Published online Jan 7, 2013. doi: 10.3748/wjg.v19.i1.42
Revised: July 31, 2012
Accepted: August 3, 2012
Published online: January 7, 2013
Abnormal vasculature, termed tumor vessels, is a hallmark of solid tumors. The degree of angiogenesis is associated with tumor aggressiveness and clinical outcome. Therefore, exact quantification of tumor vessels is useful to evaluate prognosis. Furthermore, selective detection of newly formed tumor vessels within cancer tissues using specific markers raises the possibility of molecular targeted therapy via the inhibition of tumor angiogenesis. Nestin, an intermediate filament protein, is reportedly expressed in repair processes, various neoplasms, and proliferating vascular endothelial cells. Nestin expression is detected in endothelial cells of embryonic capillaries, capillaries of the corpus luteum, which replenishes itself by angiogenesis, and proliferating endothelial progenitor cells, but not in mature endothelial cells. Therefore, expression of nestin is relatively limited to proliferating vascular endothelial cells and endothelial progenitor cells. Nestin expression is also reported in blood vessels within glioblastoma, prostate cancer, colorectal cancer, and pancreatic cancer, and its expression is more specific for newly formed blood vessels than other endothelial cell markers. Nestin-positive blood vessels form smaller vessels with high proliferation activity in tumors. Knockdown of nestin in vascular endothelial cells suppresses endothelial cell growth and tumor formation ability of pancreatic cancers in vivo. Using nestin to more accurately evaluate microvessel density in cancer specimens may be a novel prognostic indicator. Furthermore, nestin-targeted therapy may suppress tumor proliferation via inhibition of angiogenesis in numerous malignancies, including pancreatic cancer. In this review article, we focus on nestin as a novel angiogenesis marker and possible therapeutic target via inhibition of tumor angiogenesis.
- Citation: Matsuda Y, Hagio M, Ishiwata T. Nestin: A novel angiogenesis marker and possible target for tumor angiogenesis. World J Gastroenterol 2013; 19(1): 42-48
- URL: https://www.wjgnet.com/1007-9327/full/v19/i1/42.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i1.42
A hallmark of solid tumors is abnormal vasculature, termed tumor vessels, which is adjacent to the tumor itself. Greater number of tumor vessels increases the risk that tumor cells will enter systemic circulation. The basement membranes of newly formed tumor vessels are highly permeable, enabling tumor cells to penetrate them more easily than mature vessels[1]. Furthermore, tumor angiogenesis enhances the supply of oxygen and nutrients to solid tumor cells. As a result, the tumor grows more rapidly and easily when tumor vessels are formed in close proximity. After a tumor has attained a size of 1-2 mm, the induction of new blood vessel formation is required for further growth and expansion[2]. In fact, the degree of tumor angiogenesis reportedly correlates with clinical outcome, and angiogenic properties correlate with tumor aggressiveness in gastrointestinal (GI) cancers including colorectal cancers[3], gastric cancers[4], esophageal cancers[5], and pancreatic cancers[6,7]. These findings suggest that exact quantification of tumor vessels is useful to evaluate prognosis. Furthermore, selective detection of newly formed tumor vessels within cancer tissues using specific markers raises the possibility of molecular targeted therapy via the inhibition of tumor angiogenesis. Microvessel density (MVD), measured immunohistochemically in tumors, is often reported to correlate with prognosis in various malignancies[8,9], including GI cancers[10-14]. However, the clinical significance of MVD is controversial; for example, pancreatic cancer is characterized by hypovascularization[15,16]. For MVD analysis, CD34, CD31, and factor VIII are commonly used as endothelial cell markers in tumor vessels (Table 1). CD34 and CD31 are cell surface markers, and factor VIII is an essential blood clotting protein in vascular endothelial cells of normal tissues. However, these markers do not specifically identify newly formed tumor vessels, but also detect pre-existing large sized blood vessels[17]; therefore, they are not considered suitable for the sensitive analysis of tumor angiogenesis. Inclusion of pre-existing mature vessels in MVD of various tumors may cause controversial results when determining the relationship between tumor angiogenesis, clinicopathologic features, and prognosis.
| Factor VIII | CD 31 | CD 34 | Nestin | |
| Roles | Glycoprotein | Glycoprotein | Glycoprotein | Cytoskeletal protein |
| Blood coagulation | Endothelial cell intercellular junctions[57], migration | Cell-cell adhesion factor, facilitate opening of vascular lumens[58] | Cell cycle[22,59], migration | |
| Character of positive vessels | Large size | Small to large size | Small to large size | Small size[27] |
| Mature vessels | Immature to mature vessels | Immature to mature vessels | Immature vessels[26,30] | |
| High-proliferating activity[26,27] | ||||
| Clinical outcome | ||||
| Esophageal cancer | Poor prognosis[60] | Poor prognosis[61,62] | Poor prognosis[63,64] | - |
| Gastric cancer | Advanced stage[65] | No relation[66] | No relation[45] | No relation[44] |
| Colorectal cancer | No relation[68] | Poor prognosis[68] | Poor prognosis[67] | Poor prognosis[42] |
| Liver metastasis[69] | Poor prognosis[68] | |||
| Liver cancer | No relation[70] | Liver metastasis[69] | ||
| Pancreatic cancer | Poor prognosis[16] | No relation[71] | Poor prognosis[70,72-75] | |
| Lymph node meta[76] | No relation[28] | No relation[27] | ||
| Poor prognosis[77,78] | ||||
| Prostate cancer | Poor prognosis[79-81] | No relation[82] | Poor prognosis[83] | Poor prognosis[26] |
| Glioblastoma | Tumor grade[84] | No relation[85] | No relation[86] | Tumor grade[45] |
Nestin, an intermediate filament (IF) protein, has recently been recognized as a more specific marker for newly formed blood vessels and as a therapeutic target via inhibition of angiogenesis. In this review, we summarize and emphasize recent research evidences concerning nestin in tumor angiogenesis in GI cancers, including pancreatic cancer.
Nestin is a class VI IF protein originally described as a neuronal stem cell marker. After cellular differentiation, nestin expression is downregulated and replaced by neurofilament, a tissue specific IF[18]. Nestin is also expressed in non-neuronal immature or progenitor cells in some normal tissues[19]. It is a large protein (> 1600 amino acids) containing a short N terminus and an unusually long C terminus. The C terminus interacts with other IFs including vimentin, desmin, or internexin, subsequently forming heterodimers and mixed polymers[19-21]. The long C-terminal portion of nestin protrudes from the filamentous body and may function as a link or cross-bridge between IFs and microtubules. Nestin is known to contribute to the disassembly of vimentin during mitosis, inactivation of cyclin-dependent kinase 5[22], and modulation of mitosis-associated cytoplasmic reorganization during mitosis via phosphorylation at Thr316 by cdc2 kinase[23]. Neural cell-specific expression is usually regulated by the second intron, whereas nestin expression in tumor endothelium is enhanced by the first intron[24]. Although nestin is expressed at the early stages of normal development, under pathological conditions, it is re-expressed in repair processes, various neoplasms, and proliferating vascular endothelial cells[25].
Much evidence has shown that nestin expression in vascular endothelial cells is associated with proliferation and angiogenesis[24,26-29]. Nestin expression was detected in endothelial cells of embryonic capillaries[28], capillaries of the corpus luteum, which replenishes itself by angiogenesis[30], and proliferating endothelial progenitor cells, but not in mature endothelial cells[24].
At the early stage of development, high levels of nestin are identified in endothelial cells lining all blood vessels of E14-15 rat fetuses and extraembryonic (chorion, placenta, umbilical cord) and intraembryonic blood vessels. Expression of nestin by vascular endothelial cells is greatly reduced in adult tissues[28].
These findings suggest that nestin plays important roles in vasculogenesis during development.
Angiogenesis is a process of new blood vessel formation from pre-existing vessels; it is the mechanism mediating the growth and modification of a capillary network[31]. In non-cancerous diseases, nestin plays important roles in angiogenesis of wound healing in various tissues. In an adult rat necrotizing pancreatitis model, nestin is expressed in reactive stellate cells, or submesothelial cells, and endothelial cells during active angiogenesis[32], and nestin-positive cells may participate in tissue repair of the pancreas.
Nestin-expressing interfollicular blood vessel networks contribute to skin transplant survival and wound healing[33]. Scar formation following an ischemic insult to the heart is referred to as reparative fibrosis and represents an essential physiological response to damaged myocardium. Several studies reported that scar formation was associated with the recruitment of neural crest-derived cardiac resident nestin-positive cells that display characteristics consistent with a neural progenitor/stem cell phenotype[34,35]. During the reparative fibrotic response, these nestin-positive cells participate in neural remodeling and represent a novel cellular substrate of angiogenesis.
In the rat ovary, the endogenous luteinizing hormone surge induces nestin expression in capillary endothelial cells of the theca interna via the vascular endothelial growth factor (VEGF) signaling pathway[36]. Angiogenic induction of chorion-derived stem cells results in higher mRNA levels of pro-angiogenic protein, fibroblast growth factor-4, and stem cell markers ABCG-2, Sox-2, FZD9, BST-1, nestin, and Oct-4[37]. Endothelial precursors express nestin, and it participates in the formation of the cytoskeleton of newly formed endothelial cells. VEGF is essential for the differentiation of the primitive embryonic vascular system and has been implicated in the vascularization of organs. VEGF is considered a modulator involved in neurogenesis as well as angiogenesis[38,39]. Human VEGF overexpressed transgenic mice showed an increased number of nestin positive cells after temporal ischemia of the central nervous system, as compared with wild type mice[40]. Therefore, VEGF is involved in nestin expression. Furthermore, pre-eclamptic human placenta showed higher nestin expression levels[41], thus hypoxia may be involved in expression of nestin.
Expression of nestin by newly formed blood vessels in various tumor tissues, including pancreatic cancers, has been reported[26,27,30,42,43]. Its expression is not reported in mature vessels within cancer tissues. This suggests that nestin may be an angiogenesis-specific marker in malignant tumors[27,44]. Strongly nestin-positive MVD colorectal carcinomas[42] and prostate cancers[26] show a worse prognosis. In colorectal cancer, nestin is strongly localized to vascular endothelial cells, but not to cancer cells. Nestin-expressing vessels in colorectal cancer tissues are of smaller size and show greater positivity for proliferating cell nuclear antigen than CD34-expressing cells[42]. In glioblastomas, expression of nestin in both tumor cells and endothelial cells increased with tumor grade[45]. Similarly, nestin expression correlates with more advanced stage in melanoma[46]. In gastric cancers, there is no correlation between nestin-positive MVD and patient’s clinical outcome, while in patients with larger carcinomas, MVD determined by nestin correlates better with longer survival than MVD determined by CD34[44]. We previously reported that nestin-positive blood vessels in pancreatic cancer exhibit high proliferating ability, but does not correlate with prognosis[27]. Tumor angiogenesis is essential for tumor progression, but increased angiogenesis may be influenced by variations in the existing vasculature of the host organ. Compared to MVD studies using other endothelial markers, only a limited number of investigations have used nestin. Large scale studies of various different malignancies using nestin as a MVD marker are needed to determine the efficacy of nestin as a predictive and prognostic marker.
The origin of nestin-positive vascular endothelial cells in tumor tissue is still controversial. Migration of mesenchymal stem cells from the bone marrow has been proposed[47]. Dong et al[48] showed that human glioma stem/progenitor cells transdifferentiate into vascular endothelial cells in vitro and in vivo. In skin angiogenesis, nestin-positive hair follicle cells form neovasculature[49]; therefore tissue stem cells are one possible origin of nestin-positive vessels.
Recent studies showed that nestin is an effective marker for in vivo imaging of tumor angiogenesis[50,51] and vascular formation in metastatic tumors[52].
Pancreatic cancer is an aggressive malignancy with an overall five-year survival rate of less than 5%[53]. Since the majority of patients have locally advanced or metastatic disease at the time of diagnosis, there has been little progress in extending survival[54]. For over ten years, chemotherapy with gemcitabine, whose strategy is to inhibit tumor growth, has been the standard treatment for patients with advanced pancreatic cancer, prolonging survival by only five to six months.
To improve upon this modest benefit, several investigations explored other strategies aimed at inhibiting pancreatic cancer growth, including the use of anti-angiogenic agents. Tumor angiogenesis is an important factor in the proliferation, invasion, metastasis, and drug sensitivity of pancreatic cancers[6]. For a tumor to develop a highly malignant and deadly phenotype, it must first recruit and sustain its own blood supply. The process of angiogenesis is also sustained by secretion of specific angiogenic factors by cancer and non-cancer cells. Angiogenic factors can initiate a multi-step process that begins with vasodilatation, followed by the enhancement of vessel permeability and stroma degradation. Angiogenic factors can also promote endothelial cell migration and proliferation.
Several clinical agents have been developed to inhibit the angiogenesis process, including the VEGF pathway[55], and a number of phase II and phase III trials combined gemcitabine with novel anti-angiogenic agents with the hope of improving patient survival. Bevacizumab, which inhibits only the VEGF receptor, and sorafenib, which inhibits raf-1 kinase and the platelet-derived growth factor receptor tyrosine kinase in addition to the VEGF receptor, were studied in combination with gemcitabine. However, this approach has largely been unsuccessful and additional novel strategies are clearly needed.
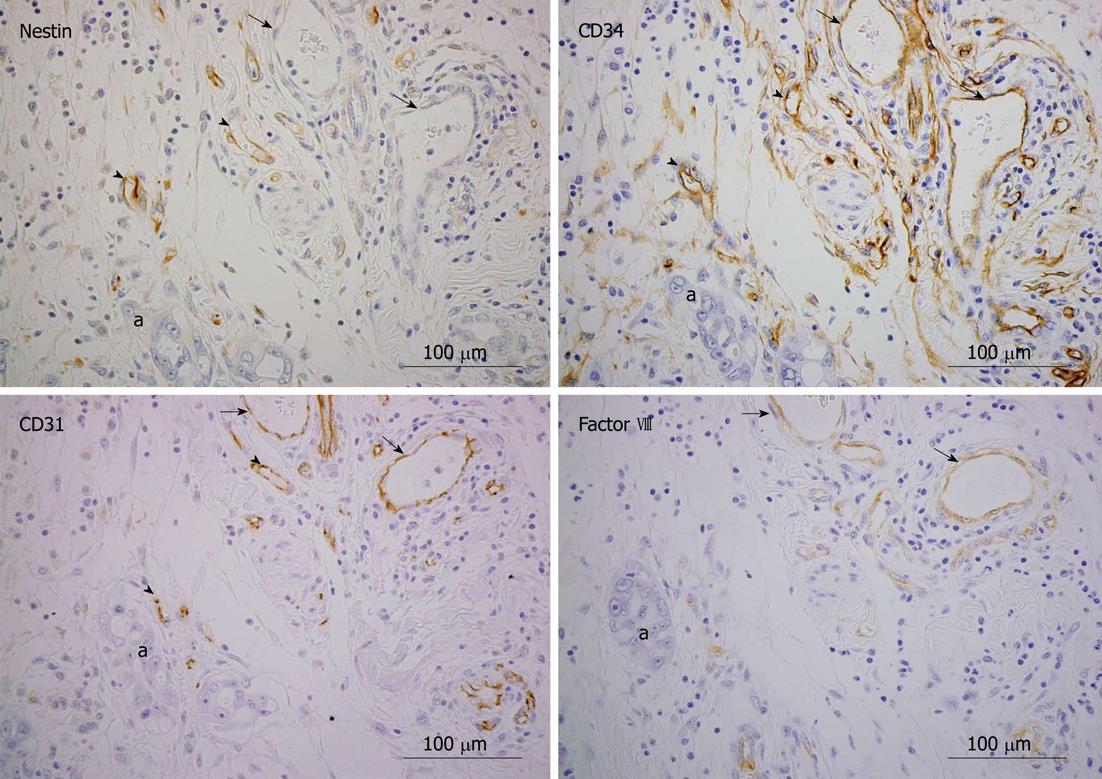
In pancreatic cancers, we found that CD34, CD31, and factor VIII, which are commonly used for MVD quantification by immunohistochemical analysis, were expressed in both newly formed small sized blood vessels and pre-existing vessels (Figure 1, arrows and arrowheads), whereas nestin was expressed only in newly formed blood vessels (arrowheads). Therefore, nestin is a potentially useful marker to distinguish newly formed blood vessels from pre-existing vessels in tumor tissues[27].
More importantly, we observed that knockdown of nestin in vascular endothelial cells suppresses cell growth and tumor formation of pancreatic cancers in vivo[27]. Therefore, nestin is a potential molecular target via inhibition of angiogenesis in pancreatic cancer.
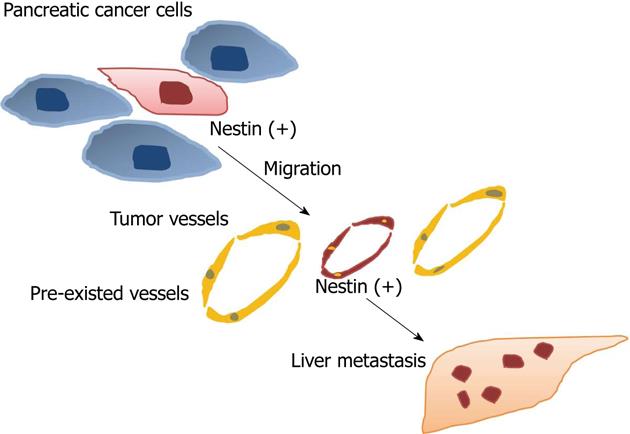
Nestin is expressed in both pancreatic cancer cells and tumor vessels. Inhibition of nestin in cancer cells reduces tumor cell migration and metastasis[56] and downregulation of nestin in tumor vessels also inhibits tumor formation[27]. These findings may indicate that nestin is a target not only for inhibition of tumor angiogenesis, but also within some cancers themselves that expression nestin, such as glioblastomas, melanomas, and pancreatic cancer (Figure 2).
More accurate estimation of MVD using nestin in GI cancers may reveal a strong relationship between tumor vessels and clinical outcome. Future studies on MVD with a large patient population are needed to clarify the efficacy of nestin as a predictive and prognostic marker. Nestin-targeting small interfering RNA (siRNA) has shown a tumor inhibitory effect in vivo via inhibition of tumor angiogenesis[27], thus nestin may be a novel therapeutic target for tumor angiogenesis. Treatment resistance often occurs with the isolated use of anti-VEGF therapy. Therefore, combination therapy that also includes nestin-targeting agents is a potentially effective therapeutic strategy. Nestin is expressed in some cancer cells, thus targeted therapy may exert inhibitory effects both on cancer cells and vascular endothelium in certain tumor types, including pancreatic cancers. In addition to small molecule compounds, emerging biotechnology including RNA aptamer and siRNA targeting nestin will possibly be adopted as new therapeutics for GI cancers, especially for pancreatic cancers.
The authors thank Dr. Kosuke Narita from Departments of Pathology and Integrative Oncological Pathology for helpful discussion.
P- Reviewer Vitiani LR S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889-894. [PubMed] |
| 2. | Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4-6. [PubMed] |
| 3. | Papamichael D. Prognostic role of angiogenesis in colorectal cancer. Anticancer Res. 2001;21:4349-4353. [PubMed] |
| 4. | Saito H, Tsujitani S. Angiogenesis, angiogenic factor expression and prognosis of gastric carcinoma. Anticancer Res. 2001;21:4365-4372. [PubMed] |
| 5. | Nakagawa S, Nishimaki T, Suzuki T, Kanda T, Kuwabara S, Hatakeyama K. Tumor angiogenesis as an independent prognostic factor after extended radical esophagectomy for invasive squamous cell carcinoma of the esophagus. Surgery. 2001;129:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 237] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Kuwahara K, Sasaki T, Kuwada Y, Murakami M, Yamasaki S, Chayama K. Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas. 2003;26:344-349. [PubMed] |
| 8. | Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti CA. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet. 1992;340:145-146. [PubMed] |
| 9. | Hansen S, Grabau DA, Rose C, Bak M, Sørensen FB. Angiogenesis in breast cancer: a comparative study of the observer variability of methods for determining microvessel density. Lab Invest. 1998;78:1563-1573. [PubMed] |
| 10. | Schoell WM, Pieber D, Reich O, Lahousen M, Janicek M, Guecer F, Winter R. Tumor angiogenesis as a prognostic factor in ovarian carcinoma: quantification of endothelial immunoreactivity by image analysis. Cancer. 1997;80:2257-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Tanigawa N, Matsumura M, Amaya H, Kitaoka A, Shimomatsuya T, Lu C, Muraoka R, Tanaka T. Tumor vascularity correlates with the prognosis of patients with esophageal squamous cell carcinoma. Cancer. 1997;79:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T. Association of tumour vasculature with tumour progression and overall survival of patients with non-early gastric carcinomas. Br J Cancer. 1997;75:566-571. [PubMed] |
| 13. | Takahashi Y, Tucker SL, Kitadai Y, Koura AN, Bucana CD, Cleary KR, Ellis LM. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg. 1997;132:541-546. [PubMed] |
| 14. | Wakui S, Furusato M, Itoh T, Sasaki H, Akiyama A, Kinoshita I, Asano K, Tokuda T, Aizawa S, Ushigome S. Tumour angiogenesis in prostatic carcinoma with and without bone marrow metastasis: a morphometric study. J Pathol. 1992;168:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 167] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Takagi K, Takada T, Amano H, Yoshida M, Miura H, Toyota N, Wada K, Takahashi I. Analysis of microvessels in pancreatic cancer: by light microscopy, confocal laser scan microscopy, and electron microscopy. J Hepatobiliary Pancreat Surg. 2008;15:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Takagi K, Takada T, Amano H. A high peripheral microvessel density count correlates with a poor prognosis in pancreatic cancer. J Gastroenterol. 2005;40:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Meert AP, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout JM, Lafitte JJ, Mascaux C, Sculier JP. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2002;87:694-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585-595. [PubMed] |
| 19. | Steinert PM, Chou YH, Prahlad V, Parry DA, Marekov LN, Wu KC, Jang SI, Goldman RD. A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J Biol Chem. 1999;274:9881-9890. [PubMed] |
| 20. | Marvin MJ, Dahlstrand J, Lendahl U, McKay RD. A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J Cell Sci. 1998;111:1951-1961. [PubMed] |
| 21. | Sjöberg G, Edström L, Lendahl U, Sejersen T. Myofibers from Duchenne/Becker muscular dystrophy and myositis express the intermediate filament nestin. J Neuropathol Exp Neurol. 1994;53:416-423. [PubMed] |
| 22. | Sahlgren CM, Mikhailov A, Vaittinen S, Pallari HM, Kalimo H, Pant HC, Eriksson JE. Cdk5 regulates the organization of Nestin and its association with p35. Mol Cell Biol. 2003;23:5090-5106. [PubMed] |
| 23. | Sahlgren CM, Mikhailov A, Hellman J, Chou YH, Lendahl U, Goldman RD, Eriksson JE. Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J Biol Chem. 2001;276:16456-16463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem. 2010;58:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 25. | Ishiwata T, Matsuda Y, Naito Z. Nestin in gastrointestinal and other cancers: effects on cells and tumor angiogenesis. World J Gastroenterol. 2011;17:409-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. Proliferation of immature tumor vessels is a novel marker of clinical progression in prostate cancer. Cancer Res. 2009;69:4708-4715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Yamahatsu K, Matsuda Y, Ishiwata T, Uchida E, Naito Z. Nestin as a novel therapeutic target for pancreatic cancer via tumor angiogenesis. Int J Oncol. 2012;40:1345-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Mokrý J, Nemecek S. Angiogenesis of extra- and intraembryonic blood vessels is associated with expression of nestin in endothelial cells. Folia Biol (Praha). 1998;44:155-161. [PubMed] |
| 29. | Mokrý J, Nemecek S. Cerebral angiogenesis shows nestin expression in endothelial cells. Gen Physiol Biophys. 1999;18 Suppl 1:25-29. [PubMed] |
| 30. | Mokrý J, Cízková D, Filip S, Ehrmann J, Osterreicher J, Kolár Z, English D. Nestin expression by newly formed human blood vessels. Stem Cells Dev. 2004;13:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364. [PubMed] |
| 32. | Ishiwata T, Kudo M, Onda M, Fujii T, Teduka K, Suzuki T, Korc M, Naito Z. Defined localization of nestin-expressing cells in L-arginine-induced acute pancreatitis. Pancreas. 2006;32:360-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Aki R, Amoh Y, Li L, Katsuoka K, Hoffman RM. Nestin-expressing interfollicular blood vessel network contributes to skin transplant survival and wound healing. J Cell Biochem. 2010;110:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | El-Helou V, Beguin PC, Assimakopoulos J, Clement R, Gosselin H, Brugada R, Aumont A, Biernaskie J, Villeneuve L, Leung TK. The rat heart contains a neural stem cell population; role in sympathetic sprouting and angiogenesis. J Mol Cell Cardiol. 2008;45:694-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Calderone A. Nestin+ cells and healing the infarcted heart. Am J Physiol Heart Circ Physiol. 2012;302:H1-H9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Takahashi N, Itoh MT, Ishizuka B. Human chorionic gonadotropin induces nestin expression in endothelial cells of the ovary via vascular endothelial growth factor signaling. Endocrinology. 2008;149:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Abdul Rahman H, Manzor NF, Tan GC, Tan AE, Chua KH. Upregulation of SOX-2, FZD9, Nestin, OCT-4 and FGF-4 expression in human chorion derived-stem cells after angiogenic induction. Med J Malaysia. 2008;63 Suppl A:57-58. [PubMed] |
| 38. | Kim BK, Kim SE, Shim JH, Woo DH, Gil JE, Kim SK, Kim JH. Neurogenic effect of vascular endothelial growth factor during germ layer formation of human embryonic stem cells. FEBS Lett. 2006;580:5869-5874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Raab S, Beck H, Gaumann A, Yüce A, Gerber HP, Plate K, Hammes HP, Ferrara N, Breier G. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Plaschke K, Staub J, Ernst E, Marti HH. VEGF overexpression improves mice cognitive abilities after unilateral common carotid artery occlusion. Exp Neurol. 2008;214:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Hwang HS, Cho NH, Maeng YS, Kang MH, Park YW, Kim YH. Differential expression of nestin in normal and pre-eclamptic human placentas. Acta Obstet Gynecol Scand. 2007;86:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Teranishi N, Naito Z, Ishiwata T, Tanaka N, Furukawa K, Seya T, Shinji S, Tajiri T. Identification of neovasculature using nestin in colorectal cancer. Int J Oncol. 2007;30:593-603. [PubMed] |
| 43. | Salehi F, Kovacs K, Cusimano MD, Horvath E, Bell CD, Rotondo F, Scheithauer BW. Immunohistochemical expression of nestin in adenohypophysial vessels during development of pituitary infarction. J Neurosurg. 2008;108:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Kim HS, Kang HS, Messam CA, Min KW, Park CS. Comparative evaluation of angiogenesis in gastric adenocarcinoma by nestin and CD34. Appl Immunohistochem Mol Morphol. 2002;10:121-127. [PubMed] |
| 45. | Hlobilkova A, Ehrmann J, Knizetova P, Krejci V, Kalita O, Kolar Z. Analysis of VEGF, Flt-1, Flk-1, nestin and MMP-9 in relation to astrocytoma pathogenesis and progression. Neoplasma. 2009;56:284-290. [PubMed] |
| 46. | Brychtova S, Fiuraskova M, Hlobilková A, Brychta T, Hinak J. Nestin expression in cutaneous melanomas and melanocytic nevi. J Cutan Pathol. 2007;34:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Schiffer D, Annovazzi L, Caldera V, Mellai M. On the origin and growth of gliomas. Anticancer Res. 2010;30:1977-1998. [PubMed] |
| 48. | Dong J, Zhao Y, Huang Q, Fei X, Diao Y, Shen Y, Xiao H, Zhang T, Lan Q, Gu X. Glioma stem/progenitor cells contribute to neovascularization via transdifferentiation. Stem Cell Rev. 2011;7:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S, Hoffman RM. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci USA. 2004;101:13291-13295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 50. | Hoffman RM. Nestin-driven green fluorescent protein as an imaging marker for nascent blood vessels in mouse models of cancer. Methods Mol Biol. 2011;689:183-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Amoh Y, Yang M, Li L, Reynoso J, Bouvet M, Moossa AR, Katsuoka K, Hoffman RM. Nestin-linked green fluorescent protein transgenic nude mouse for imaging human tumor angiogenesis. Cancer Res. 2005;65:5352-5357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Amoh Y, Bouvet M, Li L, Tsuji K, Moossa AR, Katsuoka K, Hoffman RM. Visualization of nascent tumor angiogenesis in lung and liver metastasis by differential dual-color fluorescence imaging in nestin-linked-GFP mice. Clin Exp Metastasis. 2006;23:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8226] [Article Influence: 483.9] [Reference Citation Analysis (0)] |
| 54. | Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1015] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 55. | Assifi MM, Hines OJ. Anti-angiogenic agents in pancreatic cancer: a review. Anticancer Agents Med Chem. 2011;11:464-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Matsuda Y, Naito Z, Kawahara K, Nakazawa N, Korc M, Ishiwata T. Nestin is a novel target for suppressing pancreatic cancer cell migration, invasion and metastasis. Cancer Biol Ther. 2011;11:512-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 57. | Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 717] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 58. | Strilić B, Kucera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, Dejana E, Ferrara N, Lammert E. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell. 2009;17:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 59. | Pallari HM, Lindqvist J, Torvaldson E, Ferraris SE, He T, Sahlgren C, Eriksson JE. Nestin as a regulator of Cdk5 in differentiating myoblasts. Mol Biol Cell. 2011;22:1539-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Takebayashi Y, Natugoe S, Baba M, Fukumoto T, Takao S, Akiba S, Akiyama S, Aikou T. Angiogenesis in esophageal squamous cell carcinoma. Oncol Rep. 1998;5:401-404. [PubMed] |
| 61. | Saad RS, Lindner JL, Liu Y, Silverman JF. Lymphatic vessel density as prognostic marker in esophageal adenocarcinoma. Am J Clin Pathol. 2009;131:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Saad RS, El-Gohary Y, Memari E, Liu YL, Silverman JF. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in esophageal adenocarcinoma. Hum Pathol. 2005;36:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Elpek GO, Gelen T, Aksoy NH, Erdoğan A, Dertsiz L, Demircan A, Keleş N. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol. 2001;54:940-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Igarashi M, Dhar DK, Kubota H, Yamamoto A, El-Assal O, Nagasue N. The prognostic significance of microvessel density and thymidine phosphorylase expression in squamous cell carcinoma of the esophagus. Cancer. 1998;82:1225-1232. [PubMed] |
| 65. | Du JR, Jiang Y, Zhang YM, Fu H. Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World J Gastroenterol. 2003;9:1604-1606. [PubMed] |
| 66. | Coşkun U, Akyürek N, Dursun A, Yamaç D. Peritumoral lymphatic microvessel density associated with tumor progression and poor prognosis in gastric carcinoma. J Surg Res. 2010;164:110-115. [PubMed] |
| 67. | Wei YZ, Li CF, Xue YW. [Expression of transcription factor SP1, vascular endothelial growth factor and CD34 in serosa-infiltrating gastric cancer and their relationship with biological behavior and prognosis]. Zhonghua Weichang Waike Zazhi. 2009;12:145-149. [PubMed] |
| 68. | Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823-1832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 69. | Tomisaki S, Ohno S, Ichiyoshi Y, Kuwano H, Maehara Y, Sugimachi K. Microvessel quantification and its possible relation with liver metastasis in colorectal cancer. Cancer. 1996;77:1722-1728. [PubMed] |
| 70. | Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, Wong J. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 71. | Wang SN, Yeh YT, Yang SF, Chai CY, Lee KT. Potential role of leptin expression in hepatocellular carcinoma. J Clin Pathol. 2006;59:930-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi GM, Zhang BH, Wu WZ, Shi YH, Wu B. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59:953-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 73. | Yang P, Yuan W, He J, Wang J, Yu L, Jin X, Hu Y, Liao M, Chen Z, Zhang Y. Overexpression of EphA2, MMP-9, and MVD-CD34 in hepatocellular carcinoma: Implications for tumor progression and prognosis. Hepatol Res. 2009;39:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Tseng PL, Tai MH, Huang CC, Wang CC, Lin JW, Hung CH, Chen CH, Wang JH, Lu SN, Lee CM. Overexpression of VEGF is associated with positive p53 immunostaining in hepatocellular carcinoma (HCC) and adverse outcome of HCC patients. J Surg Oncol. 2008;98:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 75. | Sun HC, Tang ZY, Li XM, Zhou YN, Sun BR, Ma ZC. Microvessel density of hepatocellular carcinoma: its relationship with prognosis. J Cancer Res Clin Oncol. 1999;125:419-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | van der Zee JA, van Eijck CH, Hop WC, van Dekken H, Dicheva BM, Seynhaeve AL, Koning GA, Eggermont AM, ten Hagen TL. Angiogenesis: a prognostic determinant in pancreatic cancer? Eur J Cancer. 2011;47:2576-2584. [PubMed] |
| 77. | Niedergethmann M, Hildenbrand R, Wostbrock B, Hartel M, Sturm JW, Richter A, Post S. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas. 2002;25:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 78. | Niedergethmann M, Hildenbrand R, Wolf G, Verbeke CS, Richter A, Post S. Angiogenesis and cathepsin expression are prognostic factors in pancreatic adenocarcinoma after curative resection. Int J Pancreatol. 2000;28:31-39. [PubMed] |
| 79. | Offersen BV, Borre M, Overgaard J. Immunohistochemical determination of tumor angiogenesis measured by the maximal microvessel density in human prostate cancer. APMIS. 1998;106:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Borre M, Offersen BV, Nerstrøm B, Overgaard J. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. Br J Cancer. 1998;78:940-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 172] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 81. | Borre M, Offersen IB, Nerstrøm B, Overgaard J. [Angiogenesis: prognostic marker in prostatic cancer]. Ugeskr Laeger. 1999;161:3832-3836. [PubMed] |
| 82. | Rubin MA, Buyyounouski M, Bagiella E, Sharir S, Neugut A, Benson M, de la Taille A, Katz AE, Olsson CA, Ennis RD. Microvessel density in prostate cancer: lack of correlation with tumor grade, pathologic stage, and clinical outcome. Urology. 1999;53:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | de la Taille A, Katz AE, Bagiella E, Buttyan R, Sharir S, Olsson CA, Burchardt T, Ennis RD, Rubin MA. Microvessel density as a predictor of PSA recurrence after radical prostatectomy. A comparison of CD34 and CD31. Am J Clin Pathol. 2000;113:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Mahzouni P, Mohammadizadeh F, Mougouei K, Moghaddam NA, Chehrei A, Mesbah A. Determining the relationship between “microvessel density” and different grades of astrocytoma based on immunohistochemistry for “factor VIII-related antigen” (von Willebrand factor) expression in tumor microvessels. Indian J Pathol Microbiol. 2010;53:605-610. [PubMed] |
| 85. | Yao Y, Kubota T, Takeuchi H, Sato K. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: comparison with an anti-CD31 monoclonal antibody. Neuropathology. 2005;25:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Preusser M, Heinzl H, Gelpi E, Schonegger K, Haberler C, Birner P, Marosi C, Hegi M, Gorlia T, Hainfellner JA. Histopathologic assessment of hot-spot microvessel density and vascular patterns in glioblastoma: Poor observer agreement limits clinical utility as prognostic factors: a translational research project of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Cancer. 2006;107:162-170. [PubMed] |