELISA reader. The number of viable cells was expressed as a percentage of the control.
Pancreatic tissues and pancreatic acini were homogenized, following which the lysates were boiled in a sample buffer [62.5 mmol Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 20% glycerol, and 10% 2-mercaptoethanol]. Proteins in the cell lysates were then separated using 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Then, the membrane was blocked with 5% skim milk in PBS-Tween-20 for 2 h at RT and then incubated with primary antibodies overnight. After washing 3 times, each blot was incubated with peroxidase-conjugated secondary antibody for 1 h, and antibody-specific proteins were visualized using an enhanced chemiluminesence detection system (Amersham, Piscataway, NJ) according to the manufacturer’s recommended protocol.
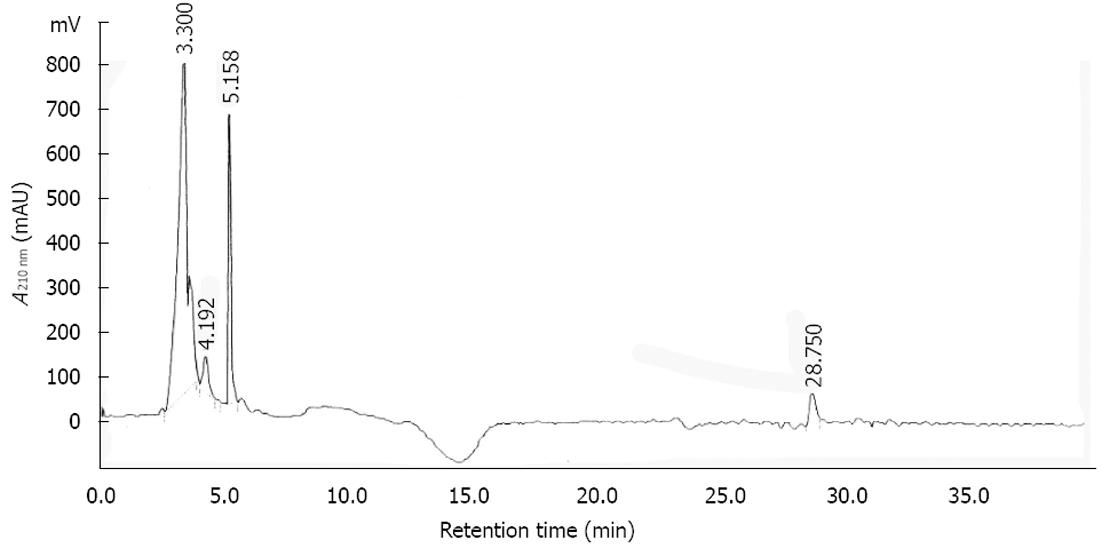
An aliquot of 5.0 mg extract powder was dissolved with 1.0 mL of methanol and then filtered through a 0.45 μm filter membrane before use. A volume of 20 μL was injected into the high-performance liquid chromatography (HPLC) sample injector system. Chromatographic experiments were performed on a SYKAM series HPLC instrument equipped with sample injector and diode-array UV/Vis detector. For all experiments a SHISEIDO CAPCELL PACK C-18 column (4.6 mm × 250 mm; 5 μm) was used as stationary phase and injection volume were set 20 μL, respectively. The mobile phase composed of water (A) and acetonitrile (B), applying gradient program starting from 10 %B to 40 %B in 40 min. The column cleaned with 10 %B for 20 min, and then the system was equilibrated for 20 min with the starting conditions. Flow rate was 0.7 mL/min, and the detection wavelength adjusted to 210 nm. The quantifications of peak are 91% (1st), 4% (2nd), 0.5% (3rd), 4.5% (4th) to total.
The results were expressed as mean ± SE. The significance of change was evaluated using the one-way analysis of variance (ANOVA). Differences between the experimental groups were evaluated by performing ANOVA. P values < 0.05 were considered statistically significant.
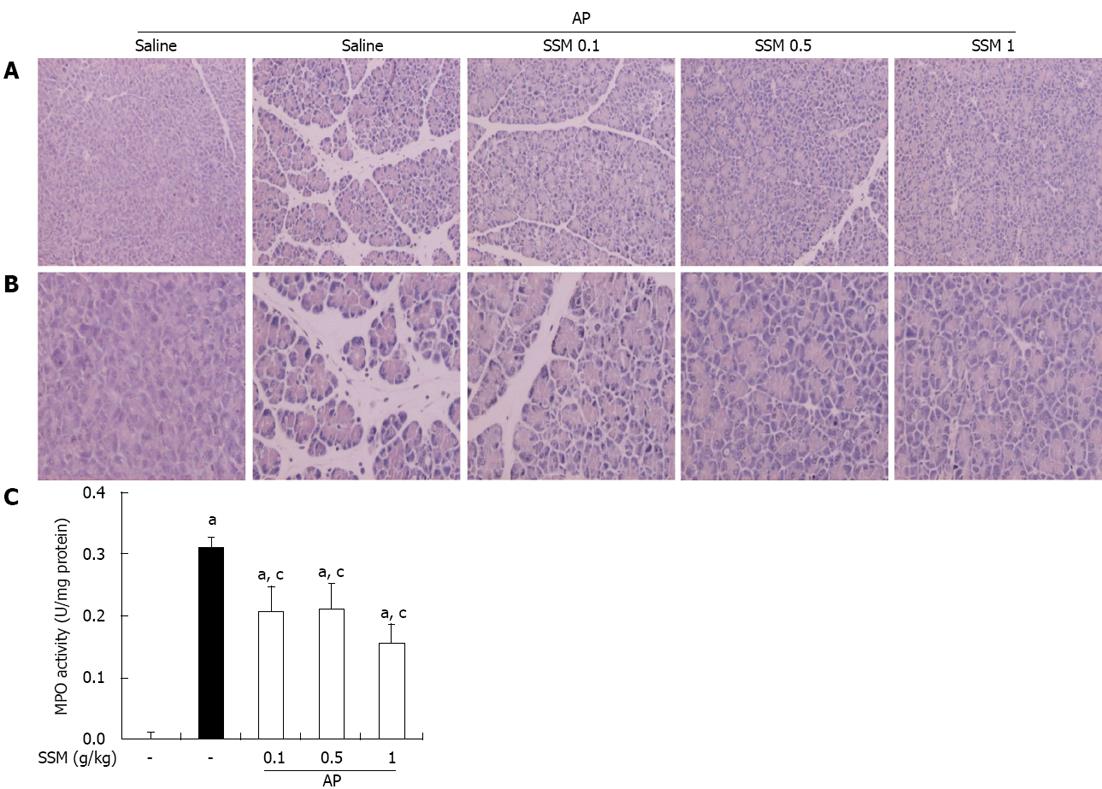
In saline-treated mice, the histological features of the pancreas showed typically normal architecture. Mice treated with intraperitoneal injections of cerulein developed AP. Histological examination of the pancreas (6 h after the final injection of cerulein) revealed tissue damage characterized by mild interstitial edema, inflammatory cell infiltration, vacuolization, and acinar cell necrosis. Compared to saline pre-treatment, SSM pre-treatment resulted in a significant reduction in pancreatic injury as shown by reduced edema, inflammation, vacuolization, and necrosis, in a dose-dependent manner (Figure 1A, B and Table 1).
| Group | Edema | Inflammation | Vacuolization | Necrosis |
| Saline | 0.1 ± 0.02 | 0.3 ± 0.05 | 0.1 ± 0.03 | 0.2 ± 0.02 |
| AP | 2.6 ± 0.03a | 2.8 ± 0.04a | 2.4 ± 0.02a | 2.5 ± 0.05a |
| SSM 0.1 + AP | 2.3 ± 0.05ac | 2.0 ± 0.02ac | 1.6 ± 0.04ac | 1.8 ± 0.01ac |
| SSM 0.5 + AP | 1.0 ± 0.02ac | 1.5 ± 0.05ac | 1.2 ± 0.02ac | 1.5 ± 0.04ac |
| SSM 1 + AP | 0.6 ± 0.01ac | 1.0 ± 0.03ac | 0.7 ± 0.05ac | 0.9 ± 0.03ac |
As an additional quantitative assessment of the severity of the inflammatory response, we measured MPO activity as an indicator of neutrophil sequestration in the pancreas, following the induction of AP. MPO activity in the pancreas of the SSM pre-treated AP mice was lesser than that in the pancreas of the saline pre-treated AP mice (Figure 1C).
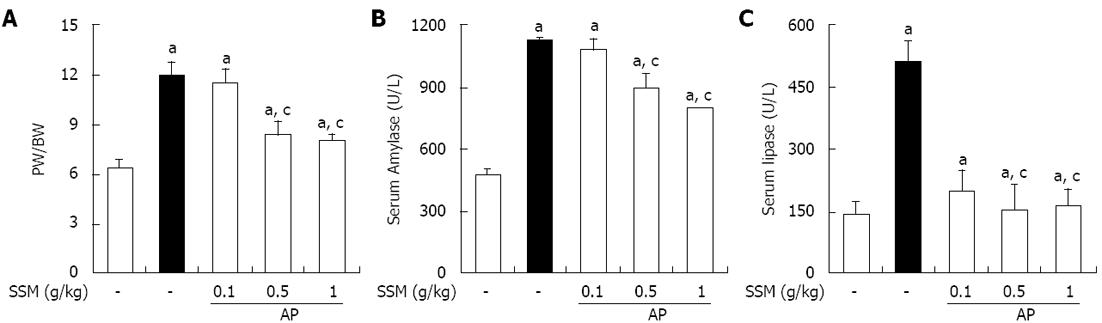
In order to assess the effect of SSM on pancreatic edema, the PW/BW was measured. As shown in Figure 2A, the PW/BW was increased in saline-treated mice with AP. SSM treatment, however, inhibited the AP-induced PW/BW ratio increase compared with the saline treated group (Figure 2A). Serum amylase and lipase levels are most commonly used biochemical markers of pancreatic disease, particularly in AP[19-21]. Therefore, we examined serum amylase and lipase levels during cerulein-induced AP. The administration of SSM significantly reduced the serum amylase and lipase levels (Figure 2B and C).
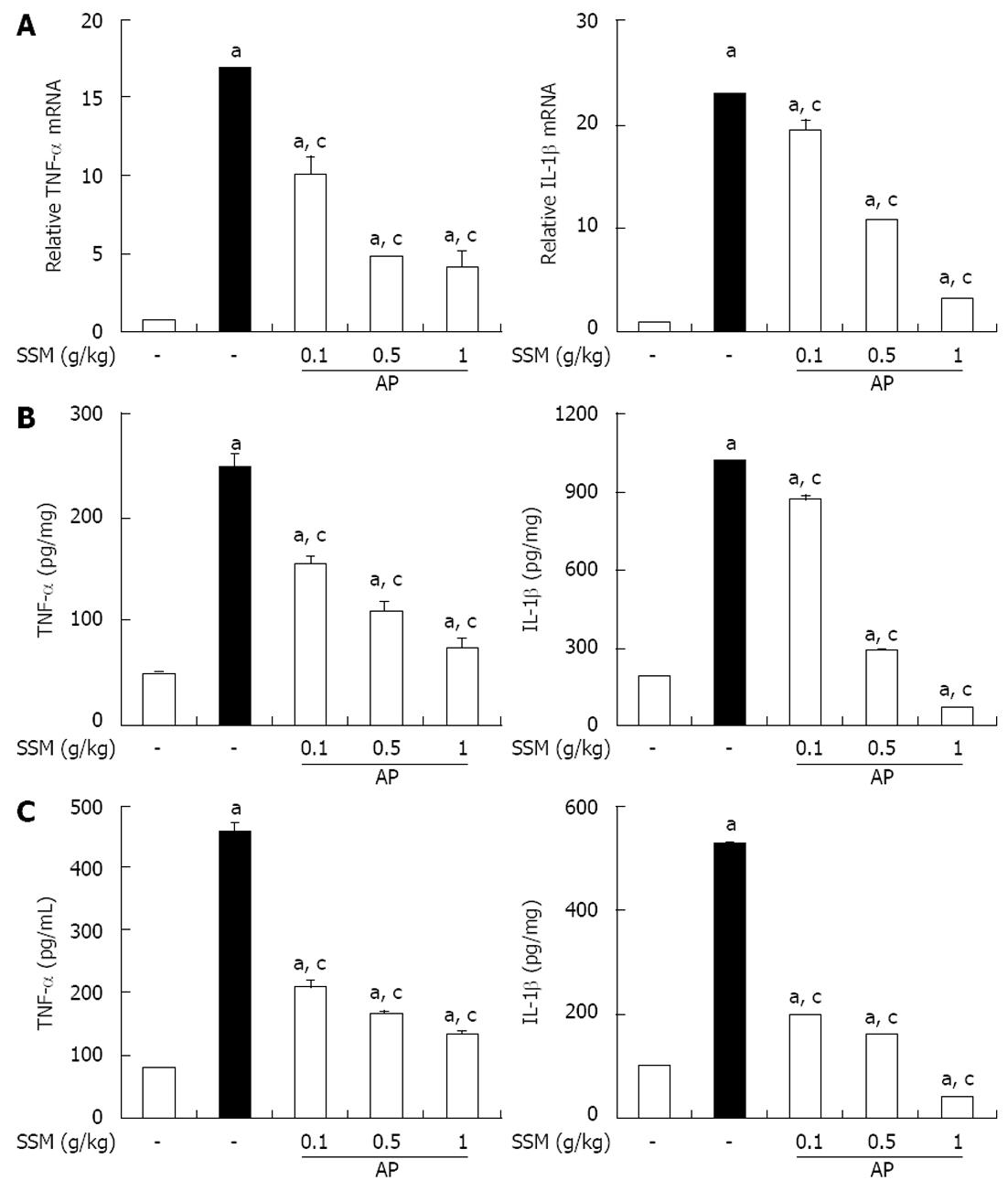
Several inflammatory mediators have been shown to increase during AP[22]. Therefore, to examine the effect of SSM on the occurrence of a systemic inflammatory response during cerulein-induced AP, we measured the level of TNF-α and IL-1β induction. Compared to control mice, mice with AP showed a significant increase in the levels of these inflammatory mediators in the pancreatic tissue and serum (Figure 3). However, SSM pre-treatment reduced the cytokine levels in both pancreatic tissue (Figure 3A and B) and serum (Figure 3C).
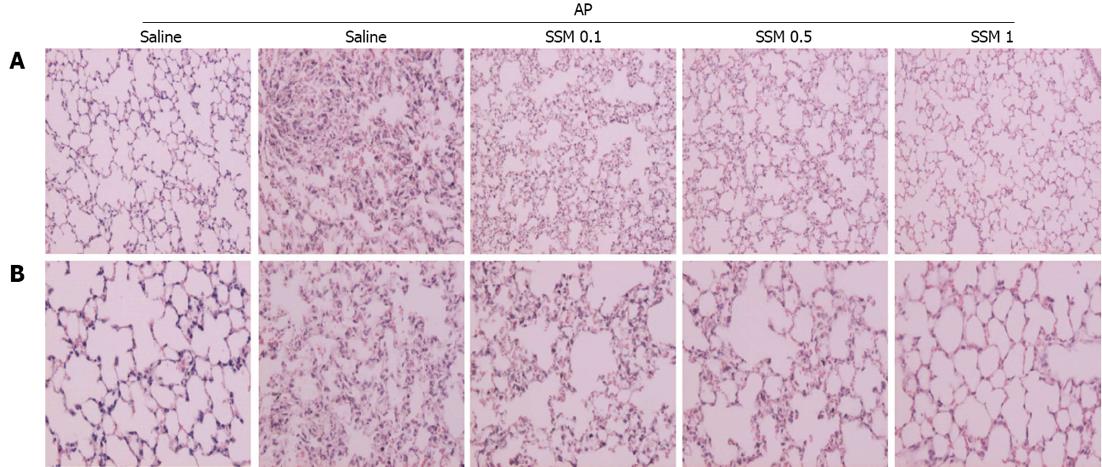
The lung is typically affected in cases of pancreatitis[23-25]. Lung injury, characterized by edema and inflammation, commonly develops early in AP[26]. Lungs from cerulein-induced AP show alveolar thickening and inflammatory cell infiltration[26]. However, these changes were significantly reduced in lungs from the SSM pre-treated group, and this effect was dose-dependent (Figure 4A, B and Table 2).
To measure the HMGB-1 expression, an IHC method was used. IHC analysis showed that HMGB-1 expression was detected in the pancreas by the presence of a brown color. As shown in Figure 5, HMGB-1 was slightly expressed in control mice, but strongly expressed in AP mice. However, compared to the saline pre-treated AP mice, SSM pre-treated AP mice showed a significant reduction in HMGB-1 expression in the pancreatic tissue (Figure 5).
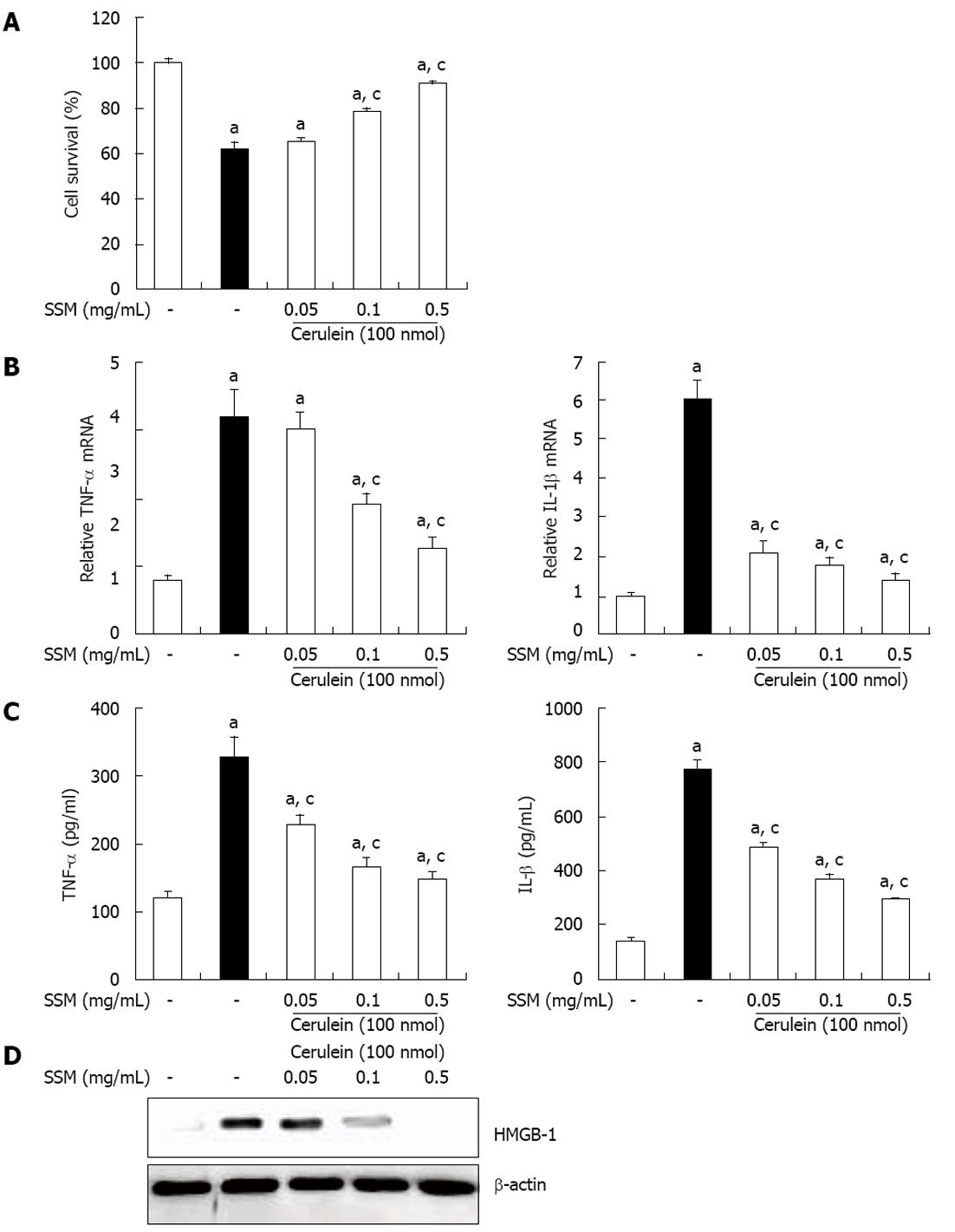
The local inflammation caused in pancreatic acinar cells results in acinar cells death and organ destruction[8]. Thus, acinar cells death can be a hallmark of AP. To assess whether SSM water extract inhibits acinar cells death, we evaluated cell viability by using the MTT assay. At 1 h after SSM pretreatment, cerulein was added for 6 h into cultured acinar cells. As shown in Figure 6A, the number of cerulein- induced acinar cells death was significantly reduced by SSM (Figure 6A). Next, we also examined cytokine production in isolated pancreatic acinar cells. Pretreatment with SSM inhibited the production of cytokines, such as TNF-α and IL-1β in a dose dependant (Figure 6C and D). In addition, SSM inhibited the cerulein-induced HMGB-1 expression, which means SSM protected the acinar cells necrosis (Figure 6E).
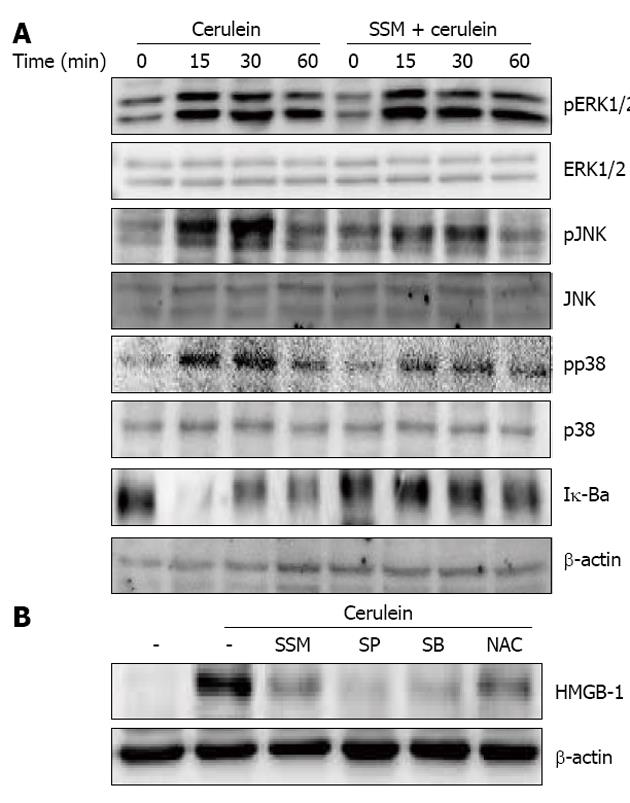
Further, to examine the inhibitory mechanism(s) against cerulein-induced responses in acinar cells, the activation of MAPKs and NF-κB were examined. We assessed the activation of MAPKs and NF-κB via phosphorylation and Iκ-Bα degradation, respectively. Cerulein treatment resulted in the phosphorylation of MAPKs and degradation of Iκ-Bα. However, SSM treatment inhibited the activation of c-Jun NH2-terminal kinase (JNK), p38, and the degradation of Iκ-Bα but not ERK1/2 (Figure 7A). To clarify whether down-regulation of the molecules in JNK, p38 and NF-κB by SSM is responsible for the reduced inflammatory responses, JNK inhibitor (SP600125), p38 inhibitor (SB239063) and NF-κB inhibitor (n-acetyl cystein; NAC) were used. The inhibition of JNK, p38 and NF-κB resulted in the reduction of HMGB-1 expression (Figure 7B).
SSM was analyzed by HPLC to characterize its main component. A chromatogram of SSM is shown in Figure 8. The peaks of the principal components of SSM have not yet been identified. Further studies to evaluate the principal components of SSM would be needed.
In this study, we have provided evidence that SSM water extract attenuated the development of cerulein-induced AP and AP-associated lung injury. Pre-treatment of mice with SSM significantly inhibited serum amylase and lipase production, TNF-α and IL-1β expression, and MPO activity. In addition, SSM pre-treatment inhibited HMGB-1 expression in the pancreas. In accordance with in vivo experiments, SSM inhibited the acinar cell death, cytokine productions, and HMGB-1 production. Furthermore, SSM inhibited the activation of JNK, p38 and NF-κB. These findings suggested that SSM protected the AP via JNK, p38 and NF-κB deactivation.
Recently, many studies have reported the anti-inflammatory activity of SSM. Wang et al[27] showed the protective effects of SSM on acute renal failure and multiple focal neuropathy, and Ren et al[28] reported the anti-inflammatory effects of SSM in Alzheimer’s disease. Therefore, to further investigate the anti-inflammatory activities of SSM, we selected to examine the effects of SSM in a cerulein-induced AP model, which has not previously been assessed. As we expected, SSM water extract significantly inhibited pancreatic and lung inflammation in a dose-dependent manner (Figures 1 and 4). We supposed that the anti-inflammatory effects of SSM on AP would be due to anti-microbial effects of SSM. Ren et al[17] reported that water soluble fraction of SSM could remove the all type of bacteria such as gram-positive, gram-negative bacteria and fungi. Because one of the main causes of AP would be bacterial infection[29,30], the removal ability of SSM would be helpful to protect AP. Thus, the anti-microbial ability of SSM might contribute to inhibition of pancreatic inflammation.
Amylase and lipase levels are used alone, or in combination, to diagnose patients with AP[31]. An increased level of serum amylase, at least 3 times over the normal limit, indicates AP. Amylase activity rises quickly during the early phase after the onset of symptoms and returns to normal quickly[31]. Serum amylase activities could reflect the exocrine pancreatic insufficiency, thus resulting in mal-digestion[32]. In comparison with serum amylase activity, serum lipase activity remains increased (up to 16-28 fold) for longer, thereby giving greater opportunity in patients with a delayed presentation. Pancreatic lipase activities are less likely to be affected by other environmental factors[33]. Thus, the serum amylase and lipase activities play a key role in determining the severity of AP. In this experiment, cerulein stimulation resulted in significant elevation in serum amylase and lipase levels. This increase was inhibited by SSM pre-treatment, suggesting that SSM is effective against the induction of AP (Figure 2).
The activation of inflammatory cells that release cytokines such as TNF-α and IL-1β is an important cascade in the pathogenesis of AP[34-36]. TNF-α and IL-1β are derived predominantly from activated macrophages and act via specific cell membrane-bound receptors. Levels of both these pro-inflammatory mediators are elevated on initiation of and during AP[37,38]. Intrapancreatic TNF-α and IL-1β can be detected 1 h after induction of AP, and the levels of these cytokines increase rapidly over the next 6 h[37,38]. Recently, many studies have reported that both TNF-α and IL-1β play an important role in AP[7,39]. In our experimental model of pancreatitis, the serum levels of TNF-α and IL-1β were elevated during AP. However, when mice were pre-treated with SSM water extract, this elevation of TNF-α and IL-1β was inhibited (Figures 3 and 6).
In this study, we examined the role of HMGB-1 as a late inflammatory mediator in AP. Generally, HMGB-1, a DNA-binding intranuclear protein, is known to be a late activator in the inflammatory cascade[10]. HMGB-1 has the capacity to induce cytokines and activate inflammatory cells when applied extracellularly[10]. This implicates that HMGB-1 is a pro-inflammatory mediator. Recent investigations reported that serum HMGB-1 levels increase in patients with sepsis/endotoxemia[40], hemorrhagic shock[41], acute lung injury[42], rheumatoid arthritis[43], and disseminated intravascular coagulation[44]. Similarly, many studies have shown the pivotal role of HMGB-1 plays in the development of pancreatic inflammation in AP[45-48]. In this study, compared to saline pre-treatment, SSM pre-treatment significantly inhibited AP-induced HMGB-1 expression (Figures 5A and 6).
Oxidative stress and pro-inflammatory cytokines trigger common signal transduction pathways involved in the inflammatory cascade, particularly through activation of MAPK[49]. We previously reported that the inhibition of MAPKs could inhibit the cytokine productions[7,8]. In the present study, acinar cells with cerulein showed increased TNF-α and IL-1β release via MAPKs and NF-κB activation. However, SSM treatment inhibited activation of JNK, p38 and NF-κB but not ERK1/2, consequently inhibiting cytokine release and HMGB-1 (Figures 6 and 7). In addition, we have shown here that cerulein-induced HMGB-1 expression was inhibited in pancreatic acinar cells by inhibition of JNK, p38 and NF-κB activation (Figure 7). These data suggest that SSM inhibits expression of HMGB-1 via inhibition of JNK, p38 and NF-κB activation.
In conclusion, this study shows that SSM attenuates the severity of cerulein-induced AP and pancreatitis-associated lung injury through the inhibition of tissue injury, pro-inflammatory cytokine production, and HMGB-1 expression. Therefore, SSM exerts potent anti-inflammatory effects in AP and could be a beneficial agent in the AP and its pulmonary complications.
Acute pancreatitis (AP) is a serious, unpredictable clinical problem, whose pathophysiology remains poorly understood. Therefore, drugs and therapies need to be developed.
Scolopendra subspinipes mutilans (SSM) is a venomous arthropod, which can be found throughout the world. SSM and its venom have been reported to exhibit many biochemical and physiological effects. In addition, SSM has been prescribed for the treatment of cardiovascular diseases in South Korea, China, and other Far Eastern Asian countries for several hundred years. However, the protective activities of SSM in cerulein-induced AP have not been examined to date. This study aimed to assess the protective effect of SSM in cerulein-induced AP.
Many studies have been tried to explore the possible candidate for treatment of acute pancreatitis (AP), but failed to find out. Nowad, the drug of AP is limited in protease inhibitors, and also the pathogenesis is not well-studied. In this paper, the authors studied the possible candidate to develop drug for AP, in line with their previous report. Also the authors provided the regulating mechanisms in AP. This finding could strengthen up the further studies of AP. Furthermore, this in vivo and in vitro studies would suggest that SSM could protect cerulein-induced AP via inhibiting high mobility group box chromosomal protein-1 release.
By understanding how SSM is effective in AP, these results could provide the clinical basis for development of drug or compound to treat AP and/or other inflammatory diseases.
AP is a sudden inflammation of the pancreas. It can have severe complications and high mortality despite treatment. While mild cases are often successfully treated with conservative measures and aggressive intravenous fluid rehydration, severe cases may require admission to the intensive care unit or even surgery to deal with complications of the disease process.
The role of SSM is mainly deleterious and also anti inflammatory and antimicrobial, what in literature has support about the protective effect of SSM in AP. The study is designed reasonably and the methods and the results seem mostly proper to show interesting protective effect of SSM on AP.
P- Reviewers Sumi S, Kumar A S- Editor Jiang L L- Editor A E- Editor Xiong L
| 1. | Zhou MT, Chen CS, Chen BC, Zhang QY, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16:2094-2099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 2. | Andersson R, Andersson B, Haraldsen P, Drewsen G, Eckerwall G. Incidence, management and recurrence rate of acute pancreatitis. Scand J Gastroenterol. 2004;39:891-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Ogawa M. Acute pancreatitis and cytokines: “second attack” by septic complication leads to organ failure. Pancreas. 1998;16:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 385] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Regnér S, Manjer J, Appelros S, Hjalmarsson C, Sadic J, Borgström A. Protease activation, pancreatic leakage, and inflammation in acute pancreatitis: differences between mild and severe cases and changes over the first three days. Pancreatology. 2008;8:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Büchler MW, Gloor B, Müller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 497] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 7. | Kim TH, Bae GS, Oh HJ, Kim MS, Park KC, Koo BS, Kim BJ, Yang YS, Park DE, Lee JH. 2’,4’,6’-Tris(methoxymethoxy) chalcone (TMMC) attenuates the severity of cerulein-induced acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2011;301:G694-G706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Bae GS, Kim MS, Jeong J, Lee HY, Park KC, Koo BS, Kim BJ, Kim TH, Lee SH, Hwang SY. Piperine ameliorates the severity of cerulein-induced acute pancreatitis by inhibiting the activation of mitogen activated protein kinases. Biochem Biophys Res Commun. 2011;410:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Yun SW, Bae GS, Kim MS, Park KC, Koo BS, Kim BJ, Kim TH, Seo SW, Shin YK, Lee SH. Melittin inhibits cerulein-induced acute pancreatitis via inhibition of the JNK pathway. Int Immunopharmacol. 2011;11:2062-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2694] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 11. | Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950-2954. [PubMed] |
| 12. | Agnello D, Wang H, Yang H, Tracey KJ, Ghezzi P. HMGB-1, a DNA-binding protein with cytokine activity, induces brain TNF and IL-6 production, and mediates anorexia and taste aversion. Cytokine. 2002;18:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Yang H, Wang H, Czura CJ, Tracey KJ. HMGB1 as a cytokine and therapeutic target. J Endotoxin Res. 2002;8:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Yang ZY, Ling Y, Yin T, Tao J, Xiong JX, Wu HS, Wang CY. Delayed ethyl pyruvate therapy attenuates experimental severe acute pancreatitis via reduced serum high mobility group box 1 levels in rats. World J Gastroenterol. 2008;14:4546-4550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Pemberton RW. Insects and other arthropods used as drugs in Korean traditional medicine. J Ethnopharmacol. 1999;65:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | You WK, Sohn YD, Kim KY, Park DH, Jang Y, Chung KH. Purification and molecular cloning of a novel serine protease from the centipede, Scolopendra subspinipes mutilans. Insect Biochem Mol Biol. 2004;34:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Ren WH, Zhang SQ, Song DX, Zhou KY. [Antibacterial activity of water soluble fraction from Scolopendra subspinipes mutilans]. Zhongyaocai. 2007;30:10-14. [PubMed] |
| 18. | Deng F, Fang H, Wang K. [Hemolysis of Scolopendra toxins]. Zhong Yao Cai. 1997;20:36-37. [PubMed] |
| 19. | Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 323] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Kaiser AM, Saluja AK, Steer ML. Repetitive short-term obstructions of the common bile-pancreatic duct induce severe acute pancreatitis in the opossum. Dig Dis Sci. 1999;44:1653-1661. [PubMed] |
| 21. | Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 597] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 22. | Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117-125. [PubMed] |
| 23. | Gerard C, Frossard JL, Bhatia M, Saluja A, Gerard NP, Lu B, Steer M. Targeted disruption of the beta-chemokine receptor CCR1 protects against pancreatitis-associated lung injury. J Clin Invest. 1997;100:2022-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 177] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Osman MO, Kristensen JU, Jacobsen NO, Lausten SB, Deleuran B, Deleuran M, Gesser B, Matsushima K, Larsen CG, Jensen SL. A monoclonal anti-interleukin 8 antibody (WS-4) inhibits cytokine response and acute lung injury in experimental severe acute necrotising pancreatitis in rabbits. Gut. 1998;43:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Ishibashi T, Zhao H, Kawabe K, Oono T, Egashira K, Suzuki K, Nawata H, Takayanagi R, Ito T. Blocking of monocyte chemoattractant protein-1 (MCP-1) activity attenuates the severity of acute pancreatitis in rats. J Gastroenterol. 2008;43:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Gross V, Leser HG, Heinisch A, Schölmerich J. Inflammatory mediators and cytokines--new aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology. 1993;40:522-530. [PubMed] |
| 27. | Wang IK, Hsu SP, Chi CC, Lee KF, Lin PY, Chang HW, Chuang FR. Rhabdomyolysis, acute renal failure, and multiple focal neuropathies after drinking alcohol soaked with centipede. Ren Fail. 2004;26:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Ren Y, Houghton P, Hider RC. Relevant activities of extracts and constituents of animals used in traditional Chinese medicine for central nervous system effects associated with Alzheimer’s disease. J Pharm Pharmacol. 2006;58:989-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Capurso G, Zerboni G, Signoretti M, Valente R, Stigliano S, Piciucchi M, Delle Fave G. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol. 2012;46 Suppl:S46-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Aydin S, Isik AT, Unal B, Comert B, Ozyurt M, Deveci S, Ozgur G, Cengiz O, Tasci I, Mas MR. Effects of infliximab on bacterial translocation in experimental acute necrotizing pancreatitis. Indian J Med Res. 2012;135:656-661. [PubMed] |
| 31. | Matull WR, Pereira SP, O’Donohue JW. Biochemical markers of acute pancreatitis. J Clin Pathol. 2006;59:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Smotkin J, Tenner S. Laboratory diagnostic tests in acute pancreatitis. J Clin Gastroenterol. 2002;34:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Tietz NW, Shuey DF. Lipase in serum--the elusive enzyme: an overview. Clin Chem. 1993;39:746-756. [PubMed] |
| 34. | Leser HG, Gross V, Scheibenbogen C, Heinisch A, Salm R, Lausen M, Rückauer K, Andreesen R, Farthmann EH, Schölmerich J. Elevation of serum interleukin-6 concentration precedes acute-phase response and reflects severity in acute pancreatitis. Gastroenterology. 1991;101:782-785. [PubMed] |
| 35. | Sun J, Bhatia M. Blockade of neurokinin-1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2007;292:G143-G153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Gukovsky I, Pandol SJ, Gukovskaya AS. Organellar dysfunction in the pathogenesis of pancreatitis. Antioxid Redox Signal. 2011;15:2699-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Fink GW, Norman JG. Specific changes in the pancreatic expression of the interleukin 1 family of genes during experimental acute pancreatitis. Cytokine. 1997;9:1023-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Norman JG, Fink GW, Franz MG. Acute pancreatitis induces intrapancreatic tumor necrosis factor gene expression. Arch Surg. 1995;130:966-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 149] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Denham W, Yang J, Fink G, Denham D, Carter G, Ward K, Norman J. Gene targeting demonstrates additive detrimental effects of interleukin 1 and tumor necrosis factor during pancreatitis. Gastroenterology. 1997;113:1741-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 930] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 41. | Ombrellino M, Wang H, Ajemian MS, Talhouk A, Scher LA, Friedman SG, Tracey KJ. Increased serum concentrations of high-mobility-group protein 1 in haemorrhagic shock. Lancet. 1999;354:1446-1447. [PubMed] |
| 42. | Mollnes TE. High mobility group box-1 protein--one step closer to the clinic? Crit Care. 2008;12:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48:971-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 384] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 44. | Hatada T, Wada H, Nobori T, Okabayashi K, Maruyama K, Abe Y, Uemoto S, Yamada S, Maruyama I. Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost. 2005;94:975-979. [PubMed] |
| 45. | Zhang ZW, Zhang QY, Zhou MT, Liu NX, Chen TK, Zhu YF, Wu L. Antioxidant inhibits HMGB1 expression and reduces pancreas injury in rats with severe acute pancreatitis. Dig Dis Sci. 2010;55:2529-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 47. | Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666-7670. [PubMed] |
| 48. | Yuan H, Jin X, Sun J, Li F, Feng Q, Zhang C, Cao Y, Wang Y. Protective effect of HMGB1 a box on organ injury of acute pancreatitis in mice. Pancreas. 2009;38:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |