Published online Jan 7, 2013. doi: 10.3748/wjg.v19.i1.115
Revised: July 2, 2012
Accepted: August 3, 2012
Published online: January 7, 2013
Vanishing bile duct syndrome (VBDS) is a group of rare disorders characterized by ductopenia, the progressive destruction and disappearance of intrahepatic bile ducts leading to cholestasis. Described in association with medications, autoimmune disorders, cancer, transplantation, and infections, the specific mechanisms of disease are not known. To date, only 4 cases of VBDS have been reported in human immunodeficiency virus (HIV) infected patients. We report 2 additional cases of HIV-associated VBDS and review the features common to the HIV-associated cases. Presentation includes hyperbilirubinemia, normal liver imaging, and negative viral and autoimmune hepatitis studies. In HIV-infected subjects, VBDS occurred at a range of CD4+ T-cell counts, in some cases following initiation or change in antiretroviral therapy. Lymphoma was associated with two cases; nevirapine, antibiotics, and viral co-infection were suggested as etiologies in the other cases. In HIV-positive patients with progressive cholestasis, early identification of VBDS and referral for transplantation may improve outcomes.
- Citation: Oppenheimer AP, Koh C, McLaughlin M, Williamson JC, Norton TD, Laudadio J, Heller T, Kleiner DE, High KP, Morse CG. Vanishing bile duct syndrome in human immunodeficiency virus infected adults: A report of two cases. World J Gastroenterol 2013; 19(1): 115-121
- URL: https://www.wjgnet.com/1007-9327/full/v19/i1/115.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i1.115
Vanishing bile duct syndrome (VBDS) is a term that refers to a group of rare disorders characterized by progressive destruction and disappearance of the intrahepatic bile ducts leading to intrahepatic cholestasis[1]. VBDS has been described in association with autoimmune disorders, medications, cancer, transplantation, and infection, though idiopathic cases have also been described[2]. Immune mediated mechanisms have been proposed[3]. The prognosis is poor with progression to biliary cirrhosis in the majority of cases.
To date only 4 previous cases in human immunodeficiency virus (HIV) infected adults have been reported[2-5]. Antiretroviral therapy (ART) has been implicated as causal in three of the cases[2,4,5]. Here we report two additional cases of biliary ductopenia consistent with VBDS seen in HIV-infected adults cared for at two academic hospitals in 2010. The previously reported cases are reviewed and the potential etiologies, pathogenesis, management and prognosis for VBDS in HIV-infected adults are discussed.
A 41-year-old man with HIV infection, diagnosed in 1989, was referred for evaluation of a 9-wk history of progressive jaundice. ART was initiated in 1999 with adherence to a regimen of tenofovir, didanosine, atazanavir, and ritonavir for at least 9 mo prior to presentation. CD4+ count was 304 cells/μL and HIV-1 viral load (VL) < 75 copies/mL two months prior to symptoms. He was taking no other medications and had no history of opportunistic infections (OI) or pancreatitis.
Eight weeks prior to referral he was evaluated for a one-week history of fatigue, nausea, abdominal pain, and scleral icterus. He denied new medications, alcohol or drug use. Laboratory studies revealed elevated alkaline phosphatase (AP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin (TB) 4.7 mg/L. Lipase was elevated at 3571 U/L. All studies were within normal ranges two months earlier. Abdominal computed tomography (CT) scan showed pancreatic enlargement consistent with pancreatitis and a normal appearing liver. Because of concern for ART-associated pancreatitis and liver injury, ART was held and he received nothing by mouth. Magnetic resonance imaging and magnetic retrograde cholangio-pancreatography (MRI/MRCP) of the liver were normal. Subsequently, his nausea and abdominal pain improved, lipase trended down, and his diet was successfully advanced. He was discharged on the third day of hospitalization.
Five days later, he reported worsening fatigue and new generalized pruritus. Laboratory studies showed worsening hyperbilirubinemia (TB 14.3 mg/dL). He was referred to the National Institutes of Health (NIH) for further evaluation.
On admission to the NIH, he was febrile (38.2 °C) with laboratory studies consistent with progressive cholestasis. CD4+ count was 164 cells/μL and HIV VL was 33 945 copies/mL. Viral hepatitis and autoimmune studies were negative. Epstein Barr virus (EBV) polymerase chain reaction (PCR) was low positive (660 genome equivalents/mL) and cytomegalovirus (CMV) PCR was low positive (< 250 genome equivalents/mL) in blood.
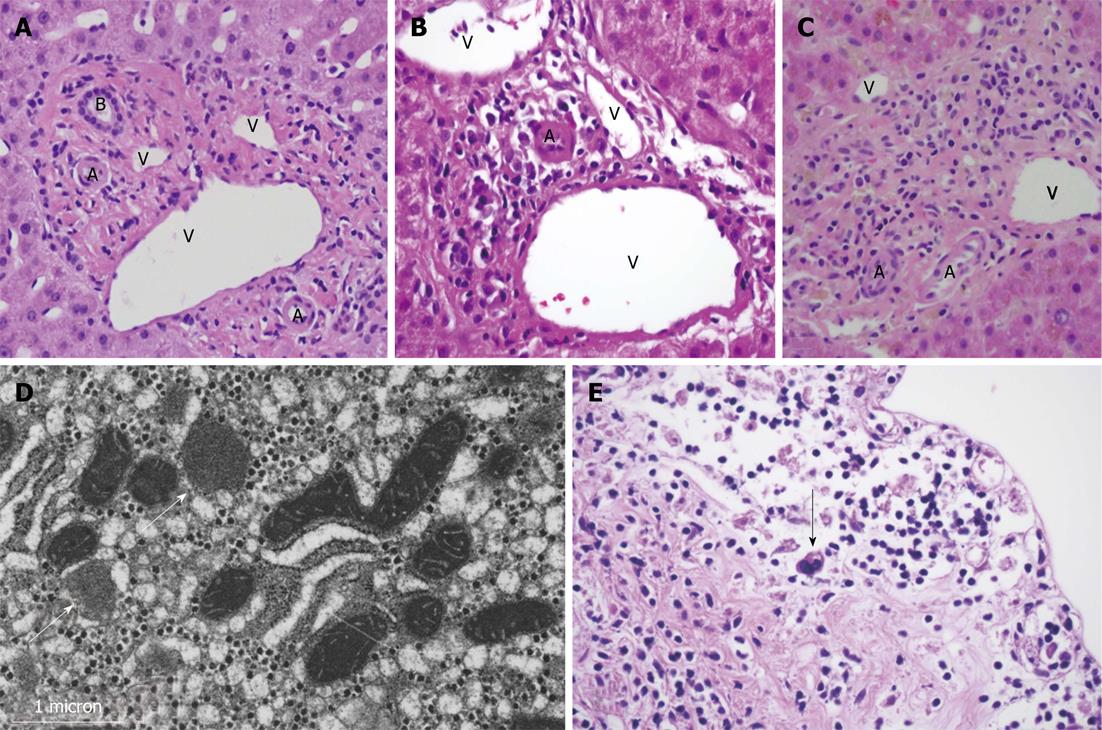
Liver biopsy revealed marked bile duct paucity associated with moderate inflammation, cholestasis, injury of residual ducts, and perivenular hepatocyte dropout with fibrosis (Figure 1B). Less than 10% of portal areas showed an intact duct, confirming the diagnosis of VBDS. No viral inclusions or granulomas were seen. Fungal and acid fast bacilli (AFB) staining were negative. Liver biopsy was negative for CMV by PCR and immunohistochemistry, and low positive for EBV by PCR, however in situ hybridization for EBV was negative. Electron microscopy showed mitochondrial injury, presumably secondary to the patient’s prior ART (Figure 1D).
ART was reinitiated three weeks after admission with lamivudine, etravirine, raltegravir and enfuvirtide[6]. Four weeks later, his CD4+ count was 133 (12%) and HIV VL < 50. The patient’s hospital course was complicated by pneumococcal sepsis, small bowel intussusception requiring surgical resection complicated by an anastomotic bleed, and renal failure. TB remained elevated, peaking at 45.6 mg/dL, and he was referred for liver transplant evaluation.
Due to poor nutritional and functional status, the patient was not deemed a candidate for liver transplant and was discharged to hospice care. He died two weeks later. Autopsy identified hepatomegaly with marked bile duct paucity, splenomegaly and bloody ascites. Classic Hodgkin’s lymphoma, lymphocyte depleted subtype, was found in the lymph nodes of the neck and mediastinum as well as in the gallbladder and common bile ducts (Figure 1E).
A 39-year-old African-American male with HIV, diagnosed in 1996, and a history of toxoplasmosis, disseminated Mycobacterium avium complex (MAC), seizure disorder, and medication non-compliance, presented with confusion. Evaluation revealed acute renal failure, hyperbilirubinemia and moderately elevated transaminases. Three months earlier, his CD4+ count was 190 cells/μL, HIV VL 136 copies/mL, AP 154 U/L, with normal serum transaminases and bilirubin. His medications included tenofovir, emtricitabine, lopinavir/ritonavir, dapsone, azithromycin, ethambutol, and divalproex. Two weeks prior, he received an unknown antibiotic for a sinus infection.
At presentation, he was awake but not following commands. Examination revealed jaundice and bibasilar rales. Laboratory studies showed pancytopenia, acute renal failure, elevated AP, AST and ALT, with TB 10 mg/dL. CT of the chest, abdomen and pelvis showed bibasilar lung infiltrates and hepatomegaly. He received cefepime for pneumonia and hemodialysis for metabolic acidosis and uremia. His prior medications were held.
MRI/MRCP showed borderline small hepatic ducts with ductal irregularity in the right lobe without definitive obstruction. Liver biopsy showed ductopenia and intrahepatic and canalicular cholestasis. Of eleven portal tracts identified, none contained bile ducts. Where a bile duct would be expected, a mild lymphoplasmacytic infiltrate was seen without resultant expansion of the portal tract or associated piecemeal necrosis (Figure 1C). Fungal and AFB staining were negative. PCR for EBV DNA was low level positive, though no viral cytopathic effect was seen. Immunohistochemistry was negative for CMV or herpes simplex virus.
With supportive care, the patient’s mental status improved. ART was reinitiated with etravirine, raltegravir, and ritonavir-boosted darunavir to improve his immune function and prevent recurrent or new OI. Secondary MAC and toxoplasmosis prophylaxis were restarted with ethambutol, azithromycin, and atovaquone. TB remained stable at 25 mg/dL. After discharge, he refused to take his medications and died 3 wk later of multisystem organ failure. Autopsy was not performed.
Case 3 was a 28-year-old African American female, 31-wk pregnant, who presented with jaundice four weeks after initiating ART with zidovudine, lamivudine, and nevirapine[4]. Prior to initiation of ART, CD4+ count was 234 cells/μL, and VL of 35 853 copies/mL. Liver biopsy showed marked ductopenia and cholestasis. ART was discontinued and the patient was treated with ursodeoxycholic acid. A repeat liver biopsy obtained 8 wk after presentation showed worsening cholestasis and persistent ductopenia. She was referred and listed for liver transplantation, however her cholestasis improved and she was ultimately removed from the transplant list (personal communication, Dr. Rajan Kochar, August 10, 2010). The authors felt that drug-induced liver injury from nevirapine was the likely cause of the VBDS.
Case 4 was a 39-year-old female with advanced acquired immunodeficiency syndrome (AIDS), hepatitis C (HCV) co-infection, and ongoing polysubstance abuse, who presented with one week of scleral icterus, pruritus, and abdominal pain[3]. She was not receiving ART and her CD4+ count was 7 cells/μL and VL 721 000 at presentation. CMV PCR in the blood was positive. Liver biopsy showed marked ductopenia and associated granulomatous inflammation. The authors imputed CMV as the culprit for VBDS, recognizing the potential contribution of HCV co-infection. The patient died one month after presentation
Case 5 was a 25-year-old African male with advanced AIDS (CD4+ 108 cells/μL, VL 97 100 copies/mL) who presented with confusion, vomiting and jaundice two weeks after starting zidovudine, lamivudine and efavirenz[2]. ART was discontinued and he received ciprofloxacin and clarithromycin for pneumonia. Three weeks later, ART was reinitiated with tenofovir, lamivudine and nevirapine. One week later, he was readmitted with fever and worsening jaundice. Liver biopsy showed bile duct damage associated with minimal inflammation and absence of ducts in 40% of the visualized portal tracts. His clinical presentation, thought to be consistent with VBDS, was attributed to medications, especially nevirapine, since his clinical deterioration occurred after starting ART. He was referred for liver transplantation evaluation but died approximately one month after referral.
Case 6, available only in abstract form, was a 41-year-old male with HIV, previously well-controlled on a regimen of zidovudine, lamivudine, abacavir and tenofovir[5]. After his HIV RNA increased from < 50 to 454 copies/mL, his ART regimen was changed to abacavir, lamivudine and nevirapine. Liver enzymes prior to ART change were normal. Four weeks later, he developed jaundice and abdominal pain. Liver biopsy showed lack of bile ducts involving all portal tracts with lymphocytic infiltration without eosinophils. Given the temporal association with initiation of nevirapine, nevirapine-induced VBDS was imputed. In subsequent follow-up, the patient was diagnosed with lymphoma and died within a year of VBDS diagnosis (personal communication, Dr. Judith Berger, May 2, 2011).
VBDS is a rare cause of progressive cholestasis, most commonly described with drugs but also seen with infection, in cancer and cancer therapy, and in liver diseases such as allograft rejection, graft-versus-host disease, primary biliary cirrhosis and primary sclerosing cholangitis. Diagnosis is made by liver biopsy when interlobular and septal bile ducts cannot be identified in at least 50% of the portal tracts in a biopsy that contains at least 10 portal tracts for assessment[7].
Clinical presentation, similar in HIV-infected and non-HIV-infected patients, varies, with some cases presenting shortly after drug exposure with acute jaundice[4] and others appearing with cholestatic cirrhosis and complications of portal hypertension[7]. Evaluation should include a detailed medication history to identify possible drug causes of cholestasis, laboratory testing to rule out viral and autoimmune hepatitis and primary biliary cirrhosis, and imaging to rule out extrahepatic biliary obstruction. Liver biopsy is required for diagnosis.
The 6 cases in HIV-infected patients described to date share many risk factors for VBDS reported previously in non-HIV patients, including immunocompromise, multiple medication exposures, viral co-infection, and Hodgkin’s lymphoma. Given the frequency of risk factors for VBDS in HIV-infected populations, it is perhaps surprising that the diagnosis is so rarely reported.
Remarkable features for the HIV-infected patients include near-total bile duct loss at the time of liver biopsy in 5 of the 6 patients described. The majority experienced irreversible progression with 5 of the 6 patients dying within months of diagnosis. Because diagnosis occurred over a range of CD4+ T-cell counts and levels of HIV viremia, it is difficult to conclude that advanced HIV disease is a predisposition. However, ART and associated immune reconstitution may play an underlying role in some cases, with 3 patients appearing shortly after antiretroviral regimen initiation or change.
Many drugs with hepatic metabolism are known to cause hepatotoxicity and cholestasis and have been associated with VBDS[8]. The growing list of drugs associated with VBDS includes most antimicrobial classes, anticonvulsants, antipsychotics, antidepressants, antihistamines, NSAIDS, and sex steroids (Table 1). VBDS has also been described as a component of severe drug reactions, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis[9,10].
| Medication class | Medication |
| Antimicrobials | Penicillins |
| Fluroquinolones | |
| Trimethoprim-sulfamethoxazole | |
| Azithromycin | |
| Tetracyclines | |
| Clindamycin | |
| Carbapenems | |
| Itraconazole | |
| Terbinafine | |
| Antiretrovirals | Nevirapine |
| Anticonvulsants | Carbamazepine |
| Valproic acid | |
| NSAIDS | Ibuprofen |
| Sex steroids | Testosterone |
| Antidepressants | Amitriptyline |
| Antipsychotics | Risperidone |
| Immunomodulating | Interleukin-2 |
| Anti-lipid Agents | Atorvastatin |
| Fenofibrate |
The prognosis of drug-induced VBDS appears to be better than with other causes of VBDS with gradual improvement in liver test abnormalities and symptoms described in the majority of reports[11,12]. However, in a portion of patients, the disease is irreversible[10], as seen in 5 of the 6 HIV-positive patients described here.
Drug-induced ductopenia is thought to occur from direct damage caused by toxins or from an immunoallergic reaction where the drug or its metabolites act as haptens that induce an immune response against the biliary epithelium, destroying the intrahepatic bile ducts and resulting in cholestasis[13]. Histologic descriptions of drug-induced VBDS include marked inflammatory infiltrates, including mononuclear cells, eosinophils, and neutrophils, surrounding bile ducts and accompanied by lobular cholestasis. Sequential biopsies demonstrate decreasing inflammation, increasing cholestasis and progressive ductopenia[13]. Interestingly, there does not appear to be any qualitative histologic difference between cases that improve and those that progress, though reports of ductopenia in allograft rejection suggest that a greater degree of bile duct loss was seen in irreversible cases[14].
Three of the HIV patients were treated with medications described in association with VBDS. Case 1 received empiric treatment for urethritis with ceftriaxone and azithromycin, two medications that could have potentially worsened his underlying liver disease. Case 2 received anti-MAC therapy and an unknown antibiotic for a respiratory infection. His antiepileptic, divalproex, is a pro-drug of valproic acid, a medication reported in association with VBDS. Case 5 received clarithromycin, ciprofloxacin, and ibuprofen. The role of these medications in causing or perpetuating the underlying liver damage is not known.
Though nevirapine has been associated with SJS and hepatoxicity, the 3 reports of VBDS in the setting of nevirapine use did not have features of SJS. However, given the temporal association of nevirapine with VBDS in cases 3 and 6, it has been added to the list of potentially causative agents.
Bile duct loss has been described in association with viral infections, most frequently HCV[15,16] and CMV,[17,18] less frequently HBV[19] and EBV[20], with most published cases occurring in the setting of transplant or other immunocompromised states.
Cholestatic presentation of HCV is well-described in liver transplant recipients[21], however ductopenia is infrequently observed in patients with chronic HCV. Rarely, this bile duct damage is associated with features of chronic cholestasis or progressive bile duct loss[16]. The mechanism of bile duct damage with HCV is not known. Proposed mechanisms include a direct cytopathic effect of HCV (HCV has been identified in bile duct epithelium[22]) and immune-mediated destruction[16]. Thus, HCV co-infection may have played a role in case 4, though other etiologies including advanced untreated HIV infection, CMV infection, and ongoing alcohol and substance abuse may also have contributed.
CMV infection is the best characterized viral cause of bile duct injury, both in neonates and adults[23,24], and is the most common viral cause of VBDS in liver transplants recipients[25]. The exact mechanism of CMV-related duct injury is not known; infection of the biliary epithelium or immune-mediated mechanisms have been proposed. Bile duct injury with CMV viral inclusions in the biliary epithelium has been shown in neonatal hepatitis and with epithelioid granulomas in adults[26]. One series of VBDS in liver transplant identified ongoing CMV replication in hepatocytes resulting in cholangiocyte death[25].
Case 4, a patient with advanced HIV disease not receiving ART and chronic HCV, had a positive CMV PCR in blood; no CMV studies were reported on the liver biopsy, however granulomatous inflammation was seen. The authors proposed CMV infection as the probable cause of the patient’s VBDS. Cases 1 and 2 had low-level positive CMV PCR in blood, however liver biopsy found no evidence of CMV cytopathic effect. Cases 1 and 2 also presented with elevations in lipase consistent with pancreatitis, the etiology of which was not determined. CMV is a recognized cause of pancreatitis in immunocompromised and immunocompetent hosts[27,28], so CMV infection may have played a role in the development of VBDS in these cases.
A role for anti-CMV therapy for the treatment of CMV-associated ductopenic liver disease has not been established. Antiviral treatment in neonatal cholestatic hepatitis remains controversial[29]. The use of antiviral prophylaxis in transplant settings is associated with a decrease in early CMV disease and allograft rejection[30], however the impact on vanishing bile duct syndrome has not been shown.
Many VBDS cases are associated with cancer and cancer therapy, frequently in association with Hodgkin’s lymphoma[31]. Postulated mechanisms for VBDS in lymphoma include lymphoma-induced toxic cytokine release, resulting in bile duct damage either directly or through recruitment of other effector cells, or occult infiltration[31]. Though remission and improvement in cholestasis have been described with lymphoma therapy, the prognosis for VBDS and lymphoma is poor, in part because of chemotherapy dose reduction required to minimize further hepatotoxicity[31,32].
Two of the cases reported here, cases 1 and 6, were diagnosed with lymphoma after diagnosis of VBDS, a pattern of presentation described in non-HIV infected patients[32]. Lymphoma may have played a role in the other fatal HIV-related cases; however, post-mortem examination was not available for cases 2, 4 and 5. Given the high rate of lymphoma in HIV-infected adults[33], suspicion for incident lymphoma in HIV patients with VBDS should be high.
EBV infection could potentially contribute to the development of both lymphoma and VBDS. One patient with Hodgkin’s lymphoma (case 1) was EBV DNA PCR positive in blood and liver, though EBV was not identified by in situ hybridization in liver.
Resolution of VBDS has been reported, both spontaneously[14,34] and in association with immunosuppressants[35,36] and ursodeoxycholic acid[9,37]. In cases of possible drug-induced cholestasis, withdrawal of the drug is recommended, however in HIV-infected patients receiving ART, the risk of viral rebound and immune decline must be weighed against potential benefit of ART discontinuation. The majority of cases progress despite these interventions, however. In progressive cases, liver transplantation is the only potentially curative option. Successful transplantation has been described in idiopathic biliary ductopenia[38], however, recurrence of disease is frequently reported in patients undergoing liver transplant for chronic cholestatic conditions and with transplant-related ductopenia[39]. For HIV-infected adults access to liver transplant remains restricted, though data suggests that HIV-infected patients with non-viral hepatitis associated liver disease have a better prognosis than those with viral hepatitis[40].
In summary, VBDS is a rare cholestatic liver disease that is usually fatal in HIV-positive patients as a result of progressive destruction and disappearance of intrahepatic bile ducts leading to liver failure. Investigations should be aimed at potential associations of VBDS in HIV-positive patients, including medications, co-infection and lymphoma. Given the association of VBDS with Hodgkin’s lymphoma, and the increased incidence of lymphoma in HIV-positive patients, evaluation for lymphoma is reasonable. In HIV-positive patients presenting with an acute cholestatic clinical picture, a high index of suspicion for VBDS, early diagnosis via liver biopsy, and referral to a liver transplant center, may improve clinical outcomes.
The authors thank Dr. Joseph A Kovacs, NIH Clinical Center, Bethesda, MD, for his helpful comments on an earlier version of the manuscript and assistance with optimizing the images included in Figure 1.
P- Reviewers Ohkohchi N, Hackert T S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Reau NS, Jensen DM. Vanishing bile duct syndrome. Clin Liver Dis. 2008;12:203-217. [PubMed] |
| 2. | Aldeen T, Davies S. Vanishing bile duct syndrome in a patient with advanced AIDS. HIV Med 2007; 8: 70-72. HIV Med. 2007;8:573-574. [PubMed] |
| 3. | Hindupur S, Yeung M, Shroff P, Fritz J, Kirmani N. Vanishing bile duct syndrome in a patient with advanced AIDS. HIV Med. 2007;8:70-72. [PubMed] |
| 4. | Kochar R, Nevah MI, Lukens FJ, Fallon MB, Machicao VI. Vanishing bile duct syndrome in human immunodeficiency virus: nevirapine hepatotoxicity revisited. World J Gastroenterol. 2010;16:3335-3338. [PubMed] |
| 5. | Stein DF, Stein B, Halton P, Tirelli R, Meyers J, Berger J, Hwang R, Kim Y, Momeni M, Culliford A. Nevirapine (NVR) Induced Vanishing Bile Duct Syndrome: A Case Report. Am J Gastroenterol. 2006;101:S310-S311. [DOI] [Full Text] |
| 6. | Pau AK, Penzak SR, Boyd SD, McLaughlin M, Morse CG. Impaired maraviroc and raltegravir clearance in a human immunodeficiency virus-infected patient with end-stage liver disease and renal impairment: a management dilemma. Pharmacotherapy. 2012;32:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Ludwig J, Wiesner RH, LaRusso NF. Idiopathic adulthood ductopenia. A cause of chronic cholestatic liver disease and biliary cirrhosis. J Hepatol. 1988;7:193-199. [PubMed] |
| 8. | Desmet VJ. Vanishing bile duct syndrome in drug-induced liver disease. J Hepatol. 1997;26 Suppl 1:31-35. [PubMed] |
| 9. | Taghian M, Tran TA, Bresson-Hadni S, Menget A, Felix S, Jacquemin E. Acute vanishing bile duct syndrome after ibuprofen therapy in a child. J Pediatr. 2004;145:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Juricic D, Hrstic I, Radic D, Skegro M, Coric M, Vucelic B, Francetic I. Vanishing bile duct syndrome associated with azithromycin in a 62-year-old man. Basic Clin Pharmacol Toxicol. 2010;106:62-65. [PubMed] |
| 11. | Gökçe S, Durmaz O, Celtik C, Aydogan A, Güllüoglu M, Sökücü S. Valproic acid-associated vanishing bile duct syndrome. J Child Neurol. 2010;25:909-911. [PubMed] |
| 12. | Vuppalanchi R, Chalasani N, Saxena R. Restoration of bile ducts in drug-induced vanishing bile duct syndrome due to zonisamide. Am J Surg Pathol. 2006;30:1619-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Degott C, Feldmann G, Larrey D, Durand-Schneider AM, Grange D, Machayekhi JP, Moreau A, Potet F, Benhamou JP. Drug-induced prolonged cholestasis in adults: a histological semiquantitative study demonstrating progressive ductopenia. Hepatology. 1992;15:244-251. [PubMed] |
| 14. | Hubscher SG, Buckels JA, Elias E, McMaster P, Neuberger JM. Reversible vanishing bile duct syndrome after liver transplantation: report of 6 cases. Transplant Proc. 1991;23:1415-1416. [PubMed] |
| 15. | Hoffmann RM, Günther C, Diepolder HM, Zachoval R, Eissner HJ, Forst H, Anthuber M, Paumgartner G, Pape GR. Hepatitis C virus infection as a possible risk factor for ductopenic rejection (vanishing bile duct syndrome) after liver transplantation. Transpl Int. 1995;8:353-359. [PubMed] |
| 16. | Kumar KS, Saboorian MH, Lee WM. Cholestatic presentation of chronic hepatitis C: a clinical and histological study with a review of the literature. Dig Dis Sci. 2001;46:2066-2073. [PubMed] |
| 17. | O’Grady JG, Alexander GJ, Sutherland S, Donaldson PT, Harvey F, Portmann B, Calne RY, Williams R. Cytomegalovirus infection and donor/recipient HLA antigens: interdependent co-factors in pathogenesis of vanishing bile-duct syndrome after liver transplantation. Lancet. 1988;2:302-305. [PubMed] |
| 18. | Martelius T, Krogerus L, Höckerstedt K, Bruggeman C, Lautenschlager I. Cytomegalovirus infection is associated with increased inflammation and severe bile duct damage in rat liver allografts. Hepatology. 1998;27:996-1002. [PubMed] |
| 19. | Umit H, Unsal G, Tezel A, Soylu AR, Pamuk GE, Turgut B, Demir M, Tucer D, Ermantas N, Cevikbas U. Vanishing bile duct syndrome in a patient with Hodgkin’s lymphoma and asymptomatic hepatitis B virus infection. Acta Gastroenterol Belg. 2009;72:277-278. [PubMed] |
| 20. | Kikuchi K, Miyakawa H, Abe K, Fujikawa H, Horiuchi T, Nagai K, Kako M. Vanishing bile duct syndrome associated with chronic EBV infection. Dig Dis Sci. 2000;45:160-165. [PubMed] |
| 21. | Narang TK, Ahrens W, Russo MW. Post-liver transplant cholestatic hepatitis C: a systematic review of clinical and pathological findings and application of consensus criteria. Liver Transpl. 2010;16:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Nouri-Aria KT, Sallie R, Mizokami M, Portmann BC, Williams R. Intrahepatic expression of hepatitis C virus antigens in chronic liver disease. J Pathol. 1995;175:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Lurie M, Elmalach I, Schuger L, Weintraub Z. Liver findings in infantile cytomegalovirus infection: similarity to extrahepatic biliary obstruction. Histopathology. 1987;11:1171-1180. [PubMed] |
| 24. | Ten Napel HH, Houthoff HJ, The TH. Cytomegalovirus hepatitis in normal and immune compromised hosts. Liver. 1984;4:184-194. [PubMed] |
| 25. | Arnold JC, Portmann BC, O’Grady JG, Naoumov NV, Alexander GJ, Williams R. Cytomegalovirus infection persists in the liver graft in the vanishing bile duct syndrome. Hepatology. 1992;16:285-292. [PubMed] |
| 26. | Nakanuma Y, Tsuneyama K, Harada K. Pathology and pathogenesis of intrahepatic bile duct loss. J Hepatobiliary Pancreat Surg. 2001;8:303-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Joe L, Ansher AF, Gordin FM. Severe pancreatitis in an AIDS patient in association with cytomegalovirus infection. South Med J. 1989;82:1444-1445. [PubMed] |
| 28. | Oku T, Maeda M, Waga E, Wada Y, Nagamachi Y, Fujita M, Suzuki Y, Nagashima K, Niitsu Y. Cytomegalovirus cholangitis and pancreatitis in an immunocompetent patient. J Gastroenterol. 2005;40:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Vancíková Z, Kucerová T, Pelikán L, Zikmundová L, Priglová M. Perinatal cytomegalovirus hepatitis: to treat or not to treat with ganciclovir. J Paediatr Child Health. 2004;40:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Rubin RH, Kemmerly SA, Conti D, Doran M, Murray BM, Neylan JF, Pappas C, Pitts D, Avery R, Pavlakis M. Prevention of primary cytomegalovirus disease in organ transplant recipients with oral ganciclovir or oral acyclovir prophylaxis. Transpl Infect Dis. 2000;2:112-117. [PubMed] |
| 31. | Hubscher SG, Lumley MA, Elias E. Vanishing bile duct syndrome: a possible mechanism for intrahepatic cholestasis in Hodgkin’s lymphoma. Hepatology. 1993;17:70-77. [PubMed] |
| 32. | Pass AK, McLin VA, Rushton JR, Kearney DL, Hastings CA, Margolin JF. Vanishing bile duct syndrome and Hodgkin disease: a case series and review of the literature. J Pediatr Hematol Oncol. 2008;30:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59-67. [PubMed] |
| 34. | Ramos AM, Gayotto LC, Clemente CM, Mello ES, Luz KG, Freitas ML. Reversible vanishing bile duct syndrome induced by carbamazepine. Eur J Gastroenterol Hepatol. 2002;14:1019-1022. [PubMed] |
| 35. | Jakab SS, West AB, Meighan DM, Brown RS, Hale WB. Mycophenolate mofetil for drug-induced vanishing bile duct syndrome. World J Gastroenterol. 2007;13:6087-6089. [PubMed] |
| 36. | Tajiri H, Etani Y, Mushiake S, Ozono K, Nakayama M. A favorable response to steroid therapy in a child with drug-associated acute vanishing bile duct syndrome and skin disorder. J Paediatr Child Health. 2008;44:234-236. [PubMed] |
| 37. | O‘Brien CB, Shields DS, Saul SH, Reddy KR. Drug-induced vanishing bile duct syndrome: response to ursodiol. Am J Gastroenterol. 1996;91:1456-1457. [PubMed] |
| 38. | Rios R, Herrero JI, Quiroga J, Sangro B, Sola I, Pardo F, Cienfuegos JA, Herraiz M, Prieto J. Idiopathic adulthood ductopenia: long-term follow-up after liver transplantation. Dig Dis Sci. 2001;46:1420-1423. [PubMed] |
| 39. | van Hoek B, Wiesner RH, Ludwig J, Paya C. Recurrence of ductopenic rejection in liver allografts after retransplantation for vanishing bile duct syndrome. Transplant Proc. 1991;23:1442-1443. [PubMed] |
| 40. | Mindikoglu AL, Regev A, Magder LS. Impact of human immunodeficiency virus on survival after liver transplantation: analysis of United Network for Organ Sharing database. Transplantation. 2008;85:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |