Published online Mar 7, 2012. doi: 10.3748/wjg.v18.i9.944
Revised: August 18, 2011
Accepted: August 27, 2011
Published online: March 7, 2012
AIM: To investigate the safety and feasibility of our original single-incision laparoscopic cholecystectomy (SILC) for acute inflamed gallbladder (AIG).
METHODS: One hundred and ten consecutive patients underwent original SILC for gallbladder disease without any selection criteria and 15 and 11 of these were diagnosed with acute cholecystitis and acute gallstone cholangitis, respectively. A retrospective review was performed not only between SILC for AIG and non-AIG, but also between SILC for AIG and traditional laparoscopic cholecystectomy (TLC) for AIG in the same period.
RESULTS: Comparison between SILC for AIG and non-AIG revealed that the operative time was longer in SILC for AIG (97.5 min vs 85.0 min, P = 0.03). The open conversion rate (2/26 vs 2/84, P = 0.24) and complication rate (1/26 vs 3/84, P = 1.00) showed no differences, but a need for additional trocars was more frequent in SILC for AIG (5/24 vs 3/82, P = 0.01). Comparison between SILC for AIG and TLC for AIG revealed no differences based on statistical analysis.
CONCLUSION: Our original SILC technique was adequately safe and feasible for the treatment of acute cholecystitis and acute gallstone cholangitis.
- Citation: Sasaki K, Watanabe G, Matsuda M, Hashimoto M. Original single-incision laparoscopic cholecystectomy for acute inflammation of the gallbladder. World J Gastroenterol 2012; 18(9): 944-951
- URL: https://www.wjgnet.com/1007-9327/full/v18/i9/944.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i9.944
Single-incision laparoscopic cholecystectomy (SILC) has recently gained popularity, just as laparoscopic cholecystectomy (LC) became popular in the early 1990s. Although LC was initially established as the treatment of choice for symptomatic cholecystolithiasis, LC for acute inflammation of the gallbladder (AIG), such as that caused by acute cholecystitis and gallstone cholangitis, was considered to be a contraindication. The complication rate for LC was believed to be higher than that for AIG. Ultimately, LC was accepted as a safe procedure for AIG, when it was performed by an expert in laparoscopic techniques[1]. As with LC, SILC for AIG is currently considered to be a contraindication because of its technical difficulty and infancy. SILC is developing, and there are a wide variety of operative techniques. The safety and feasibility of these operative techniques also varies; some are adequate for the treatment of AIG, but others are not. In the near future, SILC will probably be considered an acceptable treatment and the standard operative technique for AIG, effectively eliminating inappropriate operative techniques.
Here, we report our experience with SILC for AIG and explore the safety and feasibility of our original SILC technique.
A total of 110 consecutive patients underwent SILC for gallbladder disease from July 2009 to November 2010, without any selection criteria. Of these 110 patients, 15 and 11 were diagnosed with acute cholecystitis and acute gallstone cholangitis, respectively. We performed both SILC and traditional laparoscopic cholecystectomy (TLC) during the same period. There were four staff surgeons in our department, each of whom operated on or supervised the patients, who came to or were referred to their own outpatient clinics. Two of the four staff surgeons performed our original SILC technique routinely, and the other two performed a traditional four-port technique. All SILC operations were performed by staff surgeons only. However, several TLC procedures were performed by young surgical residents under the supervision of a staff surgeon. A surgical resident was considered eligible for performing TLC only if he/she had 2-6 years of experience in general surgery. Staff surgeons performed TLC in cases with severe inflammation or dense adhesions and in cases in which malignancy was suspected. There was no predesigned patient selection bias between the patients in the SILC and TLC groups.
A diagnosis of acute cholecystitis and the presence of acute cholangitis were determined based on the Tokyo guidelines and criteria for acute cholecystitis and cholangitis, as follows. Patients exhibiting one of the local signs of inflammation, such as a Murphy’s sign or a mass, or tenderness in the right upper quadrant, as well as one of the systemic signs of inflammation, such as fever or elevated C-reactive protein (CRP) level, were diagnosed as having acute cholecystitis. Patients in whom suspected clinical findings were confirmed by diagnostic imaging were also diagnosed with acute cholecystitis (Table 1)[2]. Patients were classified as grade I (mild), grade II (moderate), or grade III (severe), according to the severity grading of the Tokyo guidelines for acute cholecystitis (Table 1)[2].
| Diagnosis criteria |
| A: Local signs of inflammation |
| Murphy’s sign |
| Rright upper quadrant mass/pain/tenderness |
| B: Systemic signs of inflammation |
| Fever |
| Elevated C-reactive protein |
| Elevated white blood cell count |
| C: Imaging findings |
| Sonographic Murphy sign |
| Thickened gallbladder wall |
| Enlarged gallbladder |
| Pericholecystic fluid collection |
| Sonolucent layer in the gallbladder wall |
| Definite diagnosis |
| One item in A and one in B are positive |
| C confirms the diagnosis when acute cholecystitis is suspected clinically1 |
| Severity assessment |
| Mild (grade I) |
| Acute cholecystitis does not meet the criteria of severe (grade III) or moderate (grade II) acute cholecystitis or acute cholecystitis in a healthy patient with no organ dysfunction and mild inflammatory changes in the gallbladder, making cholecystectomy a safe and low risk operative procedure |
| Moderate (grade II) |
| Elevated WBC count (> 18 000/mm3) |
| Palpable tender mass in the right upper quadrant |
| Duration of complains > 72 h2 |
| Marked local inflammation (biliary peritonitis, pericholecystic abscess, hepatic abscess, gangrenous cholecystitis, emphysematous cholecystitis) |
| Severe (grade III) |
| Acute cholecystitis associated with dysfunction of any one of the following organs/systems |
| Cardiovascular dysfunction (hypotension requiring treatment with dopamine ≥ 5 μg/kg per minute, or any dose of dobutamine) |
| Neurological dysfunction (decreased level of consciousness) |
| Respiratory dysfunction (PaO2/FiO2 ratio < 300) |
| Renal dysfunction (oliguria, creatinine > 2.0 mg/dL) |
| Hepatic dysfunction (PT-INR > 1.5) |
Acute cholangitis was diagnosed if the clinical manifestations of Charcot’s triad, namely, fever and/or chills, abdominal pain (right upper quadrant or epigastric), and jaundice, were present. When not all components of the triad were present, then a definite diagnosis could be made if laboratory and imaging data supported the evidence of inflammation, and biliary obstruction was revealed (Table 2)[3]. We diagnosed patients with acute cholangitis due to gallstones and/or debris with gallstone cholangitis. Acute cholangitis patients were also classified as grade I, II or III, according to the severity grading of the Tokyo guidelines for acute cholangitis (Table 2)[3].
| Diagnosis criteria (suspected diagnosis and definite diagnosis) |
| Severity assessment |
| A: Clinical context and clinical manifestations |
| History of biliary disease |
| Fever and/or chills |
| Jaundice |
| Abdominal pain (right upper quadrant or upper abdominal) |
| B: Laboratory data |
| Evidence of inflammatory response1 |
| Abnormal liver function tests2 |
| C: Imaging findings |
| Biliary dilation, or evidence of etiology (stricture, stone, stent, etc.) |
| Two or more items in A |
| Charcot’s triad (2 + 3 + 4) |
| Two or more items in A + both items in B + C |
| Severity assessment |
| Mild (grade I) |
| Acute cholangitis that responds to initial medical treatment3 |
| Moderate (grade II) |
| Acute cholangitis that does not respond to initial medical treatmentc and is not accompanied by organ dysfunction |
| Severe (grade III) |
| Acute cholangitis that is associated with the onset of dysfunction at least in any one of the following organs/systems |
| Cardiovascular system; hypotension requiring dopamine ≥ 5 μg/kg per minute, or any dose of dobutamine |
| Nervous system: disturbance of consciousness |
| Respiratory system: PaO2/FiO2 ratio < 300 |
| Kidney: serum creatinine > 2.0 mg/dL |
| Liver: PT-INR > 1.5 |
| Hematological system: platelet count < 100 000/μL |
The general policy for acute cholecystitis in our department is delayed surgery following medical treatment, such as antibiotics or percutaneous cholecystotomy. The general policy for acute gallbladder cholangitis in our department is delayed surgery following medical treatment, with endoscopic stone extraction. The timing of surgery depends upon the extent of inflammation, and we typically perform LC after inflammation has decreased considerably.
The definition of AIG in this study was acute cholecystitis, excluding acalculous cholecystitis; acute cholangitis with gallbladder stones or/and debris; and choledocholithiasis. Even if the patient had concomitant gallstone pancreatitis, we defined the condition simply as acute gallstone cholangitis. We defined the operation for AIG as surgery that was performed within 4 mo of the primary acute inflammation.
We performed magnetic resonance cholangiopancreatography for all patients undergoing LC to gain preoperative information about the anatomy of the biliary tree and the presence of common bile duct stones. Perioperative patient care was identical between patients undergoing TLC and SILC.
A retrospective review of prospectively collected data was performed to investigate the safety and feasibility of SILC for AIG. We compared multiple variables, not only between SILC for AIG and SILC for non-AIG, but also between SILC for AIG and TLC for AIG during the same period. A comparison between SILC for AIG and SILC for non-AIG was performed to reveal the influence of AIG on SILC. In this analysis, operative findings, such as intra-abdominal adhesion and gallbladder thickening, were evaluated by the same hepatopancreatobiliary specialist. Additionally, the comparison between SILC for AIG and TLC for AIG was performed to reveal the influence of the operative method of LC on AIG. In the comparison between SILC for AIG and TLC for AIG, the maximum white blood cell (WBC) count and CRP level during the acute inflammatory phase were categorized as follows: WBC > 14 000/mm3 or not and CRP level > 10 mg/dL or not. These concrete cutoffs were determined to be indicators of severe inflammation according to the Japanese version of the Tokyo guidelines for acute cholecystitis and acute cholangitis[4].
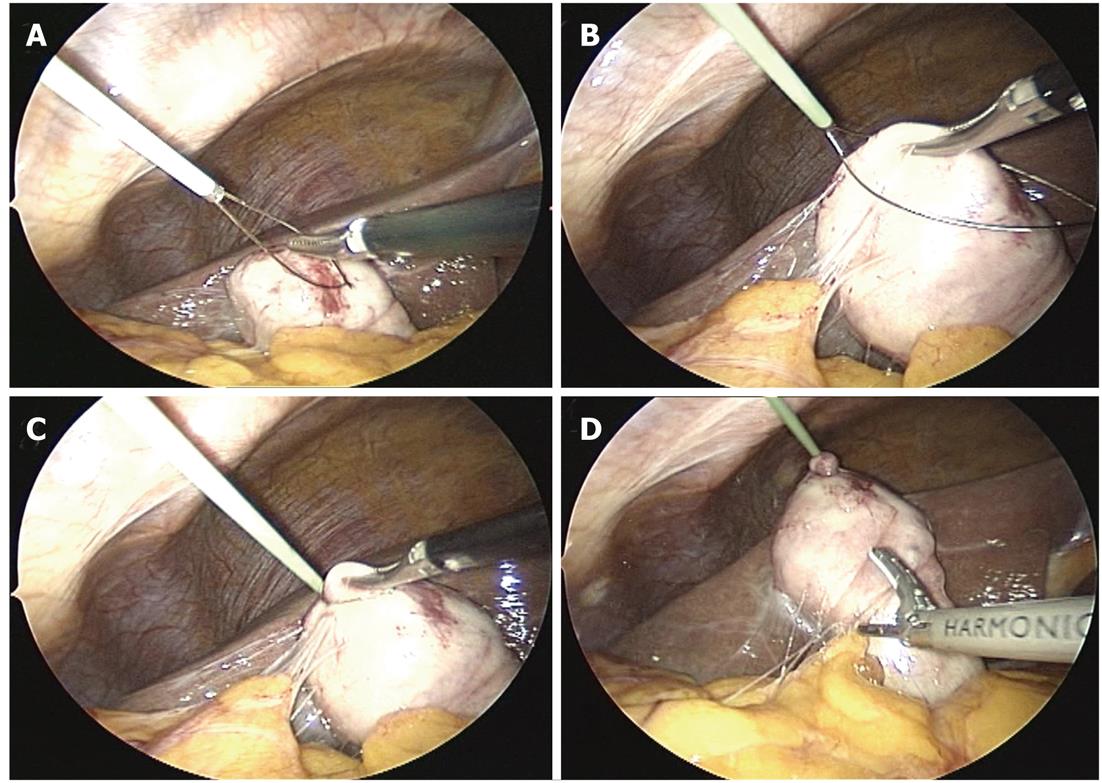
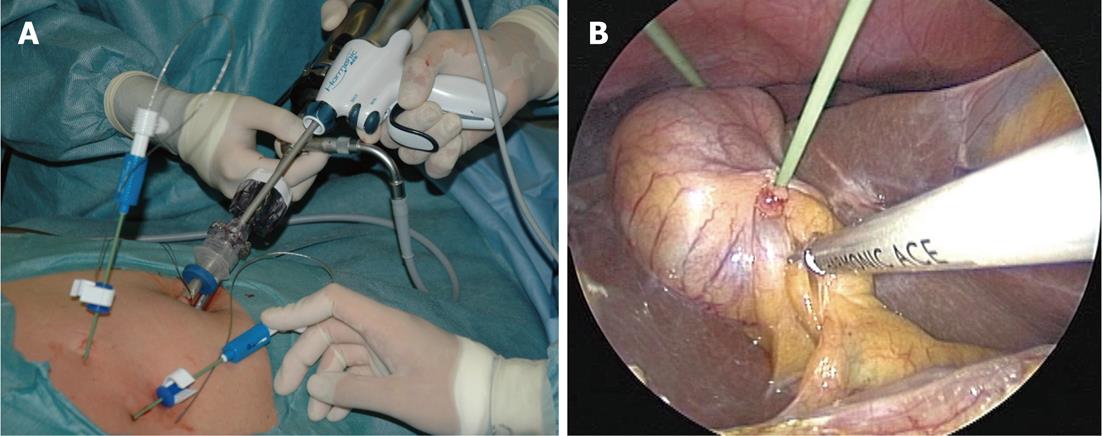
The operative technique and analysis of our original SILC technique have been described in another study[5]; here, we describe the procedure briefly as follows. The patients were placed in a low modified lithotomy position; the operator stood between the legs, the laparoscopist stood on the left side, and the second assistant stood on the right. A 10-20-mm skin incision was created by pulling out the umbilicus. After exposing the fascia, a 5-mm, 95-mm-long trocar was placed using an open approach. Pneumoperitoneum was established, and another 5-mm, 70-mm-long trocar was placed through the same skin incision but through a separate fascial incision, which was created as far as possible above the first trocar. The first trocar was for the 30-degree laparoscope, and the second trocar was for the grasper and laparoscopic coagulating shears (LCSs). After inspection of adhesions and the gallbladder, a 2-mm wire loop retractor (WLR) (Mini Loop Retractor II, Covidien, Tokyo, Japan) was inserted from the right subcostal space, and the body or fundus of the gallbladder was retracted. The WLR was used as follows: (1) the grasper was inserted into the wire, and the tissue needing retraction was grasped; and (2) the wire was wrung, and retraction was performed (Figure 1). If the gallbladder was so distended that it could not be grasped, then bile was aspirated and decompressed using a 16-gauge needle for intraoperative cholangiography (IOC). Both the dissection of the adhesions and exposure of the infundibulum of the gallbladder were performed mainly by LCSs. The second WLR was then inserted obliquely above the first to retract the neck of the gallbladder; this WLR was used as the grasper for retraction in the lateral direction (Figure 2). After visualizing the so-called “critical view of safety,” we performed routine IOC, using the catheter insertion technique. Closure of the cystic duct and dissection of the gallbladder from the liver bed were performed in the same manner as for TLC. The cystic duct was closed using a 5-mm laparoscopic clip. The gallbladder was extracted with a specimen bag through the umbilicus. The final appearances of the umbilical incision and the WLR insertion site at 3 mo after surgery were virtually scarless.
All statistical analyses were performed with SPSS II for Windows software (SPSS, Chicago, IL, United States). Parametric summary statistics are presented as mean ± SD,
whereas nonparametric summary statistics are presented as medians with interquartile ranges. Categorical data were analyzed using the χ2 test or Fisher’s exact test, as appropriate. The two-sample t test was used to test the hypothesis of equality of means, and the Mann-Whitney U test was used to test the hypothesis of equality of medians. P < 0.05 was considered statistically significant.
A total of 110 patients underwent attempted SILC and 191 patients underwent attempted TLC during the same period. A total of 23.6% (26/110) of SILCs and 28.3% (54/191) of TLCs were diagnosed and operated on as AIG. The comparison of the patients’ demographics and operative outcomes between SILC for AIG and SILC for non-AIG are shown in Table 3. Patients’ demographics between SILC for AIG and SILC for non-AIG showed no significant differences without ASA scores. SILC for AIG patients included more patients with complicated backgrounds, but there was only one ASA III patient who had severe systemic disease.
| Patient demographics | SILC for AIG | SILC for non-AIG | P value |
| n | 26 | 84 | |
| Age (yr) median (range) | 61.5 (22-81) | 56.5 (31-81) | 0.06 |
| Sex (male/female) | 12/14 | 42/42 | 0.82 |
| BMI median (range) | 22.0 (18.4-29.4) | 22.2 (16.0-30.0) | 0.85 |
| ASA score I/II/III | 14/11/1 | 65/19/0 | 0.02 |
| Previous upper abdominal surgery (yes/no) | 2/24 | 4/80 | 0.63 |
| Indication for operation | Acute cholecystitis 15 | Symptomatic gallstone 65 | |
| Acute gallstone cholangitis 11 | Choledochlithiasis 2 | ||
| No inflammation 17 | |||
| Operative outcome | |||
| Operative time (min) | 0.03 | ||
| Median (range) | 97.5 (60-163) | 85 (45-195) | |
| mean (SD) | 105.7 (31.9) | 91.0 (29.3) | |
| Intra-abdominal adhesion none to mild/moderate/severe | 8/15/3 | 52/27/15 | 0.02 |
| Gallbladder wall thickening | 16/2/8 | 66/14/4 | < 0.01 |
| none to mild/moderate/severe | |||
| IOC completion1 | 23/24 | 81/82 | 0.4 |
| Conversion to open cholecystectomy | 2 | 2 | 0.24 |
| Bile spillage | 9 | 15 | 0.1 |
| Use of additional port site | 5 | 3 | 0.01 |
| Complication (total) | 1 | 3 | 1.00 |
| Wound infection | 1 | 2 | |
| Bile duct injury | 0 | 1 |
In the operative outcomes, intra-abdominal adhesions and gallbladder wall thickening were more frequently seen in SILC for AIG. The operative time was significantly longer in SILC for AIG (97.5 min vs 85 min, P = 0.03). The open conversion rate (2/26 vs 2/84, P = 0.24) and complication rate (1/26 vs 3/84, P = 1.00) showed no significant differences, but a need for additional trocars was significantly more frequent in SILC for AIG (5/24 vs 3/82, P = 0.01). There were two cases of open conversion in SILC for AIG. The first case involved gangrenous cholecystitis with a cholecystocholedochal fistula, which we noticed when we dissected the gallbladder from the liver bed, and we converted to an open procedure to repair the fistula. This case also suffered wound infection, which was the only operative complication with SILC for AIG. The second case involved dense adhesions in a patient with severe bronchial asthma; in this case, we converted to laparotomy to shorten the operative time. Additional trocars were required in five cases of SILC for AIG; three required an additional 5-mm trocar in the right subcostal space, one required an additional 10-mm trocar in the epigastrium to perform intraoperative ultrasonography, and one required two 5-mm trocars in the subcostal spaces due to stone dissemination.
The comparison of patients’ demographics and operative outcomes between SILC for AIG and TLC for AIG is shown in Table 4. The two groups were similar with respect to sex, age, body mass index, indication for surgery, preoperative inflammation findings, severity assessment following Tokyo guidelines, and time between onset and operation. In the severity assessment, SILC for AIG included five moderate cases: three showed WBC counts > 18 000/mm3, one showed gangrenous cholecystitis, and one acute cholangitis case did not respond to initial medical treatment and required emergency endoscopic stone extraction. Furthermore, SILC for AIG included two severe cholecystitis cases; both cases showed remarkable inflammation findings (WBC > 22 000/mm3 and CRP > 25 mg/dL), cardiovascular dysfunction, and neurologic dysfunction, and required biliary drainage. Of 54 TLCs for AIG, 39 were performed by surgical residents under the supervision of staff surgeons. However, all SILCs were performed by staff surgeons.
| SILC for AIG | TLC for AIG | P value | |
| Patient demographics | |||
| n | 26 | 54 | |
| Age (yr) median (range) | 61.5 (22-81) | 61 (25-89) | 0.94 |
| Sex (male/female) | 14/12 | 34/20 | 0.47 |
| BMI median (range) | 22.0 (18.4-29.4) | 22.8 (15.4-32.0) | 0.53 |
| ASA score I/II/III | 14/11/1 | 25/25/4 | 0.73 |
| Previous upper abdominal surgery (yes/no) | 2/24 | 5/49 | 0.59 |
| Indication for operation | Acute cholecystitis 14 | Acute cholecystitis 29 | 0.81 |
| Acute gallstone cholangitis 11 | Acute gallstone cholangitis 25 | ||
| Max WBC count in acute phase | 0.78 | ||
| WBC > 14 000 | 5 | 13 | |
| WBC < 14 000 | 21 | 41 | |
| Max CRP in acute phase | 0.44 | ||
| CRP > 10 | 6 | 18 | |
| CRP < 10 | 20 | 36 | |
| Severity assessment by Tokyo Guidelines Grade I/II/III | 19/5/2 | 38/13/3 | 0.85 |
| Day from onset to operation | 19 (6-111) | 20 (8-104) | 0.82 |
| Clinical result | |||
| Operative time (min) | 0.12 | ||
| Median (range) | 97.5 (60-163) | 87.5 (35-245) | |
| mean (SD) | 105.7 (31.9) | 94.7 (34.4) | |
| Surgeon | 26/0 | 16/39 | |
| Staff surgeon/surgical resident | |||
| IOC completion1 | 23/24 | 42/49 | 0.26 |
| Conversion to open cholecystectomy | 2 | 5 | 1 |
| Bile spillage | 9 | 14 | 0.44 |
| Complication | 1 | 7 | 0.26 |
Even when the operative outcome did not reach statistical significance, the operative time of SILC for AIG was 10 min longer than that of TLC for AIG (97.5 min vs 87.5 min, P = 0.12). The open conversion rate (2/26 vs 5/54, P = 1.00) and complication rate (1/26 vs 7/54, P = 0.26) showed no significant differences. All open conversions in TLC for AIG were performed for unclear anatomic relationships due to severe adhesions. Complications in TLC for AIG were as follows: postoperative hemorrhage in two, fluid collection in two, paralytic ileus in one, intra-abdominal abscess formation in one, and wound infection in one.
At our institution, we performed SILC with very liberal selection criteria. We performed SILC without any contraindications, and we adopted SILC for AIG. The above findings clearly showed that neither acute cholecystitis nor acute gallstone cholangitis were contraindications for our original SILC technique.
LC for AIG was considered to be an absolute contraindication in the early laparoscopic era. The fear of an increased risk of complications, compared with open cholecystectomy, was unfounded based on the results of randomized controlled trials[6]. However, the conversion and complication rates of LC for AIG were greater than those of elective LC for other indications[7,8]. In the present study, the open conversion rate of SILC for AIG was 7.7% (2/26), which was a favorable result when compared with the results of TLC for AIG[6-11]. Open conversion itself is not a complication, but failure of the operative procedure is; surgeons are frequently obliged to convert due to uncertain anatomy, uncontrollable bleeding, and difficulty with manipulating swollen and thickened gallbladders. The greater the open conversion rate is, the less safe the operative technique. Thus, our original SILC for AIG was proved to be sufficiently safe given the open conversion rate. The complication rate of our SILC for AIG was 3.8% (1/26), which was also more favorable than the reported complication rates of TLC for AIG[6-11].
With regard to feasibility, even if we considered both open conversion and the requirement for additional trocars to be operative method failures, 73% (19/26) of AIG cases that fulfilled the Tokyo guidelines underwent virtually scarless operations. Considering that the reported open conversion rate in the initial experiences of TLC for AIG was 33.7%, and that it remained at 10%-25% after a decade of experience, our original SILC technique is sufficiently feasible for AIG[6-11].
However, SILC for AIG required additional trocars significantly more frequently than SILC for non-AIG. Looking back over individual cases, we especially needed additional trocars in cases with thickened gallbladder walls. In the early cases, we were inexperienced in handling WLRs and could not grasp the thickened gallbladder walls; consequently, we required additional trocars to grasp and manipulate the inflamed gallbladders. After we gained experience and became familiar with using WLRs, we could grasp even severely thickened gallbladder walls. Of the first 12 cases, four required additional trocars, but only one of the next 12 cases required an additional trocar (excluding two open conversion cases). We are convinced that, after the accumulation of another dozen cases, we will be able to perform SILC for AIG with a lower combined conversion rate (open conversion + requirement for additional trocars).
The analysis of SILC for AIG and TLC and AIG revealed no significant differences based on statistical comparison. However, all SILCs for AIG were performed by hepatopancreatobiliary specialists, and satisfactory operative results depended partly on the surgeons’ experiences.
The sufficient safety and feasibility of SILC for AIG achieved in our study were derived from some unique characteristics of our original technique. First, we employed two WLRs, which were sufficient to accomplish retraction, even in severely thickened, inflamed gallbladder walls. Second, we inserted only two trocars into the umbilical incision, which resulted in good handling of the instruments and gallbladder manipulation, without interfering with the other instruments or the laparoscope. Third, almost all dissections were performed by LCS, which allowed us to operate easily in dense fibrosis and tissue with neovascularization secondary to inflammation. Fourth, inserting two WLRs from the subcostal margin and using LCS created a triangulation of devices that allowed us to manipulate the gallbladder, as we did in TLC. All of these characteristics allowed us to employ the same operative technique and anatomical knowledge as in TLC, and ultimately, we could perform SILC without selection criteria.
In this study, we adopted the Tokyo guidelines for the diagnosis and severity assessment of gallbladder inflammation. Many reports about TLC for AIG exist in the literature, but the diagnostic criteria and severity assessment were inconsistent among the studies. Employment of the guidelines, which are based on a systemic literature review and the consensus of experts, allowed us to compare each operative result. SILC is still developing, and it has not yet been standardized. Many original procedures exist, but some may not be suitable to perform in cases of AIG. Comparisons using complication and conversion rates under the same diagnostic criteria and severity assessments should become standards of the ideal operative technique.
There are some limitations to our study. First, we did not perform early operations for acute cholecystitis, even though several prospective, randomized, controlled studies comparing early and delayed LC have concluded that early LC is safe and decreases the length of hospital stay[12]. We prefer delayed elective surgery, not only for medical reasons but also for social reasons. In our experience, we struggled with difficult bleeding from inflamed tissue in the early phase of AIG; we also struggled with dense adhesions in the delayed phase of AIG. Meticulous dissection of fibrous tissue and sure exposure of Calot’s triangle allowed us to operate safely for AIG, although the operative time was slightly longer. Regarding social reasons, our institution is a tertiary referral hospital with only four hepatopancreatobiliary specialists. Considering the availability of surgical staff, anesthesiologists, and operating rooms, we prefer to delay elective surgery unless a patient needs an emergency cholecystectomy. Similar to our institute, there has been a general reluctance to adopt this approach in the United Kingdom, despite increasing evidence supporting early cholecystectomy; currently, only 20% of surgeons perform cholecystectomies during acute cholecystitis[13].
In this study, we showed sufficient operative results for the safety and feasibility of the operative technique, even though delayed surgery is generally considered technically difficult because of acute inflammation and subsequent fibrosis, dense adhesions, and neovascularization. We are convinced that our original SILC technique can be adapted to early operations for AIG if needed. The second limitation is that we evaluated only 15 cases of SILC for acute cholecystitis and 11 cases of SILC for acute gallbladder cholangitis. These numbers of cases were too small to conclude that our SILC is statistically safe and feasible, and we must continue to analyze cases. Third, the occupied percentages of acute gallstone cholangitis in AIG in this study were 42% in SILC and 46% in TLC, which were greater than the reported prevalence of acute gallstone cholangitis[14,15]. This finding may have been because our institution is a tertiary referral hospital and there were many referrals of acute gallstone cholangitis that required endoscopic stone extraction from other institutes.
In conclusion, the significant influence of AIG on SILC in this study was due to the longer operative time and high rate of requirement for additional trocars. The open conversion rate of SILC for AIG was increased to a similar degree as that of TLC for AIG. In experienced hands, the influence of the operative method seemed to decrease, and SILC for AIG could be satisfactorily performed, comparable to TLC for AIG. Our original SILC technique was adequately safe and feasible for the treatment of AIG, with greater requirements for extra ports than non-AIG cases, and a slightly greater conversion rate. We are convinced that, in the near future, SILC will be one of the principal techniques for the management of AIG, just as TLC for AIG evolved from absolute contraindication to the first-choice standard treatment.
Single-incision laparoscopic cholecystectomy (SILC) has recently gained popularity, just as laparoscopic cholecystectomy (LC) became popular in the early 1990s. Although LC was initially established as the treatment of choice for symptomatic cholecystolithiasis, LC for acute inflammation of the gallbladder (AIG), such as that caused by acute cholecystitis and gallstone cholangitis, was considered to be a contraindication. The complication rate for LC was believed to be possibly higher than that of AIG. Ultimately, LC was accepted as a safe procedure for AIG, when it is performed by an expert at laparoscopic techniques. As with LC, SILC for AIG is currently considered to be a contraindication because of its technical difficulty and infancy.
SILC is developing, and there is a wide variety of operative techniques. There is also variety in the safety and feasibility of these operative techniques; some are adequate for the treatment of AIG, but some are not. In the near future, SILC will be considered an acceptable treatment and the standard operative technique for AIG, effectively eliminating inappropriate operative technique.
The authors investigated the feasibility and safety of their original SILC technique for AIG. The original SILC technique was proven to be adequately safe and feasible for the treatment of AIG by statistical analysis. The sufficient safety and feasibility of SILC for AIG achieved was derived from some unique characteristics of the technique. First, the authors used two wire loop retractors, which were sufficient to accomplish retraction even in severely thickened, inflamed gallbladder walls. Second, they inserted only two trocars into the umbilical incision, which resulted in good handling of instruments and gallbladder manipulation, without interfering with other instruments or the laparoscope. Third, inserting two wire loop retractor from the subcostal margin and using laparoscopic coagulating shear maintained a triangulation of devices that allowed them to manipulate the gallbladder as they did with traditional laparoscopic cholecystectomy.
This is accepted for publication because this study represent a lot of experience of SILC for AIG.
Peer reviewers: Hayrullah Derici, MD, Associate Professor, Department of General Surgery, Balıkesir University Medical Faculty, 10145 Balıkesir, Turkey; Ashok Kumar, MD, Department of Surgical Gastroenterology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, 226014 Lucknow, India
S- Editor Lv S L- Editor Kerr C E- Editor Xiong L
| 1. | 1 Yamashita Y, Takada T, Kawarada Y, Nimura Y, Hirota M, Miura F, Mayumi T, Yoshida M, Strasberg S, Pitt HA. Surgical treatment of patients with acute cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:91-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Hirota M, Takada T, Kawarada Y, Nimura Y, Miura F, Hirata K, Mayumi T, Yoshida M, Strasberg S, Pitt H. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:78-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 3. | Wada K, Takada T, Kawarada Y, Nimura Y, Miura F, Yoshida M, Mayumi T, Strasberg S, Pitt HA, Gadacz TR. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:52-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 4. | Takada T. Diagnostic criteria and severity assessment of acute cholecystitis (2005). Tokyo: Igaku Tosho Shuppan 2005; 40. |
| 5. | Watanabe G, Sasaki K, Matsuda M, Hashimoto M. Initial experience of trans-umbilical double trocars laparoscopic cholecystectomy. For achieve true minimum invasive surgery (in Japanese). Tan to Sui. 2009;30:1509-1513. |
| 6. | Johansson M, Thune A, Nelvin L, Stiernstam M, Westman B, Lundell L. Randomized clinical trial of open versus laparoscopic cholecystectomy in the treatment of acute cholecystitis. Br J Surg. 2005;92:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Suter M, Meyer A. A 10-year experience with the use of laparoscopic cholecystectomy for acute cholecystitis: is it safe? Surg Endosc. 2001;15:1187-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Poon RT, Liu CL, Lo CM, Lam CM, Yuen WK, Yeung C, Fan ST, Wong J. Management of gallstone cholangitis in the era of laparoscopic cholecystectomy. Arch Surg. 2001;136:11-16. [PubMed] |
| 9. | Sarli L, Iusco D, Sgobba G, Roncoroni L. Gallstone cholangitis: a 10-year experience of combined endoscopic and laparoscopic treatment. Surg Endosc. 2002;16:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Giger UF, Michel JM, Opitz I, Th Inderbitzin D, Kocher T, Krähenbühl L. Risk factors for perioperative complications in patients undergoing laparoscopic cholecystectomy: analysis of 22,953 consecutive cases from the Swiss Association of Laparoscopic and Thoracoscopic Surgery database. J Am Coll Surg. 2006;203:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Cox MR, Wilson TG, Luck AJ, Jeans PL, Padbury RT, Toouli J. Laparoscopic cholecystectomy for acute inflammation of the gallbladder. Ann Surg. 1993;218:630-634. [PubMed] |
| 12. | Gurusamy KS, Samraj K. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Cochrane Database Syst Rev. 2006;CD005440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Papi C, Catarci M, D'Ambrosio L, Gili L, Koch M, Grassi GB, Capurso L. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol. 2004;99:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Changchien CS, Chuah SK, Chiu KW. Is ERCP necessary for symptomatic gallbladder stone patients before laparoscopic cholecystectomy? Am J Gastroenterol. 1995;90:2124-2127. [PubMed] |
| 15. | Onken JE, Brazer SR, Eisen GM, Williams DM, Bouras EP, DeLong ER, Long TT, Pancotto FS, Rhodes DL, Cotton PB. Predicting the presence of choledocholithiasis in patients with symptomatic cholelithiasis. Am J Gastroenterol. 1996;91:762-767. [PubMed] |