Published online Jan 7, 2013. doi: 10.3748/wjg.v19.i1.72
Revised: September 17, 2012
Accepted: September 22, 2012
Published online: January 7, 2013
AIM: To evaluate the chemotherapeutic outcomes and confirm the recent improvement of prognosis for unresectable biliary tract cancer.
METHODS: A total of 186 consecutive patients with unresectable biliary tract cancer, who had been treated with chemotherapy between 2000 and 2009 at five institutions in Japan, were retrospectively analyzed. These patients were divided into three groups based on the year beginning chemotherapy: Group A (2000-2003), Group B (2004-2006), and Group C (2007-2009). The data were fixed at the end of December 2011. Overall survival and time-to-progression were analyzed and compared chronologically.
RESULTS: No patient characteristics were significantly different among the three groups. The gallbladder was involved in about half of the patients in each group, and metastatic biliary tract cancer was present in three quarters of the enrollees. In Group A, 5-fluorouracil-based chemotherapies were primarily selected as first-line chemotherapy, and only 24% were treated with second-line chemotherapy. In Group B, gemcitabine or S-1 monotherapy was mainly introduced as first-line chemotherapy, and 51% of the patients who were refractory to first-line chemotherapy were treated with second-line chemotherapy mainly with monotherapy. In Group C, the combination therapy with gemcitabine and S-1 was mainly chosen as first-line chemotherapy, and 53% of the patients refractory to first-line chemotherapy were treated with second-line chemotherapy mainly with combination therapy. The median time-to-progressions were 4.4 mo, 3.5 mo and 5.9 mo in Groups A, B and C, respectively (4.4 mo vs 3.5 mo vs 5.9 mo, P < 0.01). The median overall survivals were 7.1, 7.3, and 11.7 mo in Groups A, B and C (7.1 mo vs 7.3 mo vs 11.7 mo, P = 0.03). Induction rates of all three drugs (gemcitabine, platinum analogs, and fluoropyrimidine) in Groups A, B and C were 4%, 2% and 27% (4% vs 2% vs 27%, P < 0.01).
CONCLUSION: The prognosis of unresectable biliary tract cancer has improved recently. Using three effective drugs (gemcitabine, platinum analogs, and fluoropyrimidine) may improve the prognosis of this cancer.
- Citation: Sasaki T, Isayama H, Nakai Y, Takahara N, Sasahira N, Kogure H, Mizuno S, Yagioka H, Ito Y, Yamamoto N, Hirano K, Toda N, Tada M, Omata M, Koike K. Improvement of prognosis for unresectable biliary tract cancer. World J Gastroenterol 2013; 19(1): 72-77
- URL: https://www.wjgnet.com/1007-9327/full/v19/i1/72.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i1.72
Biliary tract cancer (BTC) is the sixth leading cause of cancer-related death in Japan according to “Cancer Statistics in Japan (2009)”. Surgery is still the only treatment that can cure this life-threatening disease. However, many patients are diagnosed at an advanced stage of disease. Therefore, chemotherapy is indispensable for the treatment of advanced BTC.
In the 21st century, many clinical trials have been conducted to investigate the use of chemotherapy for advanced BTC[1]. Regimens involving 5-fluorouracil were widely used around the year 2000. However, gemcitabine has now become a key drug based on the results of several retrospective analyses[2,3]. In Japan, gemcitabine and S-1 (an oral fluoropyrimidine) have been approved and are widely used for the treatment of BTC[4-8]. Combination therapy using gemcitabine and S-1 has produced promising results in phase II studies and is considered as one of the regimen for advanced BTC, particularly in Japan[9,10]. Meanwhile, combination therapy with gemcitabine and cisplatin has become a standard chemotherapy regimen based on the results of recent randomized controlled studies reported in 2009[11,12].
BTC include the cancers of several biliary sites, therefore the results might have strongly been affected by the patient characteristics enrolled in the clinical trial. Moreover, most of these past trials also included both unresectable and recurrent cases; however, the prognoses of these two conditions are extremely different[12]. One of the reasons for this difference might be that the tumor volume of recurrent cases was usually smaller than that of unresectable cases. We have previously reported that tumor volume affects both the tumor response and prognosis in advanced BTC patients who received chemotherapy[13]. Another reason might be that the tolerance of chemotherapeutic agents was usually poorer in recurrent cases than in unresectable cases. It is sometimes difficult to treat recurrent cases with the same treatment dose and schedule as unresectable cases because metabolism of the anti-cancer agent is often influenced by major surgery[14]. Therefore, it is better to separate these two conditions when the treatment outcomes of chemotherapy for advanced BTC are analyzed. Thus, we conducted a retrospective study to clarify the treatment outcomes of chemotherapy and confirm the recent improvement of prognosis for the treatment of unresectable BTC patients.
A total of 186 patients with unresectable BTC, who had been treated with chemotherapy between 2000 and 2009 at five institutions in Japan, were retrospectively analyzed. The patients were divided into three groups: the patients who started chemotherapy between 2000 and 2003 were enrolled as Group A, the patients who started chemotherapy between 2004 and 2006 were enrolled as Group B and the patients who started chemotherapy between 2007 and 2009 were enrolled as Group C. The eligibility criteria of this retrospective study were as follows: (1) no previous chemotherapy; (2) an Eastern Cooperative Oncology Group performance score of 0 to 3; (3) adequate bone marrow function (white blood cell count > 3000/mm3, hemoglobin > 9.0 g/dL and platelet count > 100 000/mm3), liver function [total bilirubin < three times the upper limit of normal (ULN) and aspartate/alanine transaminase levels < five times the ULN] and renal function (creatinine < 1.2 mg/dL or creatinine clearance > 50 mL/min); (4) no serious complications; (5) no uncontrolled infections; and (6) written informed consent from the patient.
First-line chemotherapy: Because there was no standard chemotherapy or efficacious key drug for advanced BTC, several 5-fluorouracil-based chemotherapies were selected between 2000 and 2003 (Group A). Chemoradiation was primarily selected in cases of locally advanced BTC. The treatment regimen was chosen by the attending doctor during this period. Starting in the middle of 2004, gemcitabine monotherapy (1000 mg/m2, days 1, 8, 15, q 4 wk) was introduced. Several clinical trials have been conducted since 2005. First, a feasibility study of S-1 monotherapy (80 mg/m2, days 1-28, q 6 wk) was started in 2005[7]. A multicenter phase II study of gemcitabine and S-1 combination therapy (gemcitabine: 1000 mg/m2, days 1, 15; S-1: 80 mg/m2, days 1-14, q 4 wk) started to enroll participants in 2007[9]. Furthermore, a randomized phase II study comparing gemcitabine and S-1 combination therapy (gemcitabine: 1000 mg/m2, days 1, 15; S-1: 80 mg/m2, days 1-14, q 4 wk) with gemcitabine monotherapy (1000 mg/m2, days 1, 8, 15, q 4 wk) started to enroll participants at the end of 2008. After the completion of this randomized study, gemcitabine and S-1 combination therapy became the first choice for clinical practice.
Second-line chemotherapy: Only certain patients were treated with second-line chemotherapy between 2000 and 2003 (Group A). Several clinical trials of second-line chemotherapy have also been conducted since 2007. A multicenter phase II study of S-1 monotherapy (80 mg/m2, days 1-28, q 6 wk) as a second-line chemotherapy started to enroll participants in 2007[8]. Moreover, a feasibility study of gemcitabine and cisplatin combination therapy (gemcitabine: 1000 mg/m2, days 1, 8; cisplatin: 25 mg/m2, days 1, 8, q 3 wk) for refractory BTC was started in 2008[15]. Recently, gemcitabine and cisplatin combination therapy are chosen as second-line chemotherapy in clinical trial.
The Mann-Whitney U test was used to compare quantitative variables. The time to progression and overall survival were calculated using the Kaplan-Meier method. The time to progression was calculated from the start of the treatment to the first date of documented disease progression. The overall survival was defined as the time from the initiation of therapy to the final follow-up or until death from any cause. The final analysis was conducted using the follow-up data through the end of December 2011 to get enough follow-up periods. The JMP 8.0 statistical software program (SAS Institute Inc., Cary, NC, United States) was used for all statistical analyses.
A total of 186 patients with unresectable BTC were treated with chemotherapy between January 2000 and December 2009. Patient characteristics are shown in Table 1. The number of patients in Group A (2000-2003), B (2004-2006) and C (2007-2009) was 25, 54 and 107, respectively. No patient characteristics were significantly different among the three groups, although the median age of each group became slightly higher with time. There were no differences in either the primary biliary site or disease status. The gallbladder was involved in about half of the patients in each group and metastatic BTC was present in three quarters of the enrollees in each group.
| Group A | Group B | Group C | P value | |
| Number of patients | 25 | 54 | 107 | |
| Age (yr) | 0.08 | |||
| Median | 64 | 66 | 68 | |
| Range | 47-81 | 35-84 | 24-89 | |
| Gender | 0.20 | |||
| Male | 11 (44) | 28 (52) | 66 (62) | |
| Female | 14 (56) | 26 (48) | 41 (38) | |
| ECOG performance score | 0.12 | |||
| 0 | 10 (40) | 13 (24) | 46 (43) | |
| 1 | 12 (48) | 37 (69) | 50 (47) | |
| 2-3 | 3 (12) | 4 (7) | 11 (10) | |
| Primary biliary site | 0.47 | |||
| Gallbladder | 14 (56) | 22 (41) | 46 (43) | |
| Intra-hepatic bile duct | 5 (20) | 21 (39) | 40 (37) | |
| Extra-hepatic bile duct | 6 (24) | 9 (16) | 20 (19) | |
| Ampulla of Vater | 0 (0) | 2 (4) | 1 (1) | |
| Disease status | 0.76 | |||
| Locally advanced | 6 (24) | 15 (28) | 24 (22) | |
| Metastatic | 19 (76) | 39 (72) | 83 (78) |
Group A (2000-2003): The regimens for first-line and second-line chemotherapy are listed in Table 2. As first-line chemotherapy, 5-fluorouracil-based chemotherapies were primarily selected during this period (sixteen patients; 64%). Eight patients (32%) were treated with chemoradiation and intra-arterial infusions were delivered to four patients (16%). In this treatment group, all of the patients progressed with first-line chemotherapy. Only six patients (24%) were treated with second-line chemotherapy; two patients were treated with gemcitabine monotherapy and one patient was treated with S-1 monotherapy, uracil-ftorafur monotherapy, 5-fluorouracil-based chemoradiation and intra-arterial infusion of gemcitabine. Of all, only one patient (4%) was treated with all three effective drugs (gemcitabine, platinum analogs and fluoropyrimidine) in this group.
| First-line treatment | |
| Group A (n = 25) | |
| 5-fluorouracil + radiation | 5 |
| 5-fluorouracil ia + radiation | 2 |
| 5-fluorouracil + cisplatin + radiation | 1 |
| 5-fluorouracil ia | 1 |
| 5-fluorouracil + doxorubicin + mitomycin C ia | 1 |
| 5-fluorouracil + cisplatin | 3 |
| 5-fluorouracil + doxorubicin + mitomycin C | 1 |
| Uracil-tegafur | 2 |
| Gemcitabine | 9 |
| Group B (n = 54) | |
| 5-fluorouracil + radiation | 2 |
| 5-fluorouracil ia + radiation | 2 |
| Uracil-tegafur + radiation | 1 |
| 5-fluorouracil | 1 |
| 5-fluorouracil + doxorubicin + mitomycin C | 2 |
| Gemcitabine | 25 |
| S-1 | 20 |
| Gemcitabine + cisplatin | 1 |
| Group C (n = 107) | |
| Gemcitabine + S-1 | 62 |
| Gemcitabine | 44 |
| S-1 | 1 |
| Second-line treatment | |
| Group A (n = 6) | |
| Gemcitabine | 2 |
| Gemcitabine ia | 1 |
| 5-fluorouracil + radiation | 1 |
| S-1 | 1 |
| Uracil-tegafur | 1 |
| Group B (n = 27) | |
| Gemcitabine | 14 |
| S-1 | 9 |
| S-1 + interferon-α | 1 |
| 5-fluorouracil | 2 |
| Mitomycin C | 1 |
| Group C (n = 51) | |
| Gemcitabine + cisplatin | 28 |
| Gemcitabine | 6 |
| S-1 | 13 |
| Cisplatin ia | 1 |
| 5-fluorouracil + interferon-α | 1 |
| Irinotecan | 1 |
| Uracil-tegafur | 1 |
Group B (2004-2006): During this period, gemcitabine or S-1 monotherapy was primarily selected for first-line chemotherapy (83%); twenty-five patients (46%) underwent gemcitabine monotherapy and twenty patients (37%) underwent S-1 monotherapy. In this treatment group, fifty-three patients progressed with first-line chemotherapy. Of the patients who were refractory to first-line chemotherapy, twenty-seven patients (51%) were treated with second-line chemotherapy, fourteen patients were treated with gemcitabine monotherapy and nine patients were treated with S-1 monotherapy. Sequential monotherapy of gemcitabine and S-1 was introduced during this period. Of all, only one patient (2%) was treated with all three effective drugs (gemcitabine, platinum analogs and fluoropyrimidine) in this group.
Group C (2007-2009): As first-line chemotherapy, combination therapy with gemcitabine and S-1 was introduced and used predominantly during this period (in 58% of patients). In this treatment group, ninety-nine patients progressed with first-line chemotherapy. Of these patients, fifty-two (53%) were treated with second-line chemotherapy; twenty-eight patients were treated with gemcitabine and cisplatin combination therapy, six patients were treated with gemcitabine monotherapy and thirteen patients were treated with S-1 monotherapy. During this period, sequential combination therapy using gemcitabine, S-1 and cisplatin was primarily prescribed. Of all, twenty-nine patients (27%) were treated with all three effective drugs (gemcitabine, platinum analogs and fluoropyrimidine) in this group.
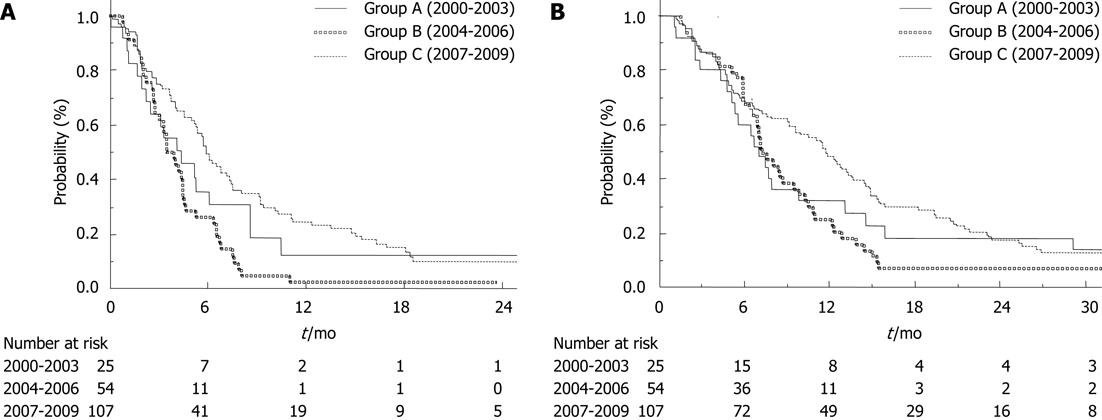
The median times-to-progression in Groups A, B and C were 4.4 mo (95% CI, 2.0-8.6 mo), 3.5 mo (95% CI, 2.8-4.5 mo) and 5.9 mo (95% CI, 5.2-7.3 mo), respectively (log-rank test; P < 0.01) (Figure 1A). There was no difference between Groups A and B (log-rank test; P = 0.20). The median overall survival times in Groups A, B and C were 7.1 mo (95% CI, 5.1-13.1 mo), 7.3 mo (95% CI, 6.6-9.5 mo) and 11.7 mo (95% CI, 9.2-13.7 mo), respectively (log-rank test; P = 0.03) (Figure 1B). There was also no difference between Groups A and B (log-rank test; P = 0.41).
After assessing the treatment outcomes of each primary biliary site, the median overall survival for patients with gallbladder cancer (n = 46), intrahepatic cholangiocarcinoma (n = 40) and extrahepatic cholangiocarcinoma (n = 20) in Group C (2007-2009) was 9.1 mo (95% CI, 5.2-12.5 mo), 12.4 mo (95% CI, 6.7-19.4 mo) and 14.9 mo (95% CI, 11.1 mo - not reached), respectively (log-rank test; P = 0.049).
Many clinical trials investigating the use of chemotherapy for advanced BTC have been reported since 2000, although most of these previous trials included both unresectable and recurrent cases[16]. However, the prognoses of these two conditions are extremely different[12]. In BT-22 study, the median overall survivals of unresectable and recurrent BTC, who were treated with the combination therapy of gemcitabine and cisplatin, were 9.4 mo and 16.1 mo, respectively. Thus, the patient characteristics strongly affect the results of clinical trial for advanced BTC, though most previous trial did not report the detail about the prognosis of unresectable BTC. In our retrospective study, we ascertained that the prognosis for unresectable BTC has actually improved in recent years (Group C; 2007-2009) and the median overall survival has reached approximately one year. We also confirmed that the prognoses for unresectable BTC at several biliary sites are actually different and that the prognosis for unresectable gallbladder cancer is still extremely poor.
The median overall survival of Group C (2007-2009) was 11.7 mo (95% CI, 9.2-13.7 mo). The difference between the median overall survival and the median time to progression increased with time (Group A, 2.7 mo; Group B, 3.8 mo; Group C, 5.8 mo). The rate of second-line chemotherapy also increased in Group B (51%) and Group C (53%). However, greater improvement was observed only in Group C, which may have been caused by the use of three effective agents (gemcitabine, platinum analogs and fluoropyrimidine). In fact, induction rates of these three effective drugs in Groups A, B and C were 4%, 2% and 27% (P < 0.01). Therefore, using three effective drugs (gemcitabine, platinum analogs and fluoropyrimidine) may improve the prognosis of unresectable BTC.
One limitation of this retrospective study is that the regimens in each group were not uniform. However, the improvement of prognosis for unresectable BTC in clinical settings was confirmed because all of the patients who underwent chemotherapy in the past ten years were included in this retrospective study. Another limitation is that gemcitabine and S-1 had mainly been used for first-line chemotherapy because the efficacy of gemcitabine and cisplatin combination therapy was reported in 2009.
In conclusion, the use of chemotherapy for unresectable BTC has made recent advances and the median overall survival has reached approximately one year. However, the prognosis of unresectable gallbladder cancer is still exceedingly poor. Using three effective drugs (gemcitabine, platinum analogs and fluoropyrimidine) may improve the prognosis of unresectable BTC. Future assessments are needed to establish the best strategy for using these three effective drugs (gemcitabine, platinum analogs and fluoropyrimidine) for the treatment of unresectable BTC.
In addition to the authors listed in the author field, following are the authors who contributed equally to this study. Kouji Miyabayashi, Keisuke Yamamoto, Dai Mohri, Kazumichi Kawakubo, Osamu Togawa and Saburo Matsubara from Department of Gastroenterology, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan; Makoto Okamoto from Department of Gastroenterology, JR Tokyo General Hospital, Tokyo, Japan; Rie Uchino, Tateo Kawase from Department of Gastroenterology, Kanto Central Hospital, Tokyo, Japan; Tsuyoshi Hamada, Yoko Yashima, Takeshi Tsujino, Ryo Nakata from Department of Gastroenterology, Japanese Red Cross Medical Center, Tokyo, Japan; Toshihiko Arizumi, Kazumi Tagawa from Department of Gastroenterology, Mitsui Memorial Hospital, Tokyo, Japan.
Biliary tract cancer (BTC) is a rare cancer worldwide. Obstructive jaundice and infection to the biliary system usually become obstacles to introduce chemotherapy. Thus, enrollment of clinical trial for advanced biliary tract cancer is more difficult than other common cancers.
Previous clinical trials investigating the efficacy of chemotherapy for advanced biliary tract cancer enrolled both unresectable and recurrent cases. Recent reports showed the differences of prognoses between these two conditions. However, the details about the prognoses of each condition have not been evaluated sufficiently.
Although the prognoses of unresectable and recurrent cases were extremely different, previous studies included both conditions. Moreover, most of these previous reports only showed the overall efficacies. Therefore, the treatment outcomes of unresectable biliary tract cancer were not fully evaluated. In our retrospective analysis, they only included unresectable biliary tract cancer and confirmed the recent improvement of prognosis in patients receiving chemotherapy.
The prognosis of unresectable biliary tract cancer has improved recently and the median overall survival has reached approximately one year. Using three effective drugs (gemcitabine, platinum analogs, and fluoropyrimidine) may improve the prognosis of unresectable biliary tract cancer. Future assessments are needed to establish the best strategy for using these three effective drugs (gemcitabine, platinum analogs, and fluoropyrimidine) for the treatment of unresectable biliary tract cancer.
Biliary tract cancer includes cholangiocarcinoma, gallbladder cancer, and ampullary carcinoma. 5-fluorouracil is a fluoropyrimidine analog used for chemotherapy. Gemcitabine is a nucleoside analog used for chemotherapy. S-1 is an oral fluoropyrimidine widely used in Japan. Cisplatin is a platinum alalog used for chemotherapy.
This paper evaluates the chemotherapeutic outcomes of patients affected by unresectable BTC. The study retrospectively analyzed 186 consecutive patients with unresectable BTC between 2000 and 2009 at five institutions in Japan. The Authors concluded that the prognosis of unresectable BTC has improved during the years and reached approximately one year. Using three effective drugs in combination (gemcitabine, platinum analogs and fluoropyrimidine) could improve the prognosis of unresectable BTC. The authors possess an expertise about the topic since published some interesting papers about the treatment of biliary and pancreatic cancer. Moreover, this paper is well written and also the number of patients is relevant.
P- Reviewers Fava G, Nath G S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Tada M, Nakai Y, Sasaki T, Hamada T, Nagano R, Mohri D, Miyabayashi K, Yamamoto K, Kogure H, Kawakubo K. Recent progress and limitations of chemotherapy for pancreatic and biliary tract cancers. World J Clin Oncol. 2011;2:158-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896-902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 351] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | Yonemoto N, Furuse J, Okusaka T, Yamao K, Funakoshi A, Ohkawa S, Boku N, Tanaka K, Nagase M, Saisho H. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol. 2007;37:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Okusaka T, Ishii H, Funakoshi A, Yamao K, Ohkawa S, Saito S, Saito H, Tsuyuguchi T. Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2006;57:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C. Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer. 2004;91:1769-1774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Furuse J, Okusaka T, Boku N, Ohkawa S, Sawaki A, Masumoto T, Funakoshi A. S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol. 2008;62:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Sasaki T, Isayama H, Yashima Y, Yagioka H, Kogure H, Arizumi T, Togawa O, Matsubara S, Ito Y, Nakai Y. S-1 monotherapy in patients with advanced biliary tract cancer. Oncology. 2009;77:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Sasaki T, Isayama H, Nakai Y, Mizuno S, Yamamoto K, Yagioka H, Yashima Y, Kawakubo K, Kogure H, Togawa O. Multicenter phase II study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine. Invest New Drugs. 2012;30:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Sasaki T, Isayama H, Nakai Y, Ito Y, Kogure H, Togawa O, Toda N, Yasuda I, Hasebe O, Maetani I. Multicenter, phase II study of gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2010;65:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Kanai M, Yoshimura K, Tsumura T, Asada M, Suzuki C, Niimi M, Matsumoto S, Nishimura T, Nitta T, Yasuchika K. A multi-institution phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2011;67:1429-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3148] [Article Influence: 209.9] [Reference Citation Analysis (1)] |
| 12. | Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, Nagino M, Kondo S, Nagaoka S, Funai J. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 570] [Cited by in RCA: 565] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 13. | Sasaki T, Isayama H, Nakai Y, Togawa O, Kogure H, Ito Y, Yamamoto K, Mizuno S, Yagioka H, Yashima Y. Prognostic factors in patients with advanced biliary tract cancer receiving chemotherapy. Cancer Chemother Pharmacol. 2011;67:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Muneoka K, Shirai Y, Sasaki M, Wakai T, Sakata J, Kanda J, Wakabayashi H, Hatakeyama K. [Effect of pylorus-preserving pancreaticoduodenectomy on serum levels of 5-fluorouracil during S-1 treatment for pancreaticobiliary malignancy]. Gan To Kagaku Ryoho. 2010;37:1503-1506. [PubMed] |
| 15. | Sasaki T, Isayama H, Nakai Y, Mizuno S, Yamamoto K, Yagioka H, Yashima Y, Kawakubo K, Kogure H, Togawa O. Feasibility study of gemcitabine and cisplatin combination chemotherapy for patients with refractory biliary tract cancer. Invest New Drugs. 2011;29:1488-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |