Published online Mar 7, 2012. doi: 10.3748/wjg.v18.i9.905
Revised: September 16, 2011
Accepted: January 18, 2012
Published online: March 7, 2012
AIM: To determine if liver regeneration (LR) could be disturbed following radiofrequency (RF) ablation and whether modification of LR by steroid administration occurs.
METHODS: Sham operation, partial hepatectomy (PH), and partial hepatectomy with radiofrequency ablation (PHA) were performed on adult Fisher 344 rats. We investigated the recovery of liver volume, DNA synthetic activities, serum cytokine/chemokine levels and signal transducers and activators of transcription 3 DNA-binding activities in the nucleus after the operations. Additionally, the effects of steroid (dexamethasone) pretreatment in the PH group (S-PH) and the PHA group (S-PHA) were compared.
RESULTS: The LR after PHA was impaired, with high serum cytokine/chemokine induction compared to PH, although the ratio of the residual liver weight to body weight was not significantly different. Steroid pretreatment disturbed LR in the S-PH group. On the other hand, low-dose steroid pretreatment improved LR and suppressed tumor necrosis factor (TNF)-α elevation in the S-PHA group, with recovery of STAT3 DNA-binding activity. On the other hand, low-dose steroid pretreatment improved LR and suppressed TNF-α elevation in the S-PHA group, with recovery of STAT3 DNA-binding activity.
CONCLUSION: LR is disturbed after RF ablation, with high serum cytokine/chemokine induction. Low-dose steroid administration can improve LR after RF ablation with TNF-α suppression.
- Citation: Shibata T, Mizuguchi T, Nakamura Y, Kawamoto M, Meguro M, Ota S, Hirata K, Ooe H, Mitaka T. Low-dose steroid pretreatment ameliorates the transient impairment of liver regeneration. World J Gastroenterol 2012; 18(9): 905-914
- URL: https://www.wjgnet.com/1007-9327/full/v18/i9/905.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i9.905
Liver resection is still one of the best curative therapies for primary or secondary liver tumors in most cases with no extrahepatic metastasis[1,2]. Various techniques and devices for liver resection have been employed to improve the perioperative outcome[3-7], although the clamp-crush technique used by a skilled surgeon still has the most favorable outcome according to recent systematic reviews[8,9]. Liver surgeons focus on reducing bleeding during liver resection, which leads to shorter operative time. Heat-assist devices such as the harmonic scalpel[10], Ligasure[11], saline-linked monopolar cautery[12], microwave coagulator[13], and radiofrequency (RF) devices[14], can seal vessels and bile ducts to avoid postoperative bleeding and bile leakage. Because these heat-assist devices can achieve firm sealing of vessels and bile ducts, unnecessary ties and clips can be avoided without any adverse events[15]. Some randomized trials have indicated the merits and demerits of using heat-assist devices[4,7].
The greatest merit is the sealing effect to reduce perioperative morbidity[7,16]. A second merit is enhancement of the surgical margins of tumors located near the cutting surface of the liver[17]. On the other hand, a necrotic zone remains in the cutting surface of the residual liver[14]. Although cryoablation causes lethal systemic responses with high levels of cytokines[18,19], RF ablation may be safe and result in only minimal release of soluble factors causing systemic responses. However, it is not known whether RF manipulation, and the residual necrotic tissue during liver resection, is beneficial or harmful to liver regeneration (LR). In addition, the molecular events of LR after the use of heating devices for liver resection are also unknown.
LR is regulated by sequential molecular events in which various humoral factors such as tumor necrosis factor (TNF)-α and IL-6 prime and facilitate hepatocyte replication[20-22]. Although the humoral factors increase and influence each step of LR in a very short time[21], each cytokine activates subsequent molecular signals to complete LR[22]. RF ablation, in particular, excessively increases plasma cytokines such as TNF-α and IL-6 compared to simple liver resection[23,24]. Due to superphysiological stimulation of these cytokines, the heat effect of RF ablation may impair liver regeneration[25]. On the other hand, fast recovery of liver function in LR after RF ablation has been reported in major clinical hepatectomy[6]. Therefore, the exact effect of RF ablation on LR remains unclear.
Steroid administration has been proved to attenuate surgical stress following liver resection[26,27]. In addition, steroid pretreatment has been proved to decrease plasma cytokine levels and the therapeutic dose of the steroid does not inhibit hepatocyte proliferation[28]. Although previous investigations showed that steroid treatment could ameliorate excessive surgical stress of extended hepatectomy[26-28], the exact benefits of steroid administration in clinical LR are largely unknown. The main aim of this study was to determine whether liver regeneration could be disturbed following RF ablation. The second was to determine the effect of steroid pretreatment on LR after RF ablation.
Animal studies were performed in compliance with institutional and National Research Council guidelines for humane care of laboratory animals.
Adult female Fisher 344 rats (250-350 g) were obtained from Charles River Japan (Kanagawa, Japan). They were housed in a climate-controlled (21 °C) room under a 12 h light-dark cycle and were given tap water and standard laboratory chow. All operations were performed between 9:00 a.m. and noon under general (ether) anesthesia using a sterile surgical technique.
Sham hepatectomy: The sham hepatectomy consisted of laparotomy and mobilization of the liver.
Partial hepatectomy: The two anterior liver lobes were removed as previously described[29,30]. In this model, removal of the two anterior lobes (68% of the liver) is known to induce the optimal proliferative response in the remnant liver mass.
Partial hepatectomy with radiofrequency ablation: Preceding partial hepatectomy (PH), the two anterior liver lobes were ablated with saline-linked electric bipolar forceps (ERBE Elektromedizin GmbH, Tübingen, Germany). After complete ablation of the two anterior lobes, they were removed the same as PH operation.
Groups of sham hepatectomy (SH), PH, and partial hepatectomy with RF ablation (PHA) rats were euthanized in batches of six at 1, 3, 5 and 7 d after surgery. A separate experiment was designed to determine the effect of steroid administration. All animals were pretreated with dexamethasone at 30 min prior to the operation. Groups of steroid pretreated PH rats (S-PH) and PHA rats (S-PHA) were euthanized in batches of six at 1 d after surgery. One hour before euthanasia, 5-bromo-2-deoxyuridine (BrdU) was injected intraperitoneally (50 μg/kg body weight)[31]. When animals were killed, part of the liver tissue was immediately frozen in liquid nitrogen for molecular analysis and part of it was dipped into cold ethanol for immunohistochemical study.
Blood samples were analyzed for activity of alanine transaminase (ALT), aspartate transaminase (AST), total protein levels, and albumin (ALB) in a clinical laboratory. White blood cells were counted with an autocalculator in the laboratory.
Serum obtained after euthanasia was kept at -80 °C until submission to a company (Upstate United States Inc., Charlottesville, VA, United States) for analysis. Briefly, multianalyte profiling was performed on a Luminex 100 system and the XY Platform (Luminex Corporation, Austin, TX, United States). Calibration microspheres for classification and reporter readings as well as sheath fluid were obtained from Luminex Corporation. Acquired fluorescence data were analyzed using MASTERPLEXTM QT (Ver. 1.2; MiraiBio Inc., South San Francisco, CA, United States). Serum concentrations of TNF-α, IL-6, IL-10, and monocyte chemoattractant protein-1 (MCP-1) were measured with an Upstate Beadlyte Mouse Multicytokine Bead master kit (Upstate United States, Inc.)[31]. All analyses were performed according to the manufacturers’ protocols.
The proliferative activity in the liver after hepatectomy was determined by measuring incorporation of BrdU as previously described[31]. Briefly, a mouse anti-BrdU antibody (X 100 dilution: DAKO A/S, Copenhagen, Denmark) was used as the primary antibody, followed by the ABC method (DAKO Co., Carpinteria, CA). Both labeled and unlabeled hepatocytes were counted in 20 fields in three different sections per time point from five different animals. Data are presented as means + SD from three independent experiments.
Growth of the residual liver lobes (right and omental lobes) was calculated as the ratio of residual liver weight/body weight (RLW/BW).
Western blotting analysis was performed using the Invitrogen NuPAGE® electrophoresis system (Invitrogen, Carlsbad, CA, United States). The samples were homogenized in phosphate buffered saline and kept at -80 °C until use. Briefly, nuclear proteins were extracted using the NE-PER® nuclear and cytoplasmic extraction protocol (Pierce Chemicals, Rockford, IL, United States). A BCA protein assay kit® (Pierce Chemicals) was used to measure the protein concentrations. Proteins (5 μg/lane) were separated on NuPAGE 4%-12% Bis-Tris gradient gels (Invitrogen). The gels were transferred to nitrocellulose membranes (Amersham Co., Buckinghamshire, United Kingdom) using an iBlot™ Gel Transfer Device (Invitrogen). Immunodetection of proteins was performed using a WesternBreeze® Chromogenic Immunodetection Kit (Invitrogen). Mouse monoclonal anti-proliferation cell nuclear antigen (PCNA) (Dako Co., Carpinteria, CA, United States) and rabbit polyclonal anti-ALB (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were used as the primary antibodies (1:250). The ECL western blotting analysis system (Amersham Co.) was used to detect signals.
Scanning densitometry was performed using a Macintosh G4 computer (Apple Computer, Cupertino, CA) and an EPSON GT-9600 scanner (Seiko Epson, Suwa, Japan). The signals were quantified using the NIH Image 1.55 Densitometric Analysis Program[30].
STAT3 activation was quantified using a TransAM™STAT3 Kit (Active Motif, Funakoshi Co., Tokyo, Japan)[32]. Briefly, 10 g/well of the nuclear cell extract from whole liver tissue (containing an activated transcription factor) was incubated in a 96-well plate on which double-stranded oligonucleotides containing the consensus sequence for the STAT3 DNA-binding site (5’-TTCCCGGAA-3’) were immobilized. The primary antibody used to detect STAT3 recognized epitopes on both the alpha and beta forms of STAT3, which are accessible only when STAT3 is activated and bound to its target DNA. After incubation with horseradish peroxidase, absorbance was recorded at 450 nm using a reference wavelength of 655 nm.
The unpaired Student’s t-test, Welch’s t-test or one-way analysis of variance (ANOVA) was used as appropriate. Data are given as mean + SD. Statistical analysis was performed using the StatView 5.0 program (SAS Institute, Cary, NC, United States) and the difference between the means was considered significant when P < 0.05.
All rats tolerated the operative procedures well and recovered uneventfully from anesthesia. Samples were collected immediately after each animal was euthanized.
Although the liver was ablated within a short time and most necrotic tissue was removed in the PHA group, postoperative serum AST and ALT levels in this group at one day after operation were significantly higher than in the PH group (Table 1). On the other hand, the white blood cell counts were not significantly different among the groups (Table 1). Total protein and albumin levels at two, three, and five days after operation in the PHA group were significantly lower than in the PH group.
| WBC (× 103μL) | Day 0 | Day 1 | Day 2 | Day 3 | Day 5 | Day 7 |
| PH | 4.95 ± 0.74 | 4.70 ± 0.81 | 6.55 ± 1.26 | 6.15 ± 0.81 | 7.43 ± 0.99 | 5.48 ± 1.14 |
| PHA | 5.80 ± 1.09 | 5.70 ± 0.84 | 7.68 ± 1.32 | 6.60 ± 1.49 | 7.65 ± 1.41 | 6.73 ± 0.81 |
| P values | NS | NS | NS | NS | NS | NS |
| TP (g/dL) | ||||||
| PH | 5.55 ± 0.24 | 4.71 ± 0.29 | 4.58 ± 0.09 | 4.87 ± 0.29 | 5.18 ± 0.21 | 5.43 ± 0.34 |
| PHA | 5.62 ± 0.17 | 4.43 ± 0.25 | 4.28 ± 0.17 | 4.05 ± 0.13 | 4.68 ± 0.24 | 5.31 ± 0.36 |
| P values | NS | NS | 0.022 | 0.002 | 0.019 | NS |
| ALB (g/dL) | ||||||
| PH | 4.10 ± 0.14 | 3.73 ± 0.21 | 3.45 ± 0.13 | 3.58 ± 0.21 | 3.80 ± 0.22 | 4.08 ± 0.15 |
| PHA | 4.20 ± 0.18 | 3.55 ± 0.21 | 3.18 ± 0.17 | 2.85 ± 0.21 | 3.10 ± 0.22 | 3.78 ± 0.28 |
| P values | NS | NS | 0.042 | 0.003 | 0.004 | NS |
| AST (U/L) | ||||||
| PH | 77.3 ± 3.3 | 591.8 ± 111.8 | 211.0 ± 23.6 | 126.8 ± 23.7 | 117.3 ± 14.5 | 105.5 ± 13.4 |
| PHA | 79.3 ± 4.6 | 2441.8 ± 501.8 | 366.5 ± 136.9 | 224.0 ± 110.2 | 163.5 ± 59.2 | 100.8 ± 11.9 |
| P values | NS | 0.001 | NS | NS | NS | NS |
| ALT (U/L) | ||||||
| PH | 48.5 ± 13.4 | 734.3 ± 187.4 | 207.8 ± 108.7 | 71.0 ± 21.1 | 59.3 ± 9.4 | 47.5 ± 13.7 |
| PHA | 49.3 ± 9.6 | 1603.3 ± 313.7 | 503.8 ± 207.8 | 102.5 ± 23.1 | 66.0 ± 8.9 | 51.3 ± 11.1 |
| P values | NS | 0.003 | 0.025 | NS | NS | NS |
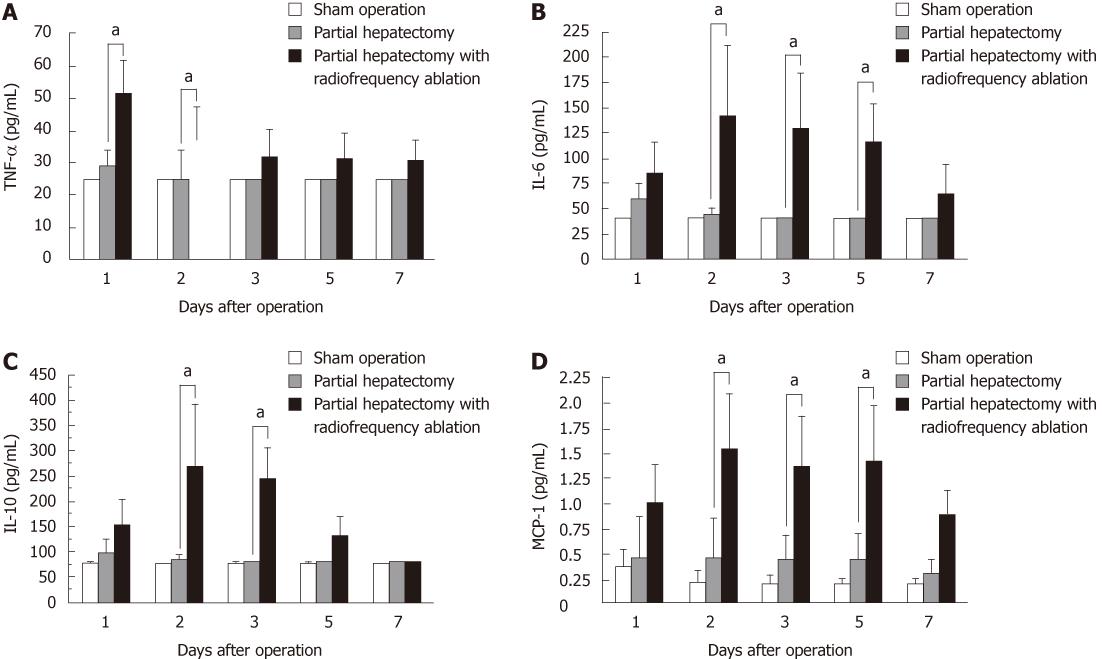
Serum cytokine and chemokine levels are shown in Figure 1. TNF-α levels in the PHA group at one and two days after operation were significantly higher than in the PH group (Figure 1A). IL-6 (Figure 1B) and MCP-1 (Figure 1D) levels in the PHA group at two, three, and five days after operation were significantly higher than in the PH group. IL-10 levels in the PHA group at two and three days after operation were significantly higher than in the PH group (Figure 1C).
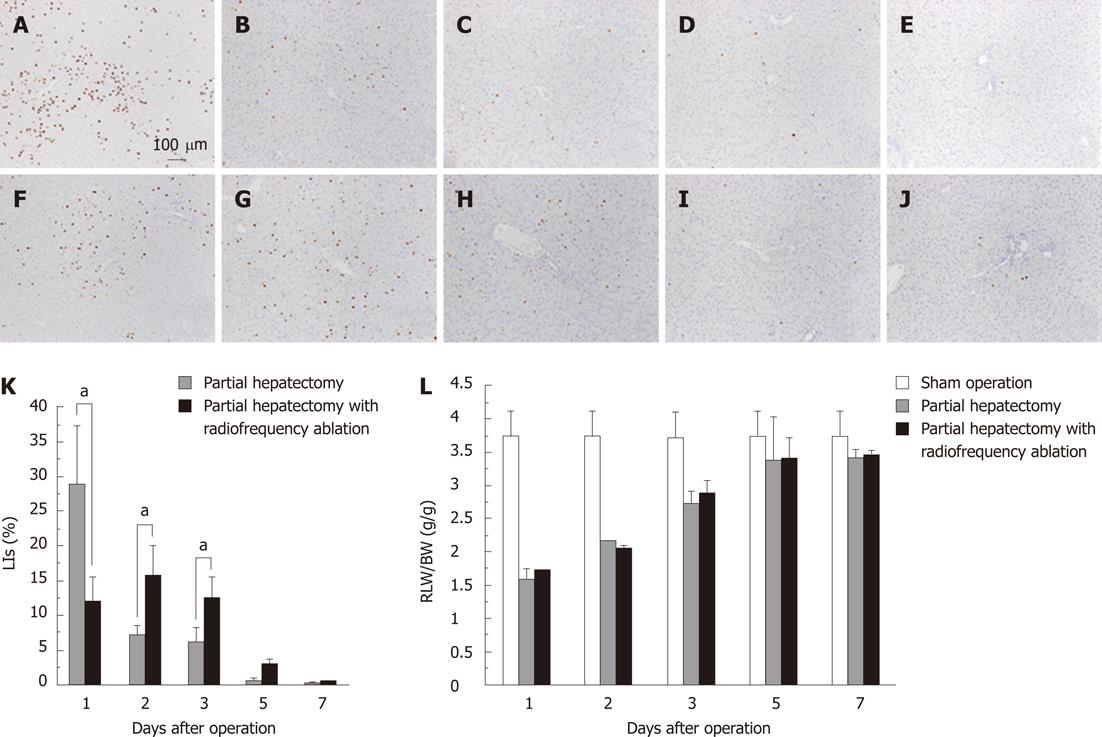
DNA synthetic activity was determined by immunohistochemistry for BrdU (Figure 2A-J) and labeling indices (LIs) (Figure 2K). The LI at one day in the PHA group was significantly lower than in the PH group (12.17 ± 3.43 vs 29.02 ± 8.47, P = 0.001). On the other hand, the LIs at two days and three days after operation in the PHA group were significantly higher than in the PH group (15.85 ± 4.18 vs 7.05 ± 1.54, P < 0.001, and 12.55 ± 3.14 vs 6.03 ± 2.11, P = 0.002, respectively). Although DNA synthetic activities between the groups are significantly different, RLW/BW ratio was not greatly significantly different among the groups (Figure 2L).
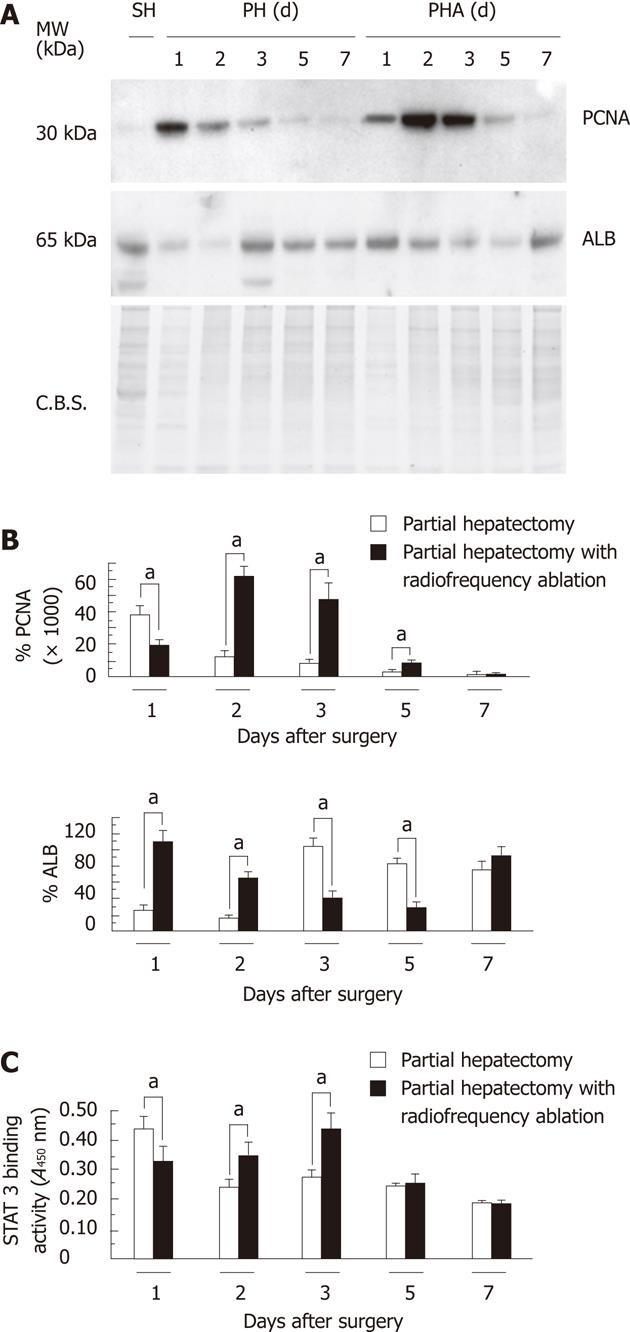
Protein expression of nuclear PCNA and cytosolic albumin is shown in Figure 3A. Densitometric analysis of the expression of each protein is shown in Figure 3B. The pattern of PCNA expression was similar to the immunohistochemistry for BrdU and LIs. The peak of BrdU expression in the PH group was seen at one day after operation and in the PHA group at two and three days after operation. ALB expression in the PH group dropped at one and two days after operation and recovered thereafter. In contrast, ALB expression in the PHA group dropped at three and five days after operation. STAT3 DNA-binding activity was also consistent with the results of BrdU, LIs, and PCNA expression (Figure 3C). The peak of STAT3 DNA-binding activity in the PH group was seen at one day after operation and in the PHA group at two and three days after operation.
We found that LR was disturbed after RF ablation in hepatectomy, with high cytokine/chemokine induction. Because steroid treatment could block cytokine elevation after hepatectomy, we tested the effects of S-PH and S-PHA groups.
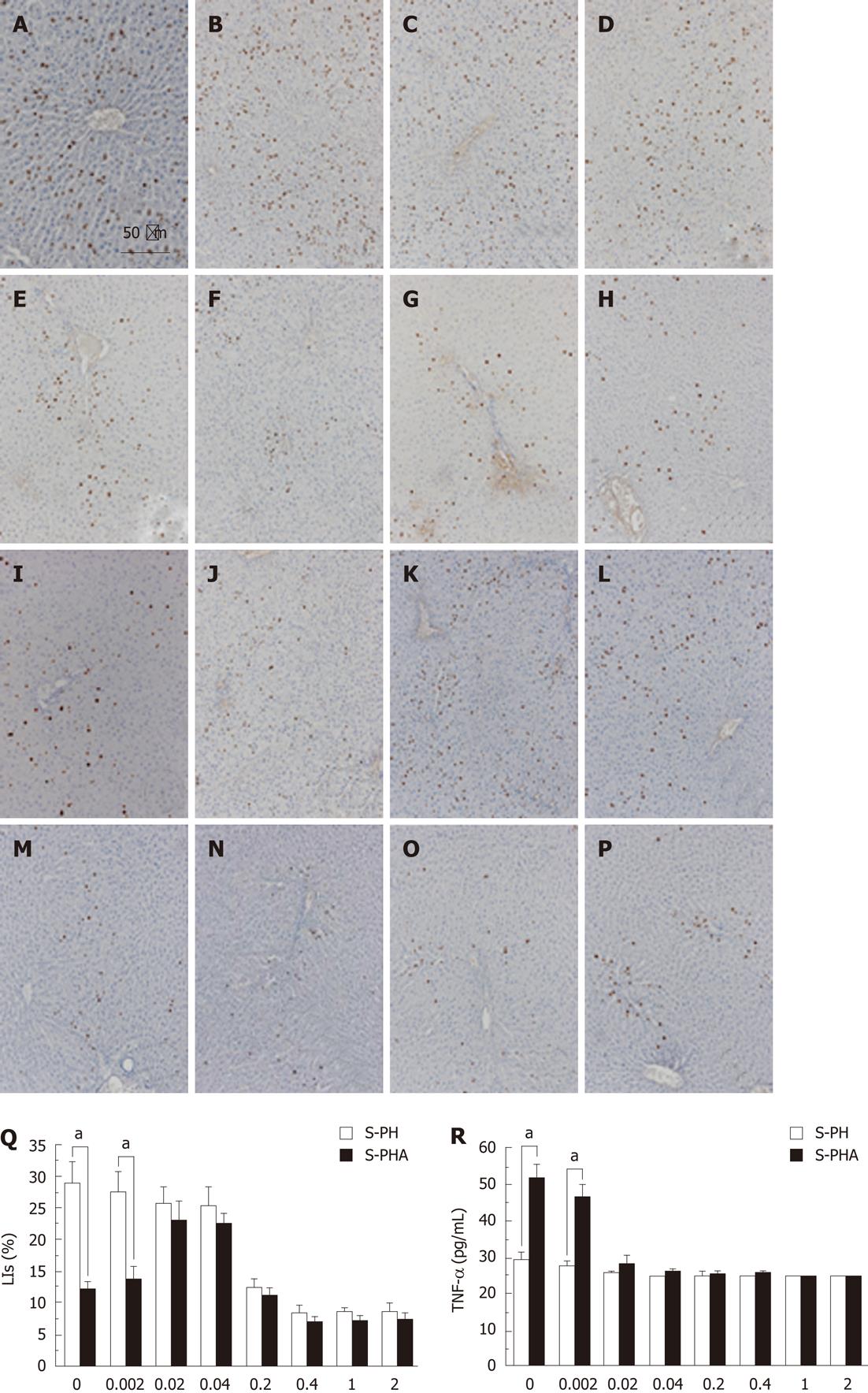
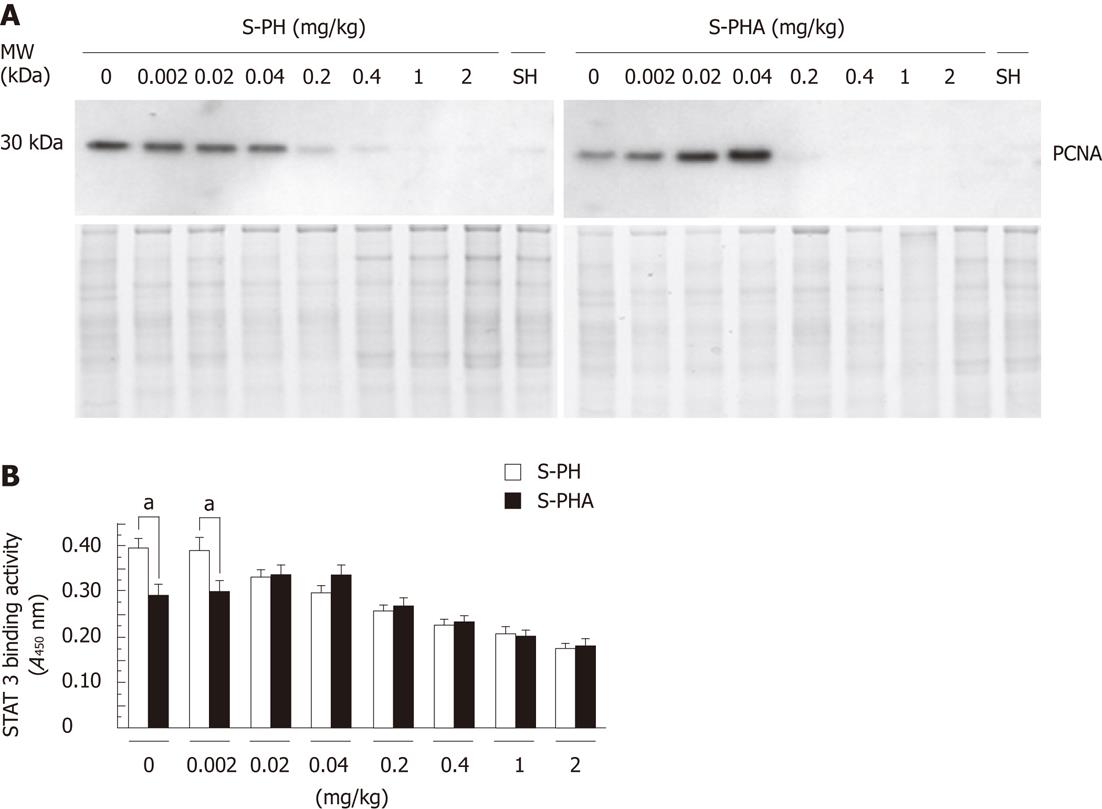
Immunohistochemistry values for BrdU (Figure 4A-P) and LIs (Figure 4Q) at 1 d after steroid administration at different concentrations are shown in Figure 4. BrdU uptake in the S-PH group (Figure 4A-H) gradually decreased when the steroid dose was increased. In contrast, that in the S-PHA group (Figure 4I-P) gradually increased when the steroid dose was increased by 0.04 mg/kg and then it decreased with further increases in dosage. Although the LI at 0.002 mg/kg steroid administration in the S-PH group was significantly higher than in the S-PHA group (29.62 ± 8.28 vs 14.87 ± 4.35, P = 0.003), the LIs at the other steroid doses were not significantly different between the groups (Figure 4Q). Among the examined cytokines and chemokines, only the TNF-α level at 0.002 mg/kg of steroid in the S-PH group (Figure 4R) was significantly lower than in the S-PHA group (27.5 ± 4.18 vs 46.5 ± 4.18, P = 0.001). In contrast, other levels were not significantly different at any steroid doses (data not shown).
The pattern of PCNA expression in the nucleus (Figure 5A) was similar to that observed in the immunohistochemistry for BrdU and LIs (Figure 4). PCNA expression in the S-PH group gradually decreased when the steroid dose was increased. In contrast, that in the S-PHA group gradually increased when the steroid dose was increased by 0.04 mg/kg and then it decreased with further dosage. STAT 3 DNA-binding activities (Figure 5B) were also consistent with the BrdU staining, LIs and PCNA expression. Only STAT3 DNA-binding activity at 0.002 mg/kg of steroid was significantly different between the groups (0.406 ± 0.042 vs 0.298 ± 0.053, P = 0.019).
We investigated the influence of RF ablation during hepatectomy on LR. We found that LR after RF ablation was disturbed, with high serum cytokine/chemokine induction. Low-dose steroid administration nearly restored LR after RF ablation during hepatectomy, and STAT3 DNA-binding activity supported this finding.
The method of liver resection should take into account both perioperative safety and oncological curability. RF ablation is one of the less invasive strategies for small liver tumors[33] and can be used for hemostasis during liver resection[34]. The one concern is that some necrotic tissue will remain in the residual liver after RF ablation and may affect LR. In addition, thermal energy itself during the operation also may affect it. Most investigations have used large animal models to study the effects of RF ablation after hepatectomy on humoral and oncological activities[25]. The use of murine models to investigate RF ablation has been limited[25,35]. Large animal models can be ideal to simulate the human response; however, they are time consuming and it is difficult to examine the molecular details compared to murine models. Although our model did not totally reproduce the human clinical situation, the postoperative course was very similar, as serum transaminases were strongly elevated at one day after operation[24]. Furthermore, high serum cytokine levels after ablation have been reported in human studies[24,36]. These clinical postoperative alterations of laboratory tests supported the idea that our model could represent the clinical phenomena of hepatectomy using RF ablation. Therefore, our model was suitable to examine the thermal effect of RF ablation after hepatectomy.
Effects of RF ablation on cancer cells in the liver could modulate the systemic immune responses, including cytokine/chemokine production and the proliferative activity of the cancer cells[37,38]. Necrotic tissue after RF ablation could also modulate the systemic immune response to specific cancer cells[39]. On the other hand, Meredith et al[40] reported that RF ablation itself did not accelerate tumor growth. In our study, RF ablation delayed the liver regenerative response, which was consistent with a previous study[25]. These differently reported proliferative responses may be due to the differences between cancer cells and normal cells. Other reasons could be differences in the amount of necrosis and the duration of the ablation. We could not distinguish between the exact effects of necrosis and ablation, but our results indicated that the heat effect of RF ablation, not necrosis, could delay LR, because most ablated tissues were removed in both groups, and the amounts of necrosis in the PH and the PHA groups were comparable in our model. Serum cytokines such as IL-6, IL-8, and IL-10 are elevated after RF ablation[24,35,36], which is also consistent with our results. Therefore, regardless of how much necrotic tissue was removed after RF ablation, the heat effect during the ablation itself could activate cytokine/chemokine responses.
LR after RF ablation was delayed without any difference in the RLW/BW ratio. This indicated that volume recovery after PH did not represent parenchymal cell proliferation itself, which was consistent with a previous report[41]. Delayed LR after RF ablation affected the serum albumin level. Albumin production was suppressed when hepatocytes began to proliferate[42]. The time lag between DNA synthesis and the serum albumin level could be due to the long half-life of serum albumin. Even though there was no critical event in our model, we need to pay attention to the albumin level, which could decrease in LR after RF ablation and be associated with delayed LR in the clinical setting.
A systemic cytokine/chemokine response was activated by RF ablation even within the short time during operation. We could not determine the specific cytokine/chemokine that disturbed hepatocyte proliferation. Excessively high levels of cytokines such as TNF-α and IL-6 are desensitized from growth stimuli[43], although knockout murine models targeting TNF-α receptor and IL-6 genes have demonstrated that these cytokine signals are necessary to accomplish LR[44,45]. The lack of DNA-binding activity of STAT3 in our results supported the finding of growth suppression in the PHA group. Even though the peaks of most cytokine/chemokine levels in the PHA model were between two days and five days after operation, DNA synthesis in PHA continued in these periods. The only distinctive alteration seen was in the TNF-α level, which gradually decreased. In addition, steroid pretreatment in the PHA group showed that only the TNF-α level was different between the PH group and PHA group at one day after operation. Therefore, DNA synthesis after RF ablation could be more affected by TNF-α than by other cytokines/chemokines. Thus, TNF-α activation should be observed within a short time after simple hepatectomy[21]. However, it could be prolonged in LR after RF ablation, as shown in our results. Though we could not determine the mechanism of the TNF-α activation after RF ablation, our results strongly suggested that the TNF-α could play a major role in LR after RF ablation. Further study is needed to determine whether TNF-α could be a molecular target to control LR in the clinical setting.
Steroids have been demonstrated to inhibit LR by inhibiting excessive TNF-α and IL-6 production[46-48], although moderate stimulation by TNF-α and IL-6 is necessary to complete LR[21]. Our results also showed that cytokine/chemokine levels decreased gradually depending on steroid administration in the S-PHA group. On the other hand, steroids can inhibit the DNA synthesis of hepatocytes directly[42]. The reason why DNA synthesis recovered after low-dose steroid administration in the S-PHA group could be related to the balance between the suppression of excessive cytokine production and the direct inhibition of DNA synthesis. In other words, LR after low-dose steroid administration could recover, escaping from the excessive cytokine production, and be nearly free from the direct inhibition by the steroid. Our results indicated the presence of an optimal threshold of the steroid concentration that facilitated LR when cytokines/chemokines were excessively activated. Therefore, our results strongly suggest that we need to pay careful attention to the clinical steroid concentration because the effects of steroid administration could be altered depending on the clinical condition.
In conclusion, LR was disturbed after RF ablation, with high serum cytokine/chemokine induction. Low-dose steroid administration could improve LR after RF ablation with TNF-α suppression. Further clinical study is needed to confirm that low-dose steroid administration has a clinical benefit for LR after RF ablation.
We thank Mr. Kim Barrymore for his help in preparing this manuscript.
Liver resection is still one of the best curative therapies for primary or secondary liver tumors. Various techniques and devices for liver resection have been employed to improve the perioperative outcome. Radiofrequency (RF) devices can seal vessels and bile ducts to avoid postoperative bleeding and bile leakage. Although some of its merits have been reported, its demerits are largely unknown.
The greatest merit of a RF device is the sealing effect to reduce perioperative morbidity. A second merit is enhancement of the surgical margins of tumors located near the cutting surface of the liver. On the other hand, a necrotic zone remains in the cutting surface of the residual liver. The research hotspot is whether RF manipulation, and the residual necrotic tissue during liver resection, is beneficial or harmful to liver regeneration (LR).
The present study showed LR after RF ablation delayed the regenerative response with high serum cytokine/chemokine induction, and low-dose steroid administration could improve LR after RF ablation with TNF-α suppression. The results indicated that the heat effect of RF ablation, not necrosis, could delay LR, because most ablated tissues were removed. Serum cytokines such as IL-6, IL-8, and IL-10 are elevated after RF ablation, which is consistent with previous studies. Therefore, regardless of how much necrotic tissue was removed after RF ablation, the heat effect during the ablation itself could activate cytokine/chemokine responses.
This study provides insights into the mechanism by which RF ablation could activate cytokines/chemokines in LR and found that steroids can be used for controlling LR. The results strongly suggest that they need to pay careful attention to the clinical steroid concentration because the effects of steroid administration could be altered depending on the clinical condition.
The aim of this study focused on liver regeneration after RF ablation is clear and interesting, and these data may provide a basis for RF ablation studies in the future. The impact of this study in this field is moderate.
Peer reviewer: Teng-Yu Lee, MD, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taichung Veterans General Hospital, 160, Sec. 3, TaichungHarbor Road, Taichung 407, Taiwan, China
S- Editor Shi ZF L- Editor Logan S E- Editor Xiong L
| 1. | Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, Marvin M, Ravindra KV, Mejia A, Lainas P. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 2. | Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Van Nhieu JT, Decaens T, Hurtova M, Memeo R, Mallat A. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 374] [Reference Citation Analysis (0)] |
| 4. | Sakamoto Y, Yamamoto J, Kokudo N, Seki M, Kosuge T, Yamaguchi T, Muto T, Makuuchi M. Bloodless liver resection using the monopolar floating ball plus ligasure diathermy: preliminary results of 16 liver resections. World J Surg. 2004;28:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242:814-22, discussion 822-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Mizuguchi T, Katsuramaki T, Nagayama M, Meguro M, Shibata T, Kaji S, Hirata K. Rapid recovery of postoperative liver function after major hepatectomy using saline-linked electric cautery. Hepatogastroenterology. 2008;55:2188-2192. [PubMed] |
| 7. | El Moghazy WM, Hedaya MS, Kaido T, Egawa H, Uemoto S, Takada Y. Two different methods for donor hepatic transection: cavitron ultrasonic surgical aspirator with bipolar cautery versus cavitron ultrasonic surgical aspirator with radiofrequency coagulator-A randomized controlled trial. Liver Transpl. 2009;15:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Pamecha V, Gurusamy KS, Sharma D, Davidson BR. Techniques for liver parenchymal transection: a meta-analysis of randomized controlled trials. HPB (Oxford). 2009;11:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Gurusamy KS, Pamecha V, Sharma D, Davidson BR. Techniques for liver parenchymal transection in liver resection. Cochrane Database Syst Rev. 2009;CD006880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Hashizume M, Takenaka K, Yanaga K, Ohta M, Kajiyama K, Shirabe K, Itasaka H, Nishizaki T, Sugimachi K. Laparoscopic hepatic resection for hepatocellular carcinoma. Surg Endosc. 1995;9:1289-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Strasberg SM, Drebin JA, Linehan D. Use of a bipolar vessel-sealing device for parenchymal transection during liver surgery. J Gastrointest Surg. 1995;6:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Poon RT, Fan ST, Wong J. Liver resection using a saline-linked radiofrequency dissecting sealer for transection of the liver. J Am Coll Surg. 2005;200:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Tabuse K, Katsumi M, Kobayashi Y, Noguchi H, Egawa H, Aoyama O, Kim H, Nagai Y, Yamaue H, Mori K. Microwave surgery: hepatectomy using a microwave tissue coagulator. World J Surg. 1985;9:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 101] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Bhardwaj N, Strickland AD, Ahmad F, Dennison AR, Lloyd DM. Liver ablation techniques: a review. Surg Endosc. 2010;24:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Ayav A, Jiao LR, Habib NA. Bloodless liver resection using radiofrequency energy. Dig Surg. 2007;24:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Kianmanesh R, Ogata S, Paradis V, Sauvanet A, Belghiti J. Heat-zone effect after surface application of dissecting sealer on the "in situ margin" after tumorectomy for liver tumors. J Am Coll Surg. 2008;206:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Seifert JK, Stewart GJ, Hewitt PM, Bolton EJ, Junginger T, Morris DL. Interleukin-6 and tumor necrosis factor-alpha levels following hepatic cryotherapy: association with volume and duration of freezing. World J Surg. 1999;23:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Ng KK, Lam CM, Poon RT, Shek TW, To JY, Wo YH, Ho DW, Fan ST. Comparison of systemic responses of radiofrequency ablation, cryotherapy, and surgical resection in a porcine liver model. Ann Surg Oncol. 2004;11:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2649] [Cited by in RCA: 2468] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 21. | Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1175] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 22. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1205] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 23. | Evrard S, Menetrier-Caux C, Biota C, Neaud V, Mathoulin-Pélissier S, Blay JY, Rosenbaum J. Cytokines pattern after surgical radiofrequency ablation of liver colorectal metastases. Gastroenterol Clin Biol. 2007;31:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Jansen MC, van Wanrooy S, van Hillegersberg R, Rijken AM, van Coevorden F, Prevoo W, van Gulik TM. Assessment of systemic inflammatory response (SIR) in patients undergoing radiofrequency ablation or partial liver resection for liver tumors. Eur J Surg Oncol. 2008;34:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Ypsilantis P, Pitiakoudis M, Souftas VD, Lambropoulou M, Tsalikidis C, Foutzitzi S, Tsigalou C, Prassopoulos P, Papadopoulos N, Simopoulos C. Liver regeneration following radiofrequency ablation. J Surg Res. 2008;150:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Yamashita Y, Shimada M, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Sugimachi K. Effects of preoperative steroid administration on surgical stress in hepatic resection: prospective randomized trial. Arch Surg. 2001;136:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 27. | Pulitanò C, Aldrighetti L, Arru M, Finazzi R, Soldini L, Catena M, Ferla G. Prospective randomized study of the benefits of preoperative corticosteroid administration on hepatic ischemia-reperfusion injury and cytokine response in patients undergoing hepatic resection. HPB (Oxford). 2007;9:183-189. [PubMed] |
| 28. | Nadal C. Dose-related opposite effects of hydrocortisone on hepatocyte proliferation in the rat. Liver. 1995;15:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Kamohara Y, Sugiyama N, Mizuguchi T, Inderbitzin D, Lilja H, Middleton Y, Neuman T, Demetriou AA, Rozga J. Inhibition of signal transducer and activator transcription factor 3 in rats with acute hepatic failure. Biochem Biophys Res Commun. 2000;273:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Mizuguchi T, Kamohara Y, Hui T, Neuman T, Mitaka T, Demetriou AA, Rozga J. Regulation of c-met expression in rats with acute hepatic failure. J Surg Res. 2001;99:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Nobuoka T, Mizuguchi T, Oshima H, Shibata T, Kaji S, Nagayama M, Meguro M, Mitaka T, Hirata K. Impaired liver regeneration with humoral and genetic disturbances in urinary trypsin inhibitor-deficient mice. Liver Int. 2009;29:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Kikuchi H, Katsuramaki T, Kukita K, Taketani S, Meguro M, Nagayama M, Isobe M, Mizuguchi T, Hirata K. New strategy for the antifibrotic therapy with oral administration of FR260330 (a selective inducible nitric oxide synthase inhibitor) in rat experimental liver cirrhosis. Wound Repair Regen. 2007;15:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 34. | Felekouras E, Prassas E, Kontos M, Papaconstantinou I, Pikoulis E, Giannopoulos A, Tsigris C, Tzivras M, Bakogiannis C, Safioleas M. Liver tissue dissection: ultrasonic or RFA energy? World J Surg. 2006;30:2210-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Ng KK, Lam CM, Poon RT, Shek TW, Ho DW, Fan ST. Safety limit of large-volume hepatic radiofrequency ablation in a rat model. Arch Surg. 2006;141:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Schell SR, Wessels FJ, Abouhamze A, Moldawer LL, Copeland EM. Pro- and antiinflammatory cytokine production after radiofrequency ablation of unresectable hepatic tumors. J Am Coll Surg. 2002;195:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | von Breitenbuch P, Köhl G, Guba M, Geissler E, Jauch KW, Steinbauer M. Thermoablation of colorectal liver metastases promotes proliferation of residual intrahepatic neoplastic cells. Surgery. 2005;138:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Nijkamp MW, van der Bilt JD, de Bruijn MT, Molenaar IQ, Voest EE, van Diest PJ, Kranenburg O, Borel Rinkes IH. Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Ann Surg. 2009;249:814-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Yu HC, Moon JI, Jin ZW, Lee DY, Kim CY, Song CH, Cho BH. Effect of radiofrequency ablation of the liver on cell-mediated immunity in rats. World J Surg. 2005;29:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Meredith K, Haemmerich D, Qi C, Mahvi D. Hepatic resection but not radiofrequency ablation results in tumor growth and increased growth factor expression. Ann Surg. 2007;245:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Temme A, Ott T, Dombrowski F, Willecke K. The extent of synchronous initiation and termination of DNA synthesis in regenerating mouse liver is dependent on connexin32 expressing gap junctions. J Hepatol. 2000;32:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Mizuguchi T, Mitaka T, Hirata K, Oda H, Mochizuki Y. Alteration of expression of liver-enriched transcription factors in the transition between growth and differentiation of primary cultured rat hepatocytes. J Cell Physiol. 1998;174:273-284. [PubMed] |
| 43. | Ahmed TA, Buzzelli MD, Lang CH, Capen JB, Shumate ML, Navaratnarajah M, Nagarajan M, Cooney RN. Interleukin-6 inhibits growth hormone-mediated gene expression in hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1793-G1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1213] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 45. | Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A. 1997;94:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 755] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 46. | Tsukamoto I, Kojo S. Effect of glucocorticoid on liver regeneration after partial hepatectomy in the rat. Gut. 1989;30:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Nagy P, Kiss A, Schnur J, Thorgeirsson SS. Dexamethasone inhibits the proliferation of hepatocytes and oval cells but not bile duct cells in rat liver. Hepatology. 1998;28:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Debonera F, Krasinkas AM, Gelman AE, Aldeguer X, Que X, Shaked A, Olthoff KM. Dexamethasone inhibits early regenerative response of rat liver after cold preservation and transplantation. Hepatology. 2003;38:1563-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |