Published online Feb 7, 2012. doi: 10.3748/wjg.v18.i5.401
Revised: June 2, 2011
Accepted: June 9, 2011
Published online: February 7, 2012
The management of complications in liver disease is often complex and challenging. Endoscopy has undergone a period of rapid expansion with numerous novel and specialized endoscopic modalities that are of increasing value in the investigation and management of the patient with liver disease. In this review, relevant literature search and expert opinions have been used to provide a brief overview and update of the current endoscopic management of patients with liver disease and portal hypertension. The main areas covered are safety of endoscopy in patients with liver disease, the use of standard endoscopy for the treatment of varices and the role of new endoscopic modalities such as endoscopic ultrasound, esophageal capsule, argon plasma coagulation, spyglass and endomicroscopy in the investigation and treatment of liver-related gastrointestinal and biliary pathology. It is clear that the role of the endoscopy in liver disease is well beyond that of just treating varices. As the technology in endoscopy expands, so does the role of the endoscopist in liver disease.
- Citation: Krystallis C, Masterton GS, Hayes PC, Plevris JN. Update of endoscopy in liver disease: More than just treating varices. World J Gastroenterol 2012; 18(5): 401-411
- URL: https://www.wjgnet.com/1007-9327/full/v18/i5/401.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i5.401
Liver disease and cirrhosis are common causes of mortality worldwide[1]. The role of endoscopy in liver disease is both diagnostic and interventional: endoscopy should be offered to patients with relevant symptoms (unsuspected liver disease may be diagnosed in this manner) and for variceal screening and treatment. Patients with liver disease can be challenging to sedate, and the complexity of endoscopy in liver disease continues to increase with rising numbers of patients with a liver transplant, and the advent of new endoscopic modalities such as capsule endoscopy and endoscopic ultrasound (EUS).
Pharmacodynamics are altered in advanced liver disease as a result of changes in hepatic conjugation and oxidation, shunting, decreased protein binding and an increased volume of distribution[2]. The common agents used for sedation in endoscopy are discussed, however, specific doses cannot be recommended because these are dependent on patient factors. We would recommend that an endoscopist or anesthetist who has experience with this patient group undertakes the sedation of liver patients.
Midazolam is the benzodiazepine of choice in most endoscopy units. It is protein bound and metabolized in the liver by cytochrome P3A4. In cirrhosis, clearance of midazolam is impaired and elimination half-life is doubled. As a result, midazolam should be used with caution in patients with cirrhosis[3].
Pethidine and fentanyl are the most commonly used analgesics for endoscopic procedures. The liver is the major site of biotransformation for most opiates. The oxidation of pethidine is reduced in patients with cirrhosis and its clearance is diminished. Therefore, there is increased bioavailability, and pethidine should be avoided in patients with liver disease[4]. The half-life of fentanyl is shorter and does not seem to be influenced by cirrhosis. Its use is preferred to pethidine[5].
The pharmacokinetics of propofol, an anesthetic agent that is widely used in endoscopy, appears to be unaffected by cirrhosis; again, perhaps secondary to its short half-life. One study has suggested that the use of propofol rather than midazolam in patients with compensated cirrhosis facilitates a faster recovery time with less exacerbation of subclinical encephalopathy[6].
Gastrointestinal (GI) bleeding in patients with liver disease may be life threatening. If bleeding varices are suspected or patients are hemodynamically unstable, there is often a low threshold for endotracheal intubation to protect the airway. There is little literature on prophylactic intubation for airway protection in such patients, and two retrospective studies[7,8] have concluded that it does not prevent cardiopulmonary complications, or pneumonia. We are of the opinion that airway protection at emergency endoscopy is extremely important in patients with suspected variceal bleeding, who present with hematemesis, and particularly in hemodynamically unstable patients and those with hepatic encephalopathy or alcohol withdrawal symptoms. In such patients, endoscopy is best undertaken in a critical care environment with immediate access to anesthetic support and endotracheal intubation[9].
Coagulopathy and thrombocytopenia are common in patients with chronic liver disease. The mechanisms behind coagulation abnormalities are often complex, and it is now thought that prolongation of prothrombin time may not directly relate to the risk of bleeding, and rather, it is the balance of pro- and antithrombotic factors that is important. In practical terms, there is currently no reliable way of quantifying this. Routine correction of coagulopathy at endoscopy is not recommended, although patients with chronic liver disease should receive vitamin K to correct any dietary deficiency that may result in coagulopathy.
It is recognized that diagnostic endoscopy is a low-risk procedure and safe in patients with altered coagulation. However, high-risk endoscopic therapeutic procedures have a significantly increased risk of hemorrhage and, as such, coagulopathy should be treated[10]. It is therefore common practice in cirrhotic patients to correct significant thrombocytopenia (< 50 × 106/mL) with platelet transfusions and to correct coagulopathy with fresh frozen plasma (FFP) if prothrombin time is > 20 s to an international normalized ratio < 1.5, before high-risk procedures. Platelet and FFP transfusions are particularly helpful during an acute bleeding episode if the prothrombin time is prolonged or platelets are low, similar to the previously mentioned values[11].
Novel treatments include recombinant factor VIIa. This has been used as a hemostatic agent in acute variceal bleeding, but failed to show efficacy in a large randomized study[12]. Another trial has demonstrated that addition of desmopressin does not improve and may worsen the efficacy of terlipressin in controlling acute variceal bleeding in cirrhotic patients[13].
Certain endoscopic investigations have been shown to be safe in coagulopathic patients with cirrhosis, despite being relatively invasive; EUS-fine needle aspiration of the liver has been shown to be a safe alternative to percutaneous liver biopsy, particularly in patients with advanced liver disease, coagulopathy and high risk of bleeding[14]. At endoscopic retrograde cholangiopancreatography (ERCP), endoscopic papillary balloon dilation is safer than endoscopic biliary sphincterotomy for the treatment of choledocholithiasis in patients with advanced cirrhosis and coagulopathy, because it has a reduced risk of bleeding[15].
The correlation between peptic ulcer disease and cirrhosis is well described. Both duodenal and gastric ulcers are more common in cirrhosis: the reported prevalence is 24.1%[16]. It is recognized that the prevalence of gastric ulceration increases with the severity of liver disease and is related to changes in the hepatic venous pressure gradient[16,17].
A high prevalence of Helicobacter pylori (H. pylori), up to 89%, in patients with cirrhosis has been reported[18]. 13C urea breath testing and gastric body histology remain highly accurate in detecting H. pylori in cirrhosis, whereas rapid urease tests and serology are less reliable than in non-cirrhotic patients[19]. A meta-analysis of seven studies with almost 1000 patients has strongly suggested that, as with non-cirrhotic patients, H. pylori infection increases the risk for peptic ulcer disease in cirrhosis[20]. H. pylori eradication therapy is effective in chronic liver disease[21]. However, two recent studies have suggested that H. pylori eradication in cirrhotic patients with duodenal ulcers is not as effective at reducing ulcer recurrence as it is in the general population. These patients require maintenance acid suppression therapy[22,23].
The development of portal hypertension and formation of portosystemic shunts is a major event in the natural history of liver disease. Measurement of the portal pressure gradient is invasive and not widely available for clinical use; instead the hepatic venous pressure gradient (HVPG) is commonly used in clinical practice and it is of prognostic value: HPVG ≥ 10 mmHg strongly predicts the development of esophageal varices[24]. Similarly, the most significant risk factor associated with failure to control bleeding or early rebleeding of esophageal varices is HVPG > 20 mmHg. This is also associated with increased mortality[25].
Gastroesophageal varices are present in > 50% of patients with portal hypertension and are more likely as liver disease progresses[26]. Ectopic varices are located in sites other than the gastroesophageal region and are more common than previously thought: duodenal or colonic varices are seen at angiography or colonoscopy in up to 40% of patients with intrahepatic portal hypertension[27].
It is recommended that all patients undergo endoscopy to assess the presence and the size of varices at the time of the diagnosis of cirrhosis. Thereafter, guidelines for the interval of endoscopic screening vary. Currently, the American Association for the Study of the Liver (AASLD) recommends that, if no varices are present at index endoscopy, this should be repeated at 2-3 years in compensated cirrhosis and annually in decompensated cirrhosis[11]. The British Society of Gastroenterology recommends annual screening if grade 1 varices are present at initial screening (Table 1, grading and treatment of esophageal varices), and an interval of 3 years if there is no evidence of varices at index endoscopy[28].
| Grade | Appearance | High-risk stigmata | Treatment |
| Grade 1: Small varices | Barely noticeable varices; disappear easily with insufflation | No red signs | No treatment |
| Grade 2: Small/medium varices | Small or medium varices; do not easily disappear with insufflation | ± Red signs | NSBB or VBL |
| Grade 3: Medium/large varices | Medium or large varies; do not disappear with insufflation | ± Red signs | NSBB or VBL |
Esophageal variceal bleeding occurs at a rate of 5%-15% per year in untreated patients. The main risk factors for bleeding are variceal size (grade 2 or 3), decompensated cirrhosis, and the presence of high-risk stigmata at endoscopy[29]. Variceal bleeding is a significant clinical event with a mortality rate of approximately 20% at 6 wk, and a recurrence rate of up to 60% at 2 years if secondary prophylaxis is not commenced[30].
The management of esophageal varices may be divided into pre-primary, primary and secondary prophylaxis and control of active bleeding. At present, there is no evidence to support treatment to prevent the development of varices in patients with liver disease (pre-primary prophylaxis)[31,24].
For primary prophylaxis of esophageal varices, there is no evidence that variceal band ligation (VBL) is superior to β-blockade. Due to issues with access to endoscopy and patient preference, non-selective β-blockade, typically with propranolol, is often first line when treatment is indicated[32]. Carvedilol is a potent non-selective β-blocker, with weak vasodilating properties. A reduction in the HVPG in the range of 10%-43% has been reported with a 12.5 mg/d dose. Carvedilol has therefore been adopted as the β-blocker of choice for primary prophylaxis of variceal bleeding in some centers[33-35]. Primary prophylaxis with VBL is recommended if there are contraindications to β-blockers, or concerns about patient compliance[11].
Secondary prophylaxis is indicated for patients who have had an episode of variceal hemorrhage. β-blocker monotherapy is not used as secondary prophylaxis, and patients should either be entered into a variceal banding program or receive a combination of a β-blocker and nitrate[36]. AASLD recommends a combination of β blockade and VBL[11]. However, there is no strong evidence to suggest that this strategy is associated with improved mortality[37] and our local practice is to use VBL alone. VBL should be repeated every 2 wk until obliteration of varices is achieved. Following this, a surveillance endoscopy at 1-3 mo to confirm eradication is required, and this should be repeated every 6-12 mo[11]. VBL is a safe technique: although asymptomatic banding ulcers are common after VBL, the rate of bleeding from these and requiring hospitalization does not exceed 5%[38].
The combination of terlipressin and VBL is the preferred treatment for acute variceal bleeding in many centers, and using terlipressin before endoscopy is not unreasonable if there is a delay to the endoscopy. Endoscopic hemostasis is usually achieved in the majority of cases[11]. Transjugular intrahepatic portal systemic shunt (TIPSS) may be considered if VBL has been unsuccessful or there is an early re-bleed (defined at Baveno V as a repeat bleed within 5 d of the index bleed). A reduction in HVPG below 12 mmHg or a 20% reduction from the baseline value, even without reaching < 12 mmHg, protects against rebleeding[39].
The use of sclerosing agents (variceal sclerotherapy) is no longer recommended as first-line treatment, because of increased mortality rates[40], nor for secondary prophylaxis because VBL treatment has been shown to be safer and more effective[41].
Recently, endoscopic placement of a specifically designed self-expanding covered metal stent has proved effective in the treatment of esophageal varices in patients in whom initial endoscopic methods have failed to achieve hemostasis[42]. This method appears to be a safe and effective means of controlling ongoing bleeding. The stent is usually removed 1 wk after the acute bleed. Currently, this technique is limited by its relative complexity of stent insertion in acute bleeding, but stenting with covered biodegradable stents when available, which do not require removal, may play an important role in the management of acute esophageal variceal bleeding[43].
Gastric varices are less prevalent than esophageal varices and less prone to bleeding: around 25% over a 2-year period[44]. There is no evidence to support the primary prophylaxis of gastric varices. The tissue adhesive cyanoacrylate (“glue”) is used widely in the management of acutely bleeding gastric varices. Cyanoacrylate is a liquid with consistency similar to water, which when added to a physiological fluid like blood, polymerizes to form a solid substance[45]. Two randomized controlled studies have compared cyanoacrylate with VBL for management of bleeding gastric varices[46,47]. In one study, cyanoacrylate was more effective than VBL in achieving homeostasis, and in the second, no difference was reported, although both reported less recurrence of bleeding. Current evidence suggests that cyanoacrylate achieves control of bleeding in 87%-93% of cases, and that bleeding-related mortality is between 6.5% and 10%[46,47].
The use of bovine or more recently, human thrombin has been described as an alternative treatment for active gastric variceal bleeding. Thrombin [activated factor II (IIa)] is a serine protease that converts soluble fibrinogen into insoluble strands of fibrin clot. It has additional effects including promotion of platelet aggregation. Initial hemostasis rates have been reported at 94%-100%, and rebleeding rates of between 23% and 25%[48,49]. A single center experience of 13 patients treated with thrombin for bleeding gastric varices has reported efficient hemostasis and an overall mortality of 38% in a median follow-up of 22 mo[50].
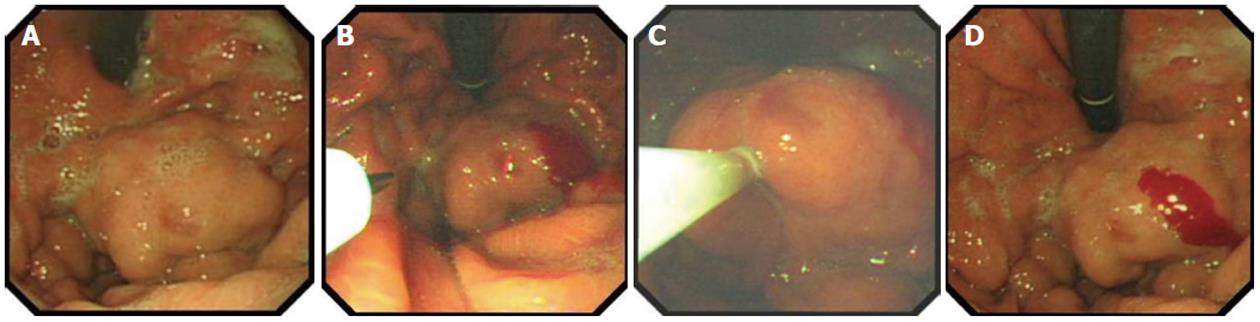
Technical difficulties that include the risk of equipment damage and reports of severe thromboembolic complications may limit the use of cyanoacrylate, and thrombin (Figure 1) may become more widespread in the future[51,52].
The role of TIPSS as primary treatment in actively bleeding gastric varices has also been explored. Although TIPSS has a comparable mortality and rebleeding rate to cyanoacrylate, it is associated with significantly higher morbidity and is not used as first-line treatment[53,54], but remains an effective treatment when endoscopy fails to control bleeding.
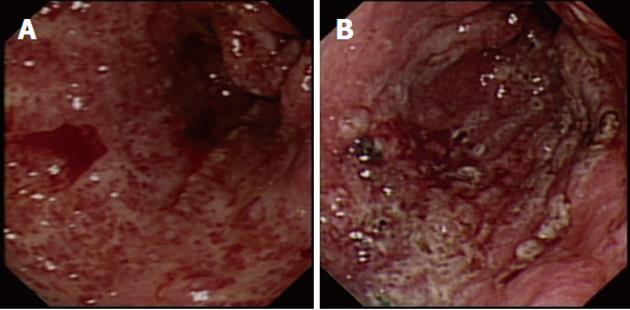
Portal hypertensive gastropathy (PHG), with its typical “snake skin” appearance, is present in approximately 80% of patients with cirrhosis[55]. PHG accounts for 8% of nonvariceal bleeds in patients with liver disease, although this condition more commonly presents with anemia[56]. Patients with cirrhosis and severe PHG-related bleeding may respond to β-blockade. Endoscopic measures such as argon plasma coagulation (APC) therapy can reduce bleeding, thus controlling anemia. TIPSS should be reserved for those patients with pharmacological treatment failure[57].
The prevalence of portal hypertensive enteropathy (PHE), determined by capsule endoscopy, is as high as 63% in patients with end-stage liver disease who also have esophageal or gastric varices[58]. Portal hypertensive duodenopathy is present in around half of patients with cirrhosis, and it is more common in patients with severe PHG[59].
Gastric antral vascular ectasia (GAVE) is related to portal hypertension in about 30% of patients, and accounts for 4% of nonvariceal upper GI bleeds[60]. Unlike PHG, GAVE does not respond well to reduction in portal pressure[61]. The Nd:YAG laser has been widely used in the treatment of GAVE and is the most commonly reported endoscopic modality in cirrhotic and non-cirrhotic patients, with a reported overall success rate of almost 90%, although the authors did not distinguish the etiology of GAVE when reporting the outcomes[62].
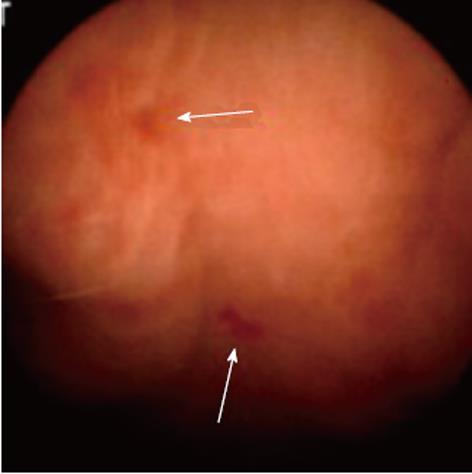
APC has also proved to be effective for the treatment of GAVE-related bleeding, and it is extensively used in our unit, with a success rate of > 85%; success is defined as control of bleeding, stabilization of hemoglobin at > 100 g/dL, or hemoglobin increase > 10% from pretreatment level, and reduction of transfusion requirements by > 50% in transfusion-dependent patients. An average of four sessions of APC is usually required (Figures 2 and 3). In two published studies, with a total of 37 patients with cirrhosis, success rates in controlling bleeding were very high, and the reported rebleeding rates were between 12% and 20% after 2 years follow-up[62,63].
Although the success rates of the two aforementioned modalities are comparable, APC treatment is probably the therapy of choice due to the technical ease, safety and low cost[61]. Other endoscopic techniques have been positively reported in small series, such as VBL[64], endoscopic mucosal ablation[65] and cryotherapy[66].
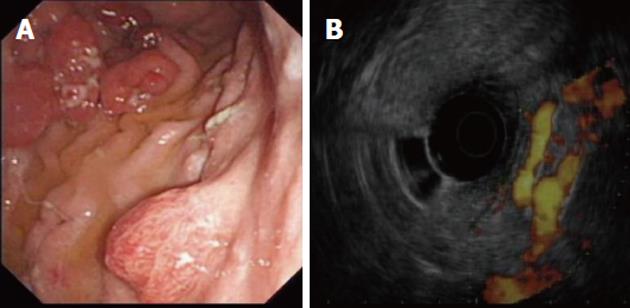
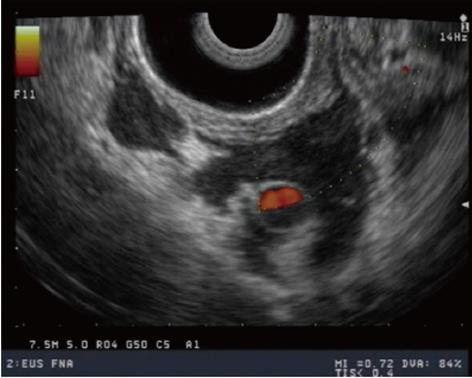
Recently, EUS has been used to assist in the management of portal hypertension. Doppler EUS is of significant value in differentiating ectopic varices from other submucosal lesions[67] (Figure 4), and several EUS-assisted techniques have been used to identify the precise site for the intravariceal injection of sclerosant agents. Linear color EUS-guided sclerotherapy has proved to be effective in the eradication of esophageal varices, in two small studies with recurrence reported at 0% and 8.3%, respectively[68,69]. EUS catheter probes and high-frequency (20 MHz) miniprobes have both been used successfully before and after esophageal variceal sclerosant injection in two different studies to assess eradication and variceal recurrence. After a mean follow-up of 24 mo, variceal recurrence was reported at 16.6% and 26.3%, respectively[70,71].
Although the overall patient numbers are small, linear EUS seems to be the superior modality in assisting treatment of esophageal varices, because it permits the targeting of the feeding vessels.
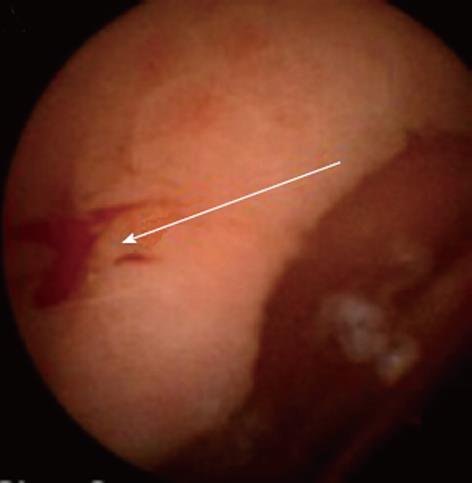
EUS-assisted injection of cyanoacrylate for the treatment of gastric varices has been described in 54 patients with a mean follow-up of 24 mo. Varices recurred in 35% of patients[72]. Furthermore, a series of 15 patients with gastric or ectopic varices treated with thrombin injection in conjunction with a variety of EUS techniques (Figure 5) has recently been reported in our unit, and this proved to be effective in controlling active bleeding and achieving variceal eradication[73].
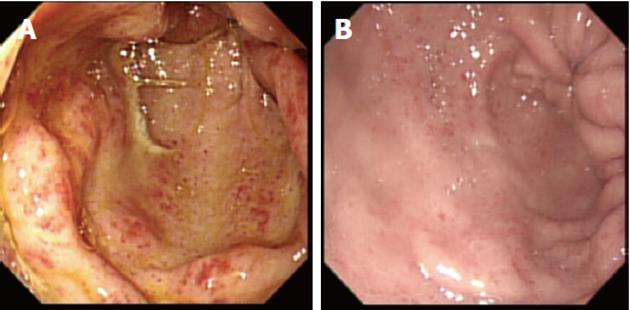
Esophageal capsule endoscopy (OCE) is an alternative to conventional upper GI endoscopy for the diagnosis of varices in complex patients with portal hypertension. In a recent meta-analysis of seven studies involving 446 patients, OCE had a sensitivity of 85.8% and specificity 80.5% in detecting esophageal varices[74]. However, a multicenter trial evaluating the efficacy of OCE in esophageal varices screening was less encouraging, because the standard of < 10% difference between capsule and conventional endoscopy was not met[75]. This is not surprising, because the two techniques differ in that during conventional endoscopy, the esophagus is inevitably insufflated with air and varices can appear more flattened than during OCE examination. Further studies to take this into account are necessary. Nevertheless, OCE remains a useful tool for screening of varices in certain patient groups; patients who poorly tolerate endoscopy or who have significant comorbidity, thus increasing the risks of repeated endoscopy, and patients with high risk of variant Creutzfeldt-Jakob disease. Although this technique is limited by availability and high costs, OCE can be cost-effective for variceal screening of patients with coagulation abnormalities (e.g., hemophilia) with coexisting liver disease, because it does not require prophylactic clotting factor administration, unlike conventional endoscopy. Serial capsule examinations in the same patient may provide significant diagnostic information regarding progression of varices.
Small bowel capsule endoscopy (SBCE) has been used to characterize PHE (Figure 6), and is of value in the diagnosis of this condition in patients with advanced liver disease who continue to bleed despite treatment of esophageal/gastric varices or portal gastropathy (Figure 7). The role of SBCE in portal hypertension has yet to be defined, but it is likely that it will remain a valuable tool in certain groups of patients with liver disease.
Primary sclerosing cholangitis (PSC) is characterized by fibrosis of both intrahepatic and/or extrahepatic biliary ducts. These patients are at risk of developing infectious cholangitis and up to 20% develop cholangiocarcinoma. A strategy of initial magnetic resonance cholangiopancreatography (MRCP) followed, if necessary, by ERCP is currently the most cost-effective approach to the work-up of patients with suspected sclerosing cholangitis[76]. ERCP plus diagnostic brushing have a sensitivity of 60%-100%, and specificity of 85%-89% in differentiating between a benign dominant stricture and cholangiocarcinoma[77,78]. Recently, two advanced cytological techniques (digital image analysis and fluorescence in situ hybridization) have been used for the detection of malignancy in PSC-related strictures and have proved to be more sensitive and equally specific to conventional cytology[79].
In addition, ERCP permits therapeutic interventions with balloon dilation or stent placement as appropriate.
Novel endoscopic modalities have been compared with conventional ERCP and brush cytology. Transpapillary cholangioscopy with tissue sampling has proved to be more sensitive (92% vs 66%) and specific (93% vs 51%) than ERCP to detect cholangiocarcinoma in PSC[80]. In a small study, narrow band imaging has demonstrated superior visualization of biliary lesions compared with conventional white light imaging[81]. In another study, transpapillary intraductal ultrasound was superior to ERCP for the detection of cholangiocarcinoma in PSC in terms of sensitivity (87.5% vs 62.5%) and specificity (90.6% vs 53.1%)[82].
SpyglassTM is a new single-operator system used for the diagnosis of a variety of pancreatobiliary disorders, such as the definition of indeterminate strictures and filling defects prior to stone extraction[83]. Although the initial experience is promising, the modality has been only been tested in a limited number of PSC-related strictures, and unlike ERCP, this is a purely diagnostic technique.
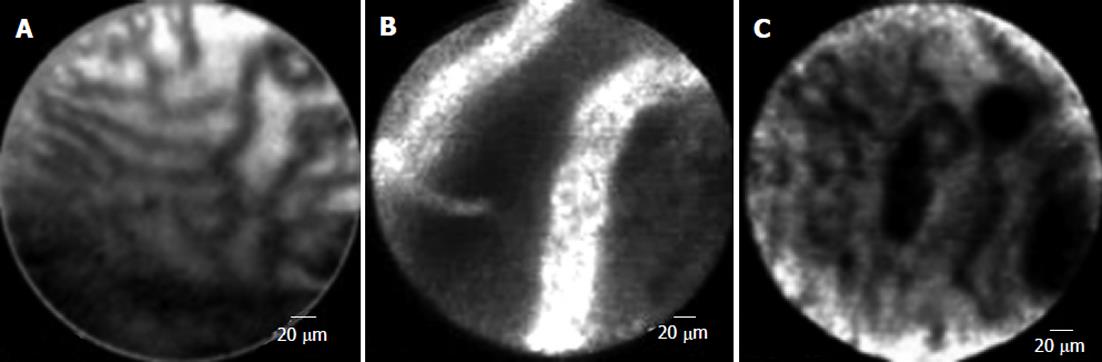
Confocal laser endomicroscopy (“miniprobe”) is a new field of endoluminal imaging that offers extremely high magnification and resolution. This technique allows visualization of pancreatic and biliary ducts. In a pilot study of 14 patients with biliary strictures, miniprobe-based microscopy after fluorescein administration proved to be more accurate than brushings and biopsy in distinguishing benign from malignant strictures (Figure 8)[84].
A novel alternative is direct cholangioscopy using ultra-slim endoscopes (4.9-5.9 mm). These endoscopes, initially developed for transnasal endoscopy, can be safely inserted into the bile duct following sphincterotomy, and not only permit high-resolution images, but also biopsy and other interventional procedures in the bile ducts, such as hydraulic lithotripsy and division of strictures in benign biliary disease. This technique is currently under development and after a full range of endoscopic accessories are available for endobiliary interventions, it could be an effective and safe approach for patients with difficult to manage biliary disease[85].
In comparison to the upper GI tract, colonic manifestations of portal hypertension much less often present with acute bleeding, and are more often found incidentally or during investigation of anemia. As such, data are sparse and less consistent. The reported prevalence of portal hypertensive colonopathy is 24%[86].
The most significant feature of portal hypertension in the colon is arguably the presence of rectal varices. These can be present in up to 44% of patients with cirrhosis at colonoscopy, although the reported prevalence varies widely. They are more frequent in patients with advanced liver disease[87].
Although bleeding from rectal varices is uncommon, it can be life threatening. Due to their rarity, no firm guidelines have been established for the management of bleeding colonic varices, and there is a limited evidence base. The most commonly used treatment modalities are sclerotherapy and band ligation. In a small retrospective comparative study of 15 patients, endoscopic injection sclerotherapy proved to be superior to endoscopic band ligation and achieved lower recurrence rates[88]. In our unit, thrombin has been successfully used to manage rectal variceal bleeding. Patients may require TIPSS if bleeding cannot be controlled endoscopically.
Peptic ulcer disease is the most common cause of GI bleeding in post-orthotopic liver transplant (OLT) recipients, accounting for 27% of all bleeding[89]. Varices rarely recur post-transplant, and if present, require investigation to exclude portal vein thrombosis or disease recurrence[90].
Liver transplant patients are at increased risk of opportunistic infections, particularly candidiasis and cytomegalovirus[91]. These often present with GI symptoms and require endoscopic evaluation with biopsies or brushings to confirm the diagnosis.
The association of PSC with ulcerative colitis is well recognized and is an additional risk factor for the development of colorectal cancer in immunosuppressed transplant patients. It is currently recommended that these patients have an annual surveillance colonoscopy commencing 10 years after the onset of bowel symptoms[92]. Colectomy is safe in patients who have undergone OLT, and in some high-risk cases, such as when high-grade dysplasia has already been identified, a prophylactic colectomy may be performed at the time of transplantation[93].
Biliary complications (biliary strictures and leaks) following liver transplantation are a challenging and common issue that affects 10%-30% of OLT patients. Biliary strictures are classified as anastomotic or non-anastomotic[94]. The initial approach in suspected post-transplant biliary strictures is usually MRCP, restricting the use of ERCP to patients who require intervention, or where MRCP results are equivocal[95]. Further imaging techniques include SpyGlassTM, which has been successfully used for the investigation of post-transplant biliary strictures[96], and contrast-enhanced ultrasound. This is a non-invasive technique for the detection of strictures relating to hepatic artery stenosis in liver transplant patients. It provides details on the presence, location, degree, and type of stricture[97]. Treatment comprises a combination of balloon dilation and stent placement, repeated if necessary until stricture resolution.
Biliary leaks can occur in up to 22% of patients. Evidence suggests that sphincterotomy with stent placement is the best treatment option for biliary leaks following OLT[94,98]. Surgical revision and biliary reconstruction with the formation of hepaticojejunostomy is indicated when endoscopic or percutaneous treatment fails[94,98].
In patients who have a roux-en-Y anastomosis, the technique of double balloon ERCP has been devised with promising results. The technique uses a double balloon colonoscope to approach the ampulla, although there are some limitations regarding endoscopic accessories. With further development of this technique, the endoscopic treatment of biliary complications may become easier and will play a larger role in the management of such patients[99].
Endoscopy has undergone rapid expansion with numerous novel endoscopic modalities and techniques directly applicable to the diagnosis and management of complications of liver disease. Although conventional upper GI endoscopy is still the modality of choice for esophageal variceal surveillance and treatment, further options are now available with the use of capsule endoscopy and EUS. Therapeutic options for the management of upper GI bleeding in portal hypertension have also been developed. Band ligation remains the treatment of choice for esophageal variceal bleeding, whereas for gastric and ectopic varices, the use of sclerosants, particularly “glue” and thrombin are increasingly being used. APC is the preferred modality for GAVE and PHG. Biliary strictures and the risk of cholangiocarcinoma are major issues in patients with PSC. ERCP is both diagnostic and therapeutic in this setting and can differentiate benign from malignant lesions in the majority of cases. Novel endoscopic techniques such as transpapillary cholangioscopy, Spyglass Direct Visualization System, confocal laser endomicroscopy (“miniprobe”) and ultra-thin cholangioscopy are increasingly being used to assist diagnosis in selected patients. Finally, in the post-liver transplant patient, upper and lower endoscopies are used to detect gastrointestinal opportunistic infections, as well as to screen for colorectal cancer in high-risk patients. Biliary complications are common after transplantation and ERCP is the modality of choice for treating such patients.
We would like to thank the Hellenic Foundation of Gastroenterology and Nutrition for providing a research scholarship to Dr. C Krystallis.
Peer reviewers: Erwin Biecker, MD, PhD, Department of Gastroenterology and Hepatology, Helios Klinikum Siegburg, Siegburg 53343, Germany; Yu-Yuan Li, Professor, Department of Gastroenterology, First Municipal People’s Hospital of Guangzhou, 1 Panfu Road, Guangzhou 510180, Guangdong Province, China
S- Editor Tian L L- Editor Kerr C E- Editor Li JY
| 1. | World Health Organization. WHO European Health for All Database. Geneva: Author 2009; . |
| 2. | Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 448] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 3. | MacGilchrist AJ, Birnie GG, Cook A, Scobie G, Murray T, Watkinson G, Brodie MJ. Pharmacokinetics and pharmacodynamics of intravenous midazolam in patients with severe alcoholic cirrhosis. Gut. 1986;27:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Neal EA, Meffin PJ, Gregory PB, Blaschke TF. Enhanced bioavailability and decreased clearance of analgesics in patients with cirrhosis. Gastroenterology. 1979;77:96-102. [PubMed] |
| 5. | Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999;37:17-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 175] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Khamaysi I, William N, Olga A, Alex I, Vladimir M, Kamal D, Nimer A. Sub-clinical hepatic encephalopathy in cirrhotic patients is not aggravated by sedation with propofol compared to midazolam: a randomized controlled study. J Hepatol. 2011;54:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Rudolph SJ, Landsverk BK, Freeman ML. Endotracheal intubation for airway protection during endoscopy for severe upper GI hemorrhage. Gastrointest Endosc. 2003;57:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Rehman A, Iscimen R, Yilmaz M, Khan H, Belsher J, Gomez JF, Hanson AC, Afessa B, Baron TH, Gajic O. Prophylactic endotracheal intubation in critically ill patients undergoing endoscopy for upper GI hemorrhage. Gastrointest Endosc. 2009;69:e55-e59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Liebler JM, Benner K, Putnam T, Vollmer WM. Respiratory complications in critically ill medical patients with acute upper gastrointestinal bleeding. Crit Care Med. 1991;19:1152-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Veitch AM, Baglin TP, Gershlick AH, Harnden SM, Tighe R, Cairns S. Guidelines for the management of anticoagulant and antiplatelet therapy in patients undergoing endoscopic procedures. Gut. 2008;57:1322-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1209] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 12. | Bosch J, Thabut D, Albillos A, Carbonell N, Spicak J, Massard J, D'Amico G, Lebrec D, de Franchis R, Fabricius S. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: A randomized, controlled trial. Hepatology. 2008;47:1604-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | de Franchis R, Arcidiacono PG, Carpinelli L, Andreoni B, Cestari L, Brunati S, Zambelli A, Battaglia G, Mannucci PM. Randomized controlled trial of desmopressin plus terlipressin vs. terlipressin alone for the treatment of acute variceal hemorrhage in cirrhotic patients: a multicenter, double-blind study. New Italian Endoscopic Club. Hepatology. 1993;18:1102-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Hollerbach S, Willert J, Topalidis T, Reiser M, Schmiegel W. Endoscopic ultrasound-guided fine-needle aspiration biopsy of liver lesions: histological and cytological assessment. Endoscopy. 2003;35:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Park DH, Kim MH, Lee SK, Lee SS, Choi JS, Song MH, Seo DW, Min YI. Endoscopic sphincterotomy vs. endoscopic papillary balloon dilation for choledocholithiasis in patients with liver cirrhosis and coagulopathy. Gastrointest Endosc. 2004;60:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Wu CS, Lin CY, Liaw YF. Helicobacter pylori in cirrhotic patients with peptic ulcer disease: a prospective, case controlled study. Gastrointest Endosc. 1995;42:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Chen LS, Lin HC, Hwang SJ, Lee FY, Hou MC, Lee SD. Prevalence of gastric ulcer in cirrhotic patients and its relation to portal hypertension. J Gastroenterol Hepatol. 1996;11:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Pellicano R, Leone N, Berrutti M, Cutufia MA, Fiorentino M, Rizzetto M, Ponzetto A. Helicobacter pylori seroprevalence in hepatitis C virus positive patients with cirrhosis. J Hepatol. 2000;33:648-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Calvet X, Sanfeliu I, Musulen E, Mas P, Dalmau B, Gil M, Bella MR, Campo R, Brullet E, Valero C. Evaluation of Helicobacter pylori diagnostic methods in patients with liver cirrhosis. Aliment Pharmacol Ther. 2002;16:1283-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Vergara M, Calvet X, Roqué M. Helicobacter pylori is a risk factor for peptic ulcer disease in cirrhotic patients. A meta-analysis. Eur J Gastroenterol Hepatol. 2002;14:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Jung SW, Lee SW, Hyun JJ, Kim DI, Koo JS, Yim HJ, Park JJ, Lee HS, Chun HJ, Um SH. Efficacy of Helicobacter pylori eradication therapy in chronic liver disease. Dig Liver Dis. 2009;41:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Lo GH, Yu HC, Chan YC, Chen WC, Hsu PI, Lin CK, Lai KH. The effects of eradication of Helicobacter pylori on the recurrence of duodenal ulcers in patients with cirrhosis. Gastrointest Endosc. 2005;62:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Tzathas C, Triantafyllou K, Mallas E, Triantafyllou G, Ladas SD. Effect of Helicobacter pylori eradication and antisecretory maintenance therapy on peptic ulcer recurrence in cirrhotic patients: a prospective, cohort 2-year follow-up study. J Clin Gastroenterol. 2008;42:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 652] [Article Influence: 32.6] [Reference Citation Analysis (1)] |
| 25. | Moitinho E, Escorsell A, Bandi JC, Salmerón JM, García-Pagán JC, Rodés J, Bosch J. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 305] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 26. | Kovalak M, Lake J, Mattek N, Eisen G, Lieberman D, Zaman A. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointest Endosc. 2007;65:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Norton ID, Andrews JC, Kamath PS. Management of ectopic varices. Hepatology. 1998;28:1154-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 254] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Jalan R, Hayes PC. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut. 2000;46 Suppl 3-4:III1-III15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 841] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 30. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [PubMed] |
| 31. | Calés P, Oberti F, Payen JL, Naveau S, Guyader D, Blanc P, Abergel A, Bichard P, Raymond JM, Canva-Delcambre V. Lack of effect of propranolol in the prevention of large oesophageal varices in patients with cirrhosis: a randomized trial. French-Speaking Club for the Study of Portal Hypertension. Eur J Gastroenterol Hepatol. 1999;11:741-745. [PubMed] |
| 32. | Schepke M, Kleber G, Nürnberg D, Willert J, Koch L, Veltzke-Schlieker W, Hellerbrand C, Kuth J, Schanz S, Kahl S. Ligation versus propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2004;40:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1027] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 34. | Tripathi D, Hayes PC. The role of carvedilol in the management of portal hypertension. Eur J Gastroenterol Hepatol. 2010;22:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Tripathi D, Ferguson JW, Kochar N, Leithead JA, Therapondos G, McAvoy NC, Stanley AJ, Forrest EH, Hislop WS, Mills PR. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 36. | de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 733] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 37. | Kumar A, Jha SK, Sharma P, Dubey S, Tyagi P, Sharma BC, Sarin SK. Addition of propranolol and isosorbide mononitrate to endoscopic variceal ligation does not reduce variceal rebleeding incidence. Gastroenterology. 2009;137:892-901, 901.e1. [PubMed] |
| 38. | García-Pagán JC, Villanueva C, Albillos A, Bañares R, Morillas R, Abraldes JG, Bosch J. Nadolol plus isosorbide mononitrate alone or associated with band ligation in the prevention of recurrent bleeding: a multicentre randomised controlled trial. Gut. 2009;58:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Bosch J, García-Pagán JC. Prevention of variceal rebleeding. Lancet. 2003;361:952-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 288] [Article Influence: 13.1] [Reference Citation Analysis (2)] |
| 40. | Prophylactic sclerotherapy for esophageal varices in men with alcoholic liver disease. A randomized, single-blind, multicenter clinical trial. The Veterans Affairs Cooperative Variceal Sclerotherapy Group. N Engl J Med. 1991;324:1779-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 143] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | de Franchis R, Primignani M. Endoscopic treatments for portal hypertension. Semin Liver Dis. 1999;19:439-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Wright G, Lewis H, Hogan B, Burroughs A, Patch D, O'Beirne J. A self-expanding metal stent for complicated variceal hemorrhage: experience at a single center. Gastrointest Endosc. 2010;71:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Zehetner J, Shamiyeh A, Wayand W, Hubmann R. Results of a new method to stop acute bleeding from esophageal varices: implantation of a self-expanding stent. Surg Endosc. 2008;22:2149-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 846] [Article Influence: 25.6] [Reference Citation Analysis (42)] |
| 45. | Binmoeller KF. Glue for gastric varices: some sticky issues. Gastrointest Endosc. 2000;52:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Tan PC, Hou MC, Lin HC, Liu TT, Lee FY, Chang FY, Lee SD. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. 2006;43:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 47. | Lo GH, Lai KH, Cheng JS, Chen MH, Chiang HT. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001;33:1060-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 304] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 48. | Przemioslo RT, McNair A, Williams R. Thrombin is effective in arresting bleeding from gastric variceal hemorrhage. Dig Dis Sci. 1999;44:778-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 49. | Yang WL, Tripathi D, Therapondos G, Todd A, Hayes PC. Endoscopic use of human thrombin in bleeding gastric varices. Am J Gastroenterol. 2002;97:1381-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Ramesh J, Limdi JK, Sharma V, Makin AJ. The use of thrombin injections in the management of bleeding gastric varices: a single-center experience. Gastrointest Endosc. 2008;68:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Roesch W, Rexroth G. Pulmonary, cerebral and coronary emboli during bucrylate injection of bleeding fundic varices. Endoscopy. 1998;30:S89-S90. [PubMed] |
| 52. | Palejwala AA, Smart HL, Hughes M. Multiple pulmonary glue emboli following gastric variceal obliteration. Endoscopy. 2000;32:S1-S2. [PubMed] |
| 53. | Procaccini NJ, Al-Osaimi AM, Northup P, Argo C, Caldwell SH. Endoscopic cyanoacrylate versus transjugular intrahepatic portosystemic shunt for gastric variceal bleeding: a single-center U.S. analysis. Gastrointest Endosc. 2009;70:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Lo GH, Liang HL, Chen WC, Chen MH, Lai KH, Hsu PI, Lin CK, Chan HH, Pan HB. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 55. | Primignani M, Carpinelli L, Preatoni P, Battaglia G, Carta A, Prada A, Cestari R, Angeli P, Gatta A, Rossi A. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis. The New Italian Endoscopic Club for the study and treatment of esophageal varices (NIEC). Gastroenterology. 2000;119:181-187. [PubMed] |
| 56. | Burak KW, Lee SS, Beck PL. Portal hypertensive gastropathy and gastric antral vascular ectasia (GAVE) syndrome. Gut. 2001;49:866-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Kamath PS, Lacerda M, Ahlquist DA, McKusick MA, Andrews JC, Nagorney DA. Gastric mucosal responses to intrahepatic portosystemic shunting in patients with cirrhosis. Gastroenterology. 2000;118:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Canlas KR, Dobozi BM, Lin S, Smith AD, Rockey DC, Muir AJ, Agrawal NM, Poleski MH, Patel K, McHutchison JG. Using capsule endoscopy to identify GI tract lesions in cirrhotic patients with portal hypertension and chronic anemia. J Clin Gastroenterol. 2008;42:844-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Barakat M, Mostafa M, Mahran Z, Soliman AG. Portal hypertensive duodenopathy: clinical, endoscopic, and histopathologic profiles. Am J Gastroenterol. 2007;102:2793-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): an update on clinical presentation, pathophysiology and treatment. Digestion. 2008;77:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 61. | Sebastian S, O'Morain CA, Buckley MJ. Review article: current therapeutic options for gastric antral vascular ectasia. Aliment Pharmacol Ther. 2003;18:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Lecleire S, Ben-Soussan E, Antonietti M, Goria O, Riachi G, Lerebours E, Ducrotté P. Bleeding gastric vascular ectasia treated by argon plasma coagulation: a comparison between patients with and without cirrhosis. Gastrointest Endosc. 2008;67:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Fuccio L, Zagari RM, Serrani M, Eusebi LH, Grilli D, Cennamo V, Laterza L, Asioli S, Ceroni L, Bazzoli F. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia-related bleeding in patients with liver cirrhosis. Digestion. 2009;79:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Wells CD, Harrison ME, Gurudu SR, Crowell MD, Byrne TJ, Depetris G, Sharma VK. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008;68:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 65. | Gross SA, Al-Haddad M, Gill KR, Schore AN, Wallace MB. Endoscopic mucosal ablation for the treatment of gastric antral vascular ectasia with the HALO90 system: a pilot study. Gastrointest Endosc. 2008;67:324-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Cho S, Zanati S, Yong E, Cirocco M, Kandel G, Kortan P, May G, Marcon N. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc. 2008;68:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Shen EF, Arnott ID, Plevris J, Penman ID. Endoscopic ultrasonography in the diagnosis and management of suspected upper gastrointestinal submucosal tumours. Br J Surg. 2002;89:231-235. [PubMed] |
| 68. | Lahoti S, Catalano MF, Alcocer E, Hogan WJ, Geenen JE. Obliteration of esophageal varices using EUS-guided sclerotherapy with color Doppler. Gastrointest Endosc. 2000;51:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | de Paulo GA, Ardengh JC, Nakao FS, Ferrari AP. Treatment of esophageal varices: a randomized controlled trial comparing endoscopic sclerotherapy and EUS-guided sclerotherapy of esophageal collateral veins. Gastrointest Endosc. 2006;63:396-402; quiz 463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Suzuki T, Matsutani S, Umebara K, Sato G, Maruyama H, Mitsuhashi O, Nakano Y, Fukamachi T, Saisho H. EUS changes predictive for recurrence of esophageal varices in patients treated by combined endoscopic ligation and sclerotherapy. Gastrointest Endosc. 2000;52:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Irisawa A, Saito A, Obara K, Shibukawa G, Takagi T, Shishido H, Sakamoto H, Sato Y, Kasukawa R. Endoscopic recurrence of esophageal varices is associated with the specific EUS abnormalities: severe periesophageal collateral veins and large perforating veins. Gastrointest Endosc. 2001;53:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Lee YT, Chan FK, Ng EK, Leung VK, Law KB, Yung MY, Chung SC, Sung JJ. EUS-guided injection of cyanoacrylate for bleeding gastric varices. Gastrointest Endosc. 2000;52:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 73. | Abstracts of the British Society of Gastroenterology Annual General Meeting. March 23-26, 2009. Glasgow, Scotland. Gut. 2009;58 Suppl 1:A1-156. [PubMed] |
| 74. | Lu Y, Gao R, Liao Z, Hu LH, Li ZS. Meta-analysis of capsule endoscopy in patients diagnosed or suspected with esophageal varices. World J Gastroenterol. 2009;15:1254-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | de Franchis R, Eisen GM, Laine L, Fernandez-Urien I, Herrerias JM, Brown RD, Fisher L, Vargas HE, Vargo J, Thompson J. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology. 2008;47:1595-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Meagher S, Yusoff I, Kennedy W, Martel M, Adam V, Barkun A. The roles of magnetic resonance and endoscopic retrograde cholangiopancreatography (MRCP and ERCP) in the diagnosis of patients with suspected sclerosing cholangitis: a cost-effectiveness analysis. Endoscopy. 2007;39:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Ponsioen CY, Vrouenraets SM, van Milligen de Wit AW, Sturm P, Tascilar M, Offerhaus GJ, Prins M, Huibregtse K, Tytgat GN. Value of brush cytology for dominant strictures in primary sclerosing cholangitis. Endoscopy. 1999;31:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 78. | Lindberg B, Arnelo U, Bergquist A, Thörne A, Hjerpe A, Granqvist S, Hansson LO, Tribukait B, Persson B, Broomé U. Diagnosis of biliary strictures in conjunction with endoscopic retrograde cholangiopancreaticography, with special reference to patients with primary sclerosing cholangitis. Endoscopy. 2002;34:909-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 80. | Tischendorf JJ, Krüger M, Trautwein C, Duckstein N, Schneider A, Manns MP, Meier PN. Cholangioscopic characterization of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Endoscopy. 2006;38:665-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Moriyasu F, Gotoda T. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos). Gastrointest Endosc. 2007;66:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 82. | Tischendorf JJ, Meier PN, Schneider A, Manns MP, Krüger M. Transpapillary intraductal ultrasound in the evaluation of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Scand J Gastroenterol. 2007;42:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 83. | Fishman DS, Tarnasky PR, Patel SN, Raijman I. Management of pancreaticobiliary disease using a new intra-ductal endoscope: the Texas experience. World J Gastroenterol. 2009;15:1353-1358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 84. | Meining A, Frimberger E, Becker V, Von Delius S, Von Weyhern CH, Schmid RM, Prinz C. Detection of cholangiocarcinoma in vivo using miniprobe-based confocal fluorescence microscopy. Clin Gastroenterol Hepatol. 2008;6:1057-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 85. | Moon JH, Ko BM, Choi HJ, Koo HC, Hong SJ, Cheon YK, Cho YD, Lee MS, Shim CS. Direct peroral cholangioscopy using an ultra-slim upper endoscope for the treatment of retained bile duct stones. Am J Gastroenterol. 2009;104:2729-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Diaz-Sanchez A, Nuñez-Martinez O, Gonzalez-Asanza C, Matilla A, Merino B, Rincon D, Beceiro I, Catalina MV, Salcedo M, Bañares R. Portal hypertensive colopathy is associated with portal hypertension severity in cirrhotic patients. World J Gastroenterol. 2009;15:4781-4787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Hosking SW, Smart HL, Johnson AG, Triger DR. Anorectal varices, haemorrhoids, and portal hypertension. Lancet. 1989;1:349-352. [RCA] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 129] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Sato T, Yamazaki K, Toyota J, Karino Y, Ohmura T, Suga T. The value of the endoscopic therapies in the treatment of rectal varices: a retrospective comparison between injection sclerotherapy and band ligation. Hepatol Res. 2006;34:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Tabasco-Minguillán J, Jain A, Naik M, Weber KM, Irish W, Fung JJ, Rakela J, Starzl TE. Gastrointestinal bleeding after liver transplantation. Transplantation. 1997;63:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 90. | Hirata M, Kita Y, Harihara Y, Hisatomi S, Sano K, Mizuta K, Yoshino H, Sugawara Y, Takayama T, Kawarasaki H. Gastrointestinal bleeding after living-related liver transplantation. Dig Dis Sci. 2002;47:2386-2388. [PubMed] |
| 91. | Sharma S, Gurakar A, Camci C, Jabbour N. Avoiding pitfalls: what an endoscopist should know in liver transplantation--part II. Dig Dis Sci. 2009;54:1386-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 808] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 93. | Vera A, Gunson BK, Ussatoff V, Nightingale P, Candinas D, Radley S, Mayer AD, Buckels JA, McMaster P, Neuberger J. Colorectal cancer in patients with inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Transplantation. 2003;75:1983-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 94. | Jue TL, Imperial JC. Management of post-liver-transplant biliary strictures: a work in progress. Gastrointest Endosc. 2008;67:886-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 95. | Boraschi P, Braccini G, Gigoni R, Sartoni G, Neri E, Filipponi F, Mosca F, Bartolozzi C. Detection of biliary complications after orthotopic liver transplantation with MR cholangiography. Magn Reson Imaging. 2001;19:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Wright H, Sharma S, Gurakar A, Sebastian A, Kohli V, Jabbour N. Management of biliary stricture guided by the Spyglass Direct Visualization System in a liver transplant recipient: an innovative approach. Gastrointest Endosc. 2008;67:1201-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Zheng RQ, Mao R, Ren J, Xu EJ, Liao M, Wang P, Lu MQ, Yang Y, Cai CJ, Chen GH. Contrast-enhanced ultrasound for the evaluation of hepatic artery stenosis after liver transplantation: potential role in changing the clinical algorithm. Liver Transpl. 2010;16:729-735. [PubMed] |
| 98. | Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 99. | Aabakken L, Bretthauer M, Line PD. Double-balloon enteroscopy for endoscopic retrograde cholangiography in patients with a Roux-en-Y anastomosis. Endoscopy. 2007;39:1068-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |