Published online Mar 7, 2012. doi: 10.3748/wjg.v18.i9.952
Revised: November 8, 2011
Accepted: January 18, 2012
Published online: March 7, 2012
AIM: To demonstrate the imaging findings of biliopancreatic and pancreatico-biliary reflux in patients with anomalous union of the pancreatico-biliary duct (AUPBD) on gadoxetic acid-enhanced functional magnetic resonance cholangiography (fMRC).
METHODS: This study included six consecutive patients (two men and four women; mean age 47.5 years) with AUPBD. All subjects underwent endoscopic retrograde cholangiopancreatography (ERCP); one subject also underwent bile sampling of the common bile duct (CBD) to measure the amylase level because his gadoxetic acid-enhanced fMRC images showed evidence of pancreatico-biliary reflux of pancreatic secretions. Of the five patients with choledochal cysts, four underwent pylorus-preserving pancreaticoduodenectomy.
RESULTS: The five cases of choledochal cysts were classified as Todani classification I. In three of the six patients with AUPBD, injected contrast media reached the distal CBD and pancreatic duct on delay images, suggesting biliopancreatic reflux. In two of these six patients, a band-like filling defect was noted in the CBD on pre-fatty meal images, which decreased in size on delayed post-fatty meal images, suggesting pancreatico-biliary reflux of pancreatic secretions, and the bile sampled from the CBD in one patient had an amylase level of 113 000 IU/L. In one of the six patients with AUPBD, contrast media did not reach the distal CBD due to multiple CBD stones.
CONCLUSION: Gadoxetic acid-enhanced fMRC successfully demonstrated biliopancreatic reflux of bile and pancreatico-biliary reflux of pancreatic secretions in patients with AUPBD with and without choledochal cysts.
- Citation: Yeom SK, Lee SW, Cha SH, Chung HH, Je BK, Kim BH, Hyun JJ. Biliary reflux detection in anomalous union of the pancreatico-biliary duct patients. World J Gastroenterol 2012; 18(9): 952-959
- URL: https://www.wjgnet.com/1007-9327/full/v18/i9/952.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i9.952
Functional hepatocytes uptake a maximum of 50% of the intravenous (IV) dose of gadoxetic acid (gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid Primovist, Bayer Schering Pharma) administered. Gadoxetic acid is excreted into the bile ducts, allowing visualization of the bile ducts on hepatobiliary phase T1-weighted images. In patients with normal hepatic function, the hepatobiliary phase usually occurs within 20 min of gadoxetic acid administration[1-3].
Hepatocyte-specific agents can be used in a wide range of hepatobiliary applications, and gadoxetic acid-enhanced T1-weighted magnetic resonance cholangiography (MRC) provides additional information to T2-weighted MRC[4,5].
At our institution, we previously evaluated the time sequence of gadoxetic acid-enhanced MRC in 40 normal healthy subjects in 2009; the study was approved by the Korea Food and Drug Administration and our institutional review board. In this previous study, we performed gadoxetic acid-enhanced MRC 60 min after contrast administration and then another 30 min after a fatty meal. In all subjects, we found complete filling of the distal common bile duct (CBD) with contrast on 30-min delayed pre-prandial images, while 50- and 60-min delayed images showed better image quality of bile ducts than early images due to increasing signal-to-noise ratio of extrahepatic bile ducts and bile duct-to-liver contrast-to-noise ratio, and post-prandial images showed gall bladder contraction and more extension of contrast excretion into the small bowel. We found no evidence of contrast media in the pancreatic duct in any subjects (unpublished data). In light of these results, 50- and 60-min delay images after gadoxetic acid administration and 10-, 20- and 30-min delayed images after fatty meal oral uptake were included in the gadoxetic acid-enhanced MRC protocol when requested by the gastroenterologist majoring in pancreatico-biliary disorders. In our experience, these images can help verify bile excretion and flow after gallbladder contraction. We were able to evaluate normal or pathological physiology of bile excretion of the liver and bile flow along the biliary tract. These experiences indicated that gadoxetic acid-enhanced functional magnetic resonance cholangiography (fMRC) could reveal the physiology of bile excretion in certain pathologic conditions, including biliopancreatic and pancreatico-biliary reflux. In this study, we present six patients with anomalous union of the pancreatico-biliary duct (AUPBD) who exhibited biliopancreatic bile reflux and pancreatico-biliary pancreatic juice reflux on gadoxetic acid-enhanced fMRC.
The purpose of this study was to investigate the value of gadoxetic acid-enhanced fMRC in the evaluation of AUPBD, with emphasis on the detection of biliopancreatic bile reflux and pancreatico-biliary pancreatic reflux.
Our institutional review board approved this retrospective study and waived the requirement to obtain informed consent. AUPBD was diagnosed radiologically as a long common channel (> 1.5 cm) or a perpendicular confluence of the CBD and the main pancreatic duct on T2-MRC images and endoscopic retrograde cholangiopancreatography (ERCP). Choledochal cysts were diagnosed when T2-MRC images and ERCP showed typical dilation of the CBD without an obstructive lesion[6-8].
After reviewing 176 patients who underwent gadoxetic acid-enhanced fMRC at our institution for evaluation of biliary pathology due to increased serum bilirubin level, incidental detection of bile duct dilatation on other imaging studies, and right upper quadrant pain between March 2009 and May 2010, we enrolled 6 patients with AUPBD. Of these participants, five had a choledochal cyst. The study group consisted of four women and two men (mean age, 47.5 years; range, 35-64 years), all six of whom underwent ERCP.
We reviewed participant medical records for pertinent clinical features, including medical history, presenting symptoms, results of other imaging studies, and operative records. Of the five patients with both AUPBD and choledochal cysts, four underwent pylorus-preserving pancreaticoduodenectomy. We reviewed these pathology reports and correlated them with imaging findings. One subject underwent bile sampling of the CBD to evaluate the amylase level because his gadoxetic acid-enhanced fMRC showed evidence of pancreatico-biliary reflux of pancreatic secretions.
All magnetic resonance imagings (MRIs) were performed on a 3-Tesla MRI machine (Achieva; Philips Medical Systems, Best, the Netherlands). Patients underwent gadoxetic acid-enhanced fMRC with hepatocyte-specific contrast agents; we obtained both contrast-enhanced and un-enhanced fat-saturated 3D gradient-echo T1-weighted images during the arterial, portal venous, and equilibrium phases. We obtained images 50 and 60 min after administration of gadoxetic acid (Primovist, Bayer Schering Pharma, Berlin, Germany) at a dose of 0.025 mmol/kg of body weight at a flow rate of 1 mL/s, followed by a 10-mL saline flush at the same flow rate, using a IV power injector (Spectris Solaris; MedRad, Indianola, PA, United States). Patients then consumed a fatty meal, and postprandial images were obtained at 10, 20 and 30 min. We used the enhanced-T1 High Resolution Isotropic Volume Examination technique to obtain 3D gradient-echo T1-weighted images. We performed 3D reconstruction using the Maximum Intensity Projection technique 60 min after gadoxetic acid administration and 30 min after the fatty meal. We obtained T2-MRC images as axial and coronal T2 single-shot sequences and maximum intensity projection (MIP) reconstruction images in all patients (Table 1).
| T2-MRC (single shot, SPAIR) | Gadoxetic acid-enhanced MRC (3D-T1-TFE, eTHRIVE) | |||||
| Axial | Coronal | MRCP slab | Axial | Coronal | MIP | |
| TR/TE (ms) | 1475/80 | 2415/235 | 10 695/920 | 3/2 | 3/2 | |
| Flip angle (°) | 90 | 90 | 90 | 10 | 10 | |
| Field of view (mm) | 300 × 350 | 350 × 350 | 250 × 250 | 304 × 330 | 350 × 350 | 315 × 315 |
| Matrix | 276 × 203 | 256 × 254 | 256 × 256 | 220 × 222 | 292 × 292 | 292 × 292 |
| Thickness (mm) | 4 | 2 | 40 | 4 | 2.4 | |
| Gap (mm) | 1.2 | 1 | 2 | 1.2 | ||
Two radiologists reviewed the image sequences of all six patients and classified choledochal cysts according to the Todani classification and AUPBD according to Kimura’s classification (B-P type: a right angle between the bile duct and pancreatic duct or the bile duct inserted into the pancreatic duct; P-B type: an acute angle between the bile duct and pancreatic duct or the pancreatic duct inserted into the bile duct)[9-11].
The radiologists evaluated by consensus the extent of injected contrast media and recorded the presence of biliopancreatic and pancreatico-biliary reflux on all image sequences. The radiologic diagnosis of biliopancreatic bile reflux was made when biliary-excreted contrast media was visible in the main pancreatic duct on gadoxetic acid-enhanced fMRC. The diagnosis of pancreaticobiliary reflux of pancreatic secretions was made when a filling defect was present on pre-prandial images, which decreased in size or was absent on post-fatty meal images. Based on an unpublished study we conducted on normal volunteers, which found that the CBD was filled with excreted gadoxetic acid 30 min after contrast administration and a fatty meal uptake can make more extension of biliary excreted contrast media by contraction of the gallbladder, we therefore considered a filling defect in the distal CBD on 50 and 60 min delayed images as evidence of reflux of pancreatic secretions.
The results of our study are summarized in Table 2. Of the six patients, four had B-P type AUPBD and two had P-B type AUPBD. All five choledochal cysts were Todani classification I (four patients with Ia and one patient with Ic), with confined dilation of the extrahepatic bile duct.
| T2-MRC | Gd-EOB-DTPA-enhanced MRC | ||||||||
| Sex | Age | Type of choledochal cyst1 | Type of AUPBD2 | Surgery | CBD stone | BP | PB | Travel extent of contrast on pre-prandial images3 | Travel extent of contrast on post-prandial images4 |
| F | 52 | Ia | B-P | Yes | No | Yes | NI | MPD | MPD |
| M | 64 | Ia | B-P | Yes | No | Yes | NI | CBD | MPD |
| M | 46 | None | B-P | No | No | NI | Yes | CHD5 | CBD |
| F | 35 | Ia | P-B | Yes | Yes | NI | NI | CHD | CHD |
| F | 48 | Ia | B-P | Yes | No | Yes | NI | CBD | MPD |
| F | 40 | Ic | P-B | Yes | No | NI | Yes | CHD6 | CBD |
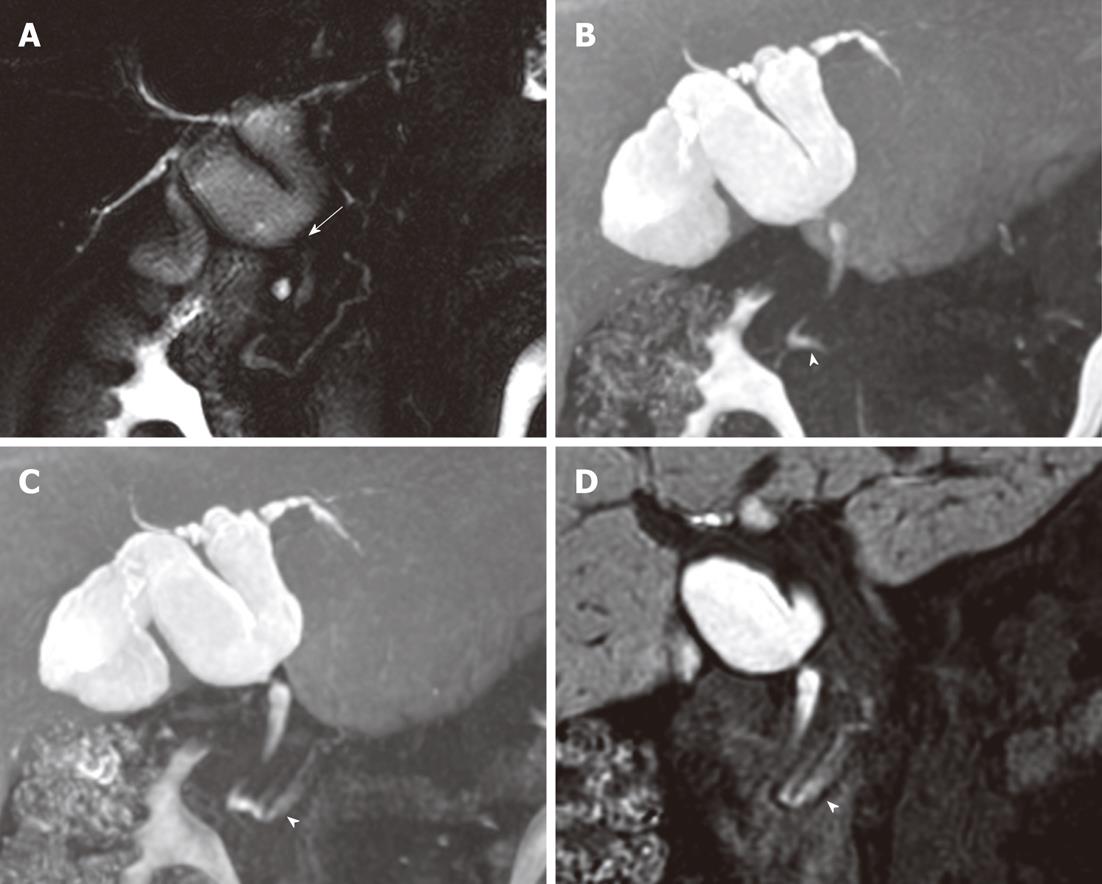
In three of the six patients with AUPBD, injected contrast media reached the distal CBD and pancreatic duct, suggesting biliopancreatic reflux. We observed extension of contrast to the pancreatic duct on images taken 20 min after a fatty meal in two patients (Figure 1). In one patient, we observed contrast extending to the pancreatic duct on images taken 50 min after contrast administration; later images showed contrast extending to the more distal portion of the pancreatic duct (Figure 2). These three patients all had Todani type Ia choledochal cysts and B-P type AUPBD. The one patient with AUPBD without a choledochal cyst had a history of recurrent acute pancreatitis with no history of alcohol abuse.
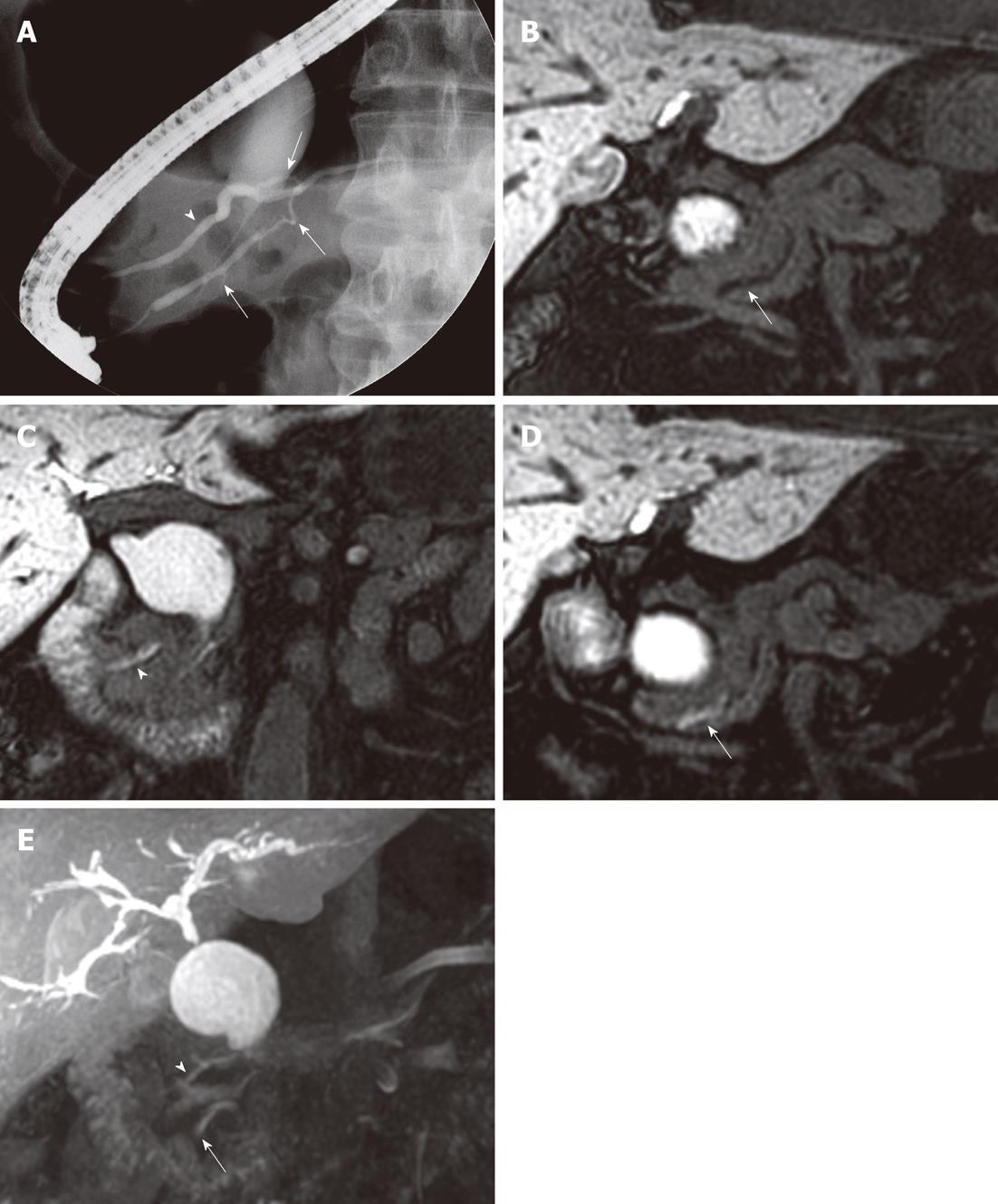
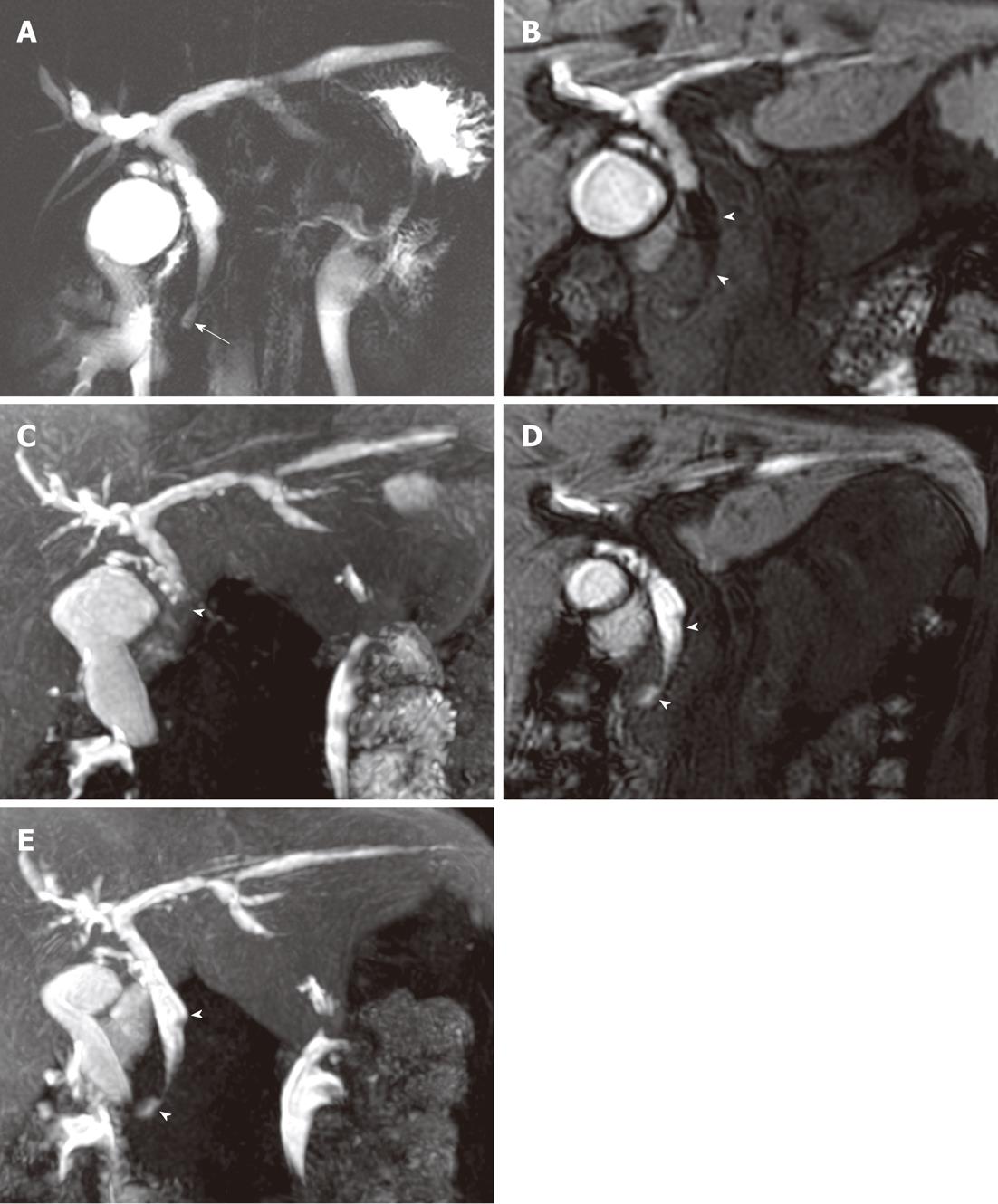
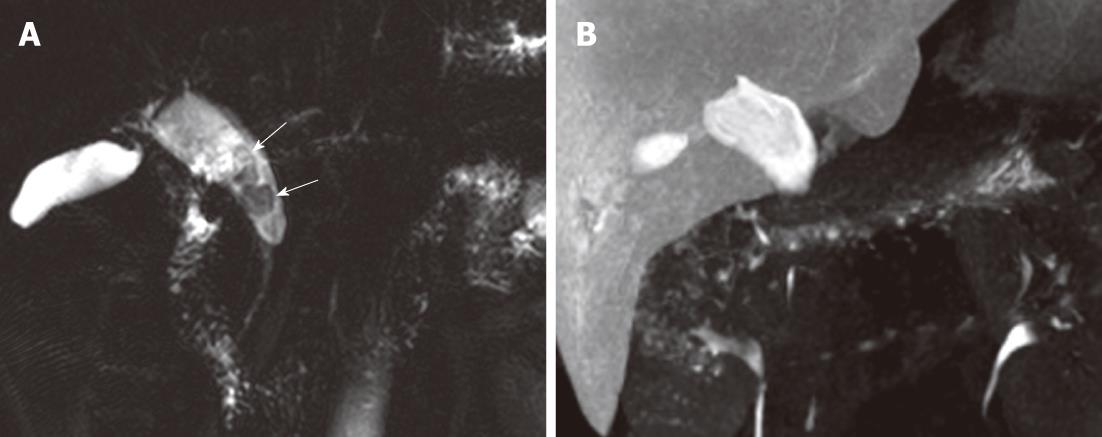
In two patients, we observed a band-like filling defect in the central portion of the distal CBD on pre-fatty meal images. The filling defect decreased in size on post-fatty meal images, which we considered evidence of pancreatico-biliary reflux of pancreatic secretions. One of these two patients had B-P type AUPBD with no combined bile duct dilatation, and the other had P-B type AUPBD with a Todani type Ic choledochal cyst. A bile amylase level of 113 000 IU/L was measured from the CBD in the latter patient (Figure 3). In one patient with a choledochal cyst (Todani Ia and Kimura P-B), contrast did not reach the distal CBD and did not appear to enter the duodenum. We noted multiple CBD stones in this patient (Figure 4).
AUPBD is defined as an anomalous union of the bile and pancreatic ducts outside the duodenal wall proximal to the sphincter of Oddi. The diagnostic criteria for AUPBD include the radiological and anatomical detection of the extramural location of the junction of the pancreatic and biliary ducts in the duodenal wall, as well as radiological detection of a long common channel (> 1.5 cm). Detection of the extramural location is difficult in AUPBD patients with a short common duct (less than 1 cm in length)[6]. The sphincter of Oddi, which regulates the outflow of bile and pancreatic secretions, is deficient in AUPBD due to the long common channel, allowing two-way regurgitation: Pancreatico-biliary reflux of pancreatic secretions and biliopancreatic bile reflux. This reflux can result in various pathological conditions including choledocholithiasis, cholangitis, gallstones, acute pancreatitis, bile duct cancer, gallbladder cancer, and pancreatic ductal carcinoma[12,13]. It is known that choledochal cysts are embryologically associated with AUPBD, and their various clinical signs and symptoms have been shown to be closely related to the presence of AUPBD[14].
Various methods have been reported to confirm biliopancreatic reflux, including operative or postoperative T-tube cholangiography, computered tomography combined with drip infusion cholangiography, histological detection of gallbladder cancer cells in the main pancreatic duct, and bile reflux on the cut surface of the pancreas[12,15,16]. Furthermore, pancreatico-biliary reflux of pancreatic secretions has been confirmed by measurement of bile amylase in the bile duct, secretin-stimulated dynamic MRC, and pancreatography via the minor duodenal papilla[15,17]. However, these methods are limited by their levels of invasiveness, the time required, patient discomfort, and adverse effects of contrast materials[18-20].
Gadoxetic acid is widely used because of its diagnostic efficacy in focal lesions of the liver and its safety[21-24]. In our study, there were no serious adverse effects related to the use of contrast material that required medical management. Although our protocol for functional MR cholangiography to detect biliary reflux in AUPBD patients required an additional 90 min and 70 min when compared with non-contrast enhanced T2-MRC and Gd-enhanced T1-MRC, respectively, it is easy to perform and does not augment the risk of contrast agent-related adverse effects.
As previously described, AUPBD was diagnosed by identifying biliopancreatic bile reflux and pancreatico-biliary pancreatic reflux on gadoxetic acid-enhanced fMRC in addition to the detection of a long common channel on T2-MRC or the customary 20-min delayed gadoxetic acid-enhanced MRC. Delayed hepatobiliary MRC (greater than a 30 min delay) after contrast administration, and MRC of gallbladder contraction induced by a fatty meal were required to detect both types of reflux.
Some researchers report that, if the passage of contrast material through the ampulla of Vater takes longer than 30 to 60 min, it can be considered delayed. In comparison, excretion of contrast material past the ampulla in less than 20 to 30 min is considered normal[4-5]. There have been many reports on gadoxetic acid-enhanced MRC which showed that bile excretion could be visualized by capturing images during the hepatobiliary phase approximately 20 or 30 min after contrast administration[4,5,25,26]. Images taken one hour after contrast administration and again after a fatty meal allow bile to travel further along the normal or anomalous pathway, providing additional information about the patient’s biliary system. Since there are no studies which looked into the optimal phase to observe AUPBD, further study is warranted to simplify the study protocol.
Our fMRC protocol requires additional time for delayed images compared with contrast enhanced T1-MRC. However, gadoxetic acid-enhanced fMRC allows the assessment of bile excretion and pancreas secretion physiology in addition to visualization of bile duct and pancreatic duct morphology, thus obviating the need for additional imaging studies such as hepatobiliary scan and ERCP.
It is difficult to generalize the results of our study due to its small sample size. Nonetheless, one patient in our study was found to have a filling defect in the distal CBD along with an abnormally high amylase level (113 000 IU/L) in the CBD. One study reported the biliary amylase levels of patients with biliopancreatic disease to range widely from less than 10 to 300 000 IU/L[27]. Sai et al[28] reported a mean biliary amylase level of 238 IU/L in patients with no reflux of pancreatic secretions into the bile duct, while Horaguchi et al[27] adopted 168 IU/L as the upper limit of a normal biliary amylase level. Our previous study of gadoxetic acid-enhanced MRC in 40 normal volunteers found no filling defects in the distal CBD up to one hour after contrast administration or after a fatty meal (unpublished data). Taken together, we used these data to presume pancreatico-biliary reflux in the case of a central filling defect in the CBD that diminished after a fatty meal.
In normal physiology, the pressure in the pancreatic duct exceeds the choledochal pressure, allowing pancreatic secretions to flow into the biliary tract rather than reflux into the pancreatic duct where they can cause biliary complications[29,30]. In the case of AUPBD, however, bile can reflux into the pancreatic duct under conditions such as increased pressure in the bile duct due to bile stasis in a choledochal cyst or cholangitis[31].
A study of 2980 patients undergoing ERCP found a 1.7% prevalence of a long common channel. In that study, 13 patients underwent intraoperative cholangiography, 11 of whom were found to have biliopancreatic reflux with an elevated biliary amylase level[32]. In our study, all three patients with biliopancreatic reflux were found to have B-P type AUPBD as well as Todani type Ia choledochal cysts. We hypothesize that bile duct stasis in the dilated bile duct resulted in elevated choledochal pressure, resulting in biliopancreatic bile reflux. Of the two patients with pancreatico-biliary reflux of pancreatic secretions, one had B-P type AUPBD and the other had P-B type AUPBD.
Our study had several limitations. First, the sample size is too small to allow for generalization. Second, the type of choledochal cysts in our study were limited to Todani classification type I, meaning a cystic or fusiform dilation of the extrahepatic bile duct. Further studies that include larger sample sizes and several types of choledochal cysts are required to generalize the imaging findings of biliopancreatic and pancreatico-biliary reflux, as well as to evaluate the diagnostic accuracy of these findings for AUPBD in patients with choledochal cysts.
In conclusion, gadoxetic acid-enhanced fMRC can show biliopancreatic bile reflux and pancreatico-biliary reflux of pancreatic secretions in patients with AUPBD with and without combined Todani type I choledochal cysts.
Gadoxetic acid has both properties of extracellular contrast media, which make dynamic study possible, through the organic anion-transporting polypeptide and is excreted into the bile ducts, allowing visualization of the bile ducts on hepatobiliary phase T1-weighted images. In patients with normal hepatic function, the hepatobiliary phase usually occurs within 20 min of gadoxetic acid administration. From a previous study in normal healthy patients, we know that images obtained after a delayed time period and after a fatty meal allow bile to travel further along the normal or anomalous pathway, providing additional information about the patient’s biliary system.
Gadoxetic acid has hepatobiliary properties which mediate specific uptake of the agent into the hepatocytes. Gadoxetic acid-enhanced magnetic resonance cholangiography (MRC) can visualize the physiology of bile excretion directly, in contrast to conventional T2-weighted MRC which can visualize fluid filled space by heavily T2-weighted and fat-suppressed images. The usefulness of gadoxetic acid-enhanced MRC was demonstrated in many reports in a wide range of hepatobiliary applications including evaluation of biliary tract anomalies, the diagnosis of acute cholecystitis, assessment of postsurgical anatomy and complications, and to determine whether fluid collections communicate with the biliary tree.
The present study clearly showed that biliopancreatic bile reflux and pancreatico-biliary reflux of pancreatic secretions in patients with anomalous union of the pancreatico-biliary duct (AUPBD) could be easily diagnosed using the convenient and safe imaging method of gadoxetic acid-enhanced MR cholangiography.
The present study revealed that biliopancreatic bile reflux and pancreatico-biliary reflux of pancreatic secretions in patients with AUPBD could be diagnosed with gadoxetic acid-enhanced MRC. Further studies which include larger sample sizes and several types of choledochal cysts are required to generalize the imaging findings of biliopancreatic and pancreatico-biliary reflux, as well as to evaluate the diagnostic accuracy of these findings.
Although gadoxetic acid-enhanced functional MRC is not a new method to observe the bile system, it is reasonable and interesting to use it to detect bilio-pancreatic and pancreatico-biliary reflux in patients with AUPBD.
Peer reviewer: Xiao-Peng Zhang, Professor, Peking University School of Oncology, Beijing Cancer Hospital and Institute, No. 52 Haidian District, Beijing 100000, China
S- Editor Gou SX L- Editor Webster JR E- Editor Xiong L
| 1. | Carlos RC, Branam JD, Dong Q, Hussain HK, Francis IR. Biliary imaging with Gd-EOB-DTPA: is a 20-minute delay sufficient? Acad Radiol. 2002;9:1322-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Hamm B, Staks T, Mühler A, Bollow M, Taupitz M, Frenzel T, Wolf KJ, Weinmann HJ, Lange L. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995;195:785-792. [PubMed] |
| 3. | Ringe KI, Husarik DB, Gupta RT, Boll DT, Merkle EM. Hepatobiliary transit times of gadoxetate disodium (Primovist®) for protocol optimization of comprehensive MR imaging of the biliary system--what is normal? Eur J Radiol. 2011;79:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Gupta RT, Brady CM, Lotz J, Boll DT, Merkle EM. Dynamic MR imaging of the biliary system using hepatocyte-specific contrast agents. AJR Am J Roentgenol. 2010;195:405-413. [PubMed] |
| 5. | Lee NK, Kim S, Lee JW, Lee SH, Kang DH, Kim GH, Seo HI. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics. 2009;29:1707-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Matsumoto Y, Fujii H, Itakura J, Matsuda M, Nobukawa B, Suda K. Recent advances in pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 2002;9:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Cha SW, Park MS, Kim KW, Byun JH, Yu JS, Kim MJ, Kim KW. Choledochal cyst and anomalous pancreaticobiliary ductal union in adults: radiological spectrum and complications. J Comput Assist Tomogr. 2008;32:17-22. [PubMed] |
| 8. | The Japanese Study Group On Pancreaticobiliary Maljunction. Diagnostic criteria of pancreaticobiliary maljunction. Journal of Hepato-Biliary-Pancreatic Surgery. 1994;1:219-221. [RCA] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 104] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Kimura K, Ohto M, Ono T, Tsuchiya Y, Saisho H, Kawamura K, Yogi Y, Karasawa E, Okuda K. Congenital cystic dilatation of the common bile duct: relationship to anomalous pancreaticobiliary ductal union. AJR Am J Roentgenol. 1977;128:571-577. [PubMed] |
| 10. | Todani T, Watanabe Y, Toki A, Morotomi Y. Classification of congenital biliary cystic disease: special reference to type Ic and IVA cysts with primary ductal stricture. J Hepatobiliary Pancreat Surg. 2003;10:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 11. | Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263-269. [PubMed] |
| 12. | Kamisawa T, Kurata M, Honda G, Tsuruta K, Okamoto A. Biliopancreatic reflux-pathophysiology and clinical implications. J Hepatobiliary Pancreat Surg. 2009;16:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Kamisawa T, Honda G, Kurata M, Tokura M, Tsuruta K. Pancreatobiliary disorders associated with pancreaticobiliary maljunction. Dig Surg. 2010;27:100-104. [PubMed] |
| 14. | Okada A. [Pancreatico-biliary maljunction and congenital dilatation of bile duct]. Nihon Geka Gakkai Zasshi. 1996;97:589-593. [PubMed] |
| 15. | Kamisawa T, Okamoto A. Biliopancreatic and pancreatobiliary refluxes in cases with and without pancreaticobiliary maljunction: diagnosis and clinical implications. Digestion. 2006;73:228-236. [PubMed] |
| 16. | Fumino S, Tokiwa K, Katoh T, Ono S, Iwai N. New insight into bile flow dynamics in anomalous arrangement of the pancreaticobiliary duct. Br J Surg. 2002;89:865-869. [PubMed] |
| 17. | Hosoki T, Hasuike Y, Takeda Y, Michita T, Watanabe Y, Sakamori R, Tokuda Y, Yutani K, Sai C, Mitomo M. Visualization of pancreaticobiliary reflux in anomalous pancreaticobiliary junction by secretin-stimulated dynamic magnetic resonance cholangiopancreatography. Acta Radiol. 2004;45:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Takahashi M, Saida Y, Itai Y, Gunji N, Orii K, Watanabe Y. Reevaluation of spiral CT cholangiography: basic considerations and reliability for detecting choledocholithiasis in 80 patients. J Comput Assist Tomogr. 2000;24:859-865. [PubMed] |
| 19. | Shimada K, Yanagisawa J, Nakayama F. Increased lysophosphatidylcholine and pancreatic enzyme content in bile of patients with anomalous pancreaticobiliary ductal junction. Hepatology. 1991;13:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Aoki T, Tsuchida A, Kasuya K, Endo M, Kitamura K, Koyanagi Y. Is preventive resection of the extrahepatic bile duct necessary in cases of pancreaticobiliary maljunction without dilatation of the bile duct? Jpn J Clin Oncol. 2001;31:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Gschwend S, Ebert W, Schultze-Mosgau M, Breuer J. Pharmacokinetics and imaging properties of Gd-EOB-DTPA in patients with hepatic and renal impairment. Invest Radiol. 2011;46:556-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Raman SS, Leary C, Bluemke DA, Amendola M, Sahani D, McTavish JD, Brody J, Outwater E, Mitchell D, Sheafor DH. Improved characterization of focal liver lesions with liver-specific gadoxetic acid disodium-enhanced magnetic resonance imaging: a multicenter phase 3 clinical trial. J Comput Assist Tomogr. 2010;34:163-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, Kamura T, Gabata T, Murakami T, Ito K. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010;45:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 24. | Döhr O, Hofmeister R, Treher M, Schweinfurth H. Preclinical safety evaluation of Gd-EOB-DTPA (Primovist). Invest Radiol. 2007;42:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Lee MJ, Kim MJ, Yoon CS. MR cholangiopancreatography findings in children with spontaneous bile duct perforation. Pediatr Radiol. 2010;40:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Dahlström N, Persson A, Albiin N, Smedby O, Brismar TB. Contrast-enhanced magnetic resonance cholangiography with Gd-BOPTA and Gd-EOB-DTPA in healthy subjects. Acta Radiol. 2007;48:362-368. [PubMed] |
| 27. | Horaguchi J, Fujita N, Noda Y, Kobayashi G, Ito K, Takasawa O, Obana T, Endo T, Nakahara K, Ishida K. Amylase levels in bile in patients with a morphologically normal pancreaticobiliary ductal arrangement. J Gastroenterol. 2008;43:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Sai JK, Suyama M, Kubokawa Y, Tadokoro H, Sato N, Maehara T, Iida Y, Kojima K. Occult pancreatobiliary reflux in patients with a normal pancreaticobiliary junction. Gastrointest Endosc. 2003;57:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Csendes A, Kruse A, Funch-Jensen P, Oster MJ, Ornsholt J, Amdrup E. Pressure measurements in the biliary and pancreatic duct systems in controls and in patients with gallstones, previous cholecystectomy, or common bile duct stones. Gastroenterology. 1979;77:1203-1210. [PubMed] |
| 30. | Carr-Locke DL, Gregg JA. Endoscopic manometry of pancreatic and biliary sphincter zones in man. Basal results in healthy volunteers. Dig Dis Sci. 1981;26:7-15. [PubMed] |
| 31. | Sugiyama M, Atomi Y, Kuroda A. Pancreatic disorders associated with anomalous pancreaticobiliary junction. Surgery. 1999;126:492-497. [PubMed] |
| 32. | Kamisawa T, Amemiya K, Tu Y, Egawa N, Sakaki N, Tsuruta K, Okamoto A, Munakata A. Clinical significance of a long common channel. Pancreatology. 2002;2:122-128. [PubMed] |