Published online Dec 14, 2012. doi: 10.3748/wjg.v18.i46.6771
Revised: September 10, 2012
Accepted: September 19, 2012
Published online: December 14, 2012
Glucose and other carbohydrates are transported into cells using members of a family of integral membrane glucose transporter (GLUT) molecules. To date 14 members of this family, also called the solute carrier 2A proteins have been identified which are divided on the basis of transport characteristics and sequence similarities into several families (Classes 1 to 3). The expression of these different receptor subtypes varies between different species, tissues and cellular subtypes and each has differential sensitivities to stimuli such as insulin. The liver is a contributor to metabolic carbohydrate homeostasis and is a major site for synthesis, storage and redistribution of carbohydrates. Situations in which the balance of glucose homeostasis is upset such as diabetes or the metabolic syndrome can lead metabolic disturbances that drive chronic organ damage and failure, confirming the importance of understanding the molecular regulation of hepatic glucose homeostasis. There is a considerable literature describing the expression and function of receptors that regulate glucose uptake and release by hepatocytes, the most import cells in glucose regulation and glycogen storage. However there is less appreciation of the roles of GLUTs expressed by non parenchymal cell types within the liver, all of which require carbohydrate to function. A better understanding of the detailed cellular distribution of GLUTs in human liver tissue may shed light on mechanisms underlying disease pathogenesis. This review summarises the available literature on hepatocellular expression of GLUTs in health and disease and highlights areas where further investigation is required.
- Citation: Karim S, Adams DH, Lalor PF. Hepatic expression and cellular distribution of the glucose transporter family. World J Gastroenterol 2012; 18(46): 6771-6781
- URL: https://www.wjgnet.com/1007-9327/full/v18/i46/6771.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i46.6771
Provision of a regular supply of glucose and other carbohydrates for fuel is vital for human survival and these are transported into cells using members of a family of integral membrane glucose transporter (GLUT) molecules[1]. To date 14 members of this family, also called the solute carrier 2A (SLC2A) proteins have been identified which can be divided on the basis of transport characteristics (intrinsic or inducible, specificities) and sequence similarities[2] into several families (Classes 1 to 3)[3,4]. The expression of these different receptor subtypes varies between different species, tissues and cellular subtypes and each has differential sensitivities to stimuli such as insulin.
The liver is a contributor to metabolic carbohydrate homeostasis and is a major site for synthesis, storage and redistribution of carbohydrates. At its simplest, after a meal hepatocyte GLUTs take up glucose from the portal bloodstream and it is converted to glycogen for storage. In a glucose-depleted state, this glycogen can then be converted back to glucose for fuel with up to 70% of total hepatic glucose production arising via this route[5]. Situations in which the balance of glucose homeostasis is upset such as diabetes or the metabolic syndrome can lead metabolic disturbances that drive chronic organ damage and failure, which confirms the importance of understanding the molecular regulation of glucose homeostasis. The liver is the major store of glycogen, regulates the availability of glucose and acute liver failure is associated with profound hypoglycemia. This has led to a large body of work investigating the expression and function of receptors that regulate glucose uptake and release by hepatocytes, the most import cells in glucose regulation and glycogen storage but there is less appreciation of the roles of GLUTs expressed by other cell types within the liver. Thus to date expression of GLUT-1, GLUT-2[6,7], GLUT-9[8] and GLUT-10[9] has been documented on hepatocytes but little is known about their expression or function on other cell types. However all cells require carbohydrate to function and there is evidence that non-parenchymal cells may contribute to glucose disposal. For example sinusoidal endothelial cells bind insulin with high affinity, and endothelial insulin-responses may be rate-limiting for glucose uptake[10]. Thus a better understanding of the detailed cellular distribution of GLUTs in human liver tissue may shed light on mechanisms underlying disease pathogenesis. We begin by discussing the extrahepatic expression and functions of these proteins.
This family contains the proteins GLUTs 1 to 4 and 14 (SLC2A1-4, 14). The gene for GLUT-1 (SLC2A1) the most ubiquitous transporter is located on chromosome 1p35-p31.3 and generates a 54 Kd protein in humans and rodents[11]. It has a high Km for glucose (Km = 1-2 mmol/L) and is mainly responsible for basal glucose and uptake[12], but can also transport other hexose carbohydrates including mannose, galactose, glucosamine, 3-O-methylglucose and 2-deoxy-d-glucose. GLUT-1 is, expressed in most cells[13] at low levels, with highest expression reported on erythrocytes, the blood brain barrier, neuronal membranes, eye, placenta and lactating mammary glands[14-16]. Murine embryonic expression also suggests a developmental role[17,18]. Over expression of GLUT-1 has been documented in a variety of tumours[19] and is associated with increased proliferation rates and increased mortality[20] leading to its use as a diagnostic/prognostic marker in some cancers[21,22].
The GLUT-2 (SLC2A2) gene located on chromosome 3q26-1-q26.2 encodes a 524 amino acid protein. GLUT-2 can efficiently transport sugars due to its high Vmax and Km for glucose, and is well suited to managing large bi-directional fluxes of glucose in and out of cells[23]. It also transports other dietary sugars such as galactose, mannose and fructose with a high affinity for glucosamine[11,24,25]. GLUT-2 is highly expressed in the liver, pancreatic beta cells, and on the basolateral surface of kidney and small intestine epithelia[26,27] with expression regulated by sugars and hormones[23,28]. Glycogenosis in the rare autosomal recessive disorder Fanconi-Bickel Syndrome has been associated with mutations in GLUT-2[29], and diabetes mellitus in patients with prolonged hepatitis C virus (HCV) infection has been linked to virally-induced reduction in hepatocyte expression of GLUT-2[30].
GLUT-3 (SLC2A3) was initially identified from muscle cell cDNA[31]. Expression localises to the membrane of slow twitch muscle fibres[32] and it is implicated in muscle regeneration and cell fusion[33]. However its major role is in neurons, supplying the high glucose demands in the brain[11,34] and it is increased in brain tumour cells[35]. The gene for GLUT-3 is located on chromosome 12p13.3 and encodes a 496aa protein[12] which transports glucose with a high affinity (Km = 1.8 mmol/L) and maltose, xylose, dehydroascorbic acid, mannose and galactose[25]. It is also present in fat, kidney, heart, placenta and liver at lower levels[36], and is vital for the supply of substrate to early post-implanted embryos[37]. White blood cells, which need an increased supply of glucose to fuel immune functions, express several GLUTs including GLUT-3[34] the expression of which is decreased in diabetes[38].
GLUT-4 (SLC2A4) was cloned and sequenced by several groups in 1989[39-41]. It is a 55kDa protein responsible for more than 50% of all body glucose uptake[42]. In the absence of insulin it is sequestered in intracellular vesicles and rapidly translocated to the plasma membrane in response to insulin. GLUT-4 transports glucose (Km = 5-6 mmol/L), dehydroascorbic acid and glucosamine[11,24]. Highest levels of expression are detected in insulin sensitive tissues such as skeletal and cardiac muscle, brown and white adipose tissue[11] and endothelial cells[43]. Expression has also been documented in monocytes, and like GLUT-3 is reduced in insulin-resistant individuals[44]. Mutations in the gene have been associated with diabetes.
GLUT-14 (SLC2A14) was identified and cloned by Wu X et al[45] in 2002. It is located on chromosome 12p13.3, has a high sequence similarity to GLUT-3 and may have arisen as a result of gene duplication. The protein contains sugar transporter signature motifs predicted to exhibit glucose transport activity[45]. Two splice variants have been identified in the testis[45]. Mutations of GLUT-14 and its drosophila homologue have been associated with Alzheimers disease in genome wide association studies in patients and insect models[46,47] and may explain the reported brain-specific dyregulation of glucose metabolism seen in this condition.
This family contains the transporters GLUT-5, GLUT-7, GLUT-9 and GLUT-11. GLUT-5 (SLC2A5) mRNA is detected mainly in the small intestine were it is found at both the apical and basolateral membranes and functions to absorb dietary fructose (Km= 6 mmol/L)[11,48,49]. It is also expressed at lower levels in the human kidney, microglial cells, adipocytes, muscle, brain, and testes[49,50], and in common with other transporters, expression is increased in human malignant tumours[51]. The protein exhibits no activity for glucose transport in humans or mouse[11,52] and its localization is not regulated by insulin[50,53]. There is a growing interest in fructose consumption and its link with the metabolic syndrome, type 11 diabetes and obesity[49] since consuming foods and beverages which contain excessive amounts of fructose has been linked to nonalcoholic fatty liver disease (NAFLD)[54]. The thiazolidinedione drug pioglitazone[55], which is used to treat type II diabetes, decreases GLUT-5 mRNA (52%) and protein (40%) in muscle fibres of type II diabetic subjects.
The GLUT-7 (SLC2A7), which was originally cloned from a human intestinal cDNA library[56], has considerable sequence similarity to GLUT-5[57] and is involved in uptake of sugars via facilitative diffusion mechanisms. Like GLUT-5 it has substrate specificity for both glucose and fructose and a key Ile-314 residue confers hexose specificity and is essential for fructose transport[58]. GLUT-7 mRNA is detected in the small and large intestines at the brush border membrane of enterocytes[11,49]; it is also detected in the prostate and testis[56]. Interestingly, disparities between the localisation of expression within the small intestine and glucose and fructose substrate availabilities suggest that alternate ligands may exist[57].
GLUT-9 (SLC2A9) shares sequence homology[59,60] and substrate specificities[58] with GLUT-5, GLUT-7 and GLUT-11 and, together with GLUT-2, is important for glucose-sensing by pancreatic B-cells[61]. Expression is localised to liver, kidneys, leukocytes[62], pancreas[61], placenta, lung[63], testis and adrenal gland. Two alternate isoforms have been identified, termed GLUT-9a and GLUT-9b[64,65], and alternative splicing results in differential subcellular localisation. Both isoforms have also been reported to transport urate with high affinity[66], and polymorphisms in the GLUT-9 gene are linked with an increased predisposition to gout[67]. Some polymorphisms have also been associated with an increased incidence of diabetes in Chinese populations[68]. GLUT-9a expression increases in pregestational and gestational diabetes, and GLUT-9b is increased by insulin[59]. In mouse, three isoforms of this transporter are reported, and similarly elevated in diabetes[8].
Three distinct isoforms of GLUT-11 (SLC2A11) have been identified in humans, with distinct but overlapping tissue expression patterns. Thus GLUT-11-A is expressed in skeletal muscle, kidney and heart, GLUT-11-B in adipose tissue, kidney and placenta, and GLUT-11-C in pancreas, heart, adipose tissue and skeletal muscle[69-72]. Muscle expression is localised to slow twitch fibres[73] and appears to be involved in myeloma cell viability and proliferation[74]. All three variants of GLUT-11 exhibit transport activity for both glucose and fructose but not galactose when expressed in Xenopus oocytes[58,70].
This family constitutes the evenly numbered transporters GLUT-6, GLUT-8, GLUT-10, GLUT-12 and GLUT-13. GLUT-6 (SCL2A6)[62] is widely expressed in normal and malignant tissue. mRNA has been detected in peripheral leucocytes, brain[72] and spleen[11] as well as in pancreas, testis, colon[62] and adipose tissue[75]. Subcellular protein expression varies with plasma membrane localisation in renal collecting tubule cells and cytoplasmic localisation in germinal cells of the testis and smooth muscle. GLUT-6 has significant sequence identity with GLUT-3 and may have arisen through insertion of GLUT-3 sequence into another gene on chromosome 5[76].
GLUT-8 (SLC2A8) is a high capacity intracellular GLUT[77] composed of 447 amino acids containing an N-terminal dileucine motif that permits trafficking via adaptor proteins to different organelles[77,78]. Expression is highest in the testis and[79], following insulin stimulation increases in the mid-piece of mature spermatozoa and translocates to the acrosome where the spermatozoa take up glucose to drive motility and the acrosome reaction. GLUT-8 may compensate for a lack of GLUT-4 in spermatozoa[80,81] and the preimplantation blastocyst, which demonstrates insulin stimulated glucose uptake via GLUT-8 translocation[82]. GLUT-8 is also found in some insulin receptive tissues including adipose tissue, muscle, brain, adrenal glands, spleen, heart and the liver[62,83,84], but not adipocytes[75] or neuronal cells[85].
GLUT-10 (SLC2A10) is a 541aa protein in humans and 513aa in zebrafish[11,86], which transports both glucose and galactose with high affinity[87]. It is expressed in the brain, lungs, adipose tissue[88], heart, placenta, and skeletal muscle with highest expression in the liver and pancreas[9,87]. The GLUT-10 gene, located on chromosome 20q12-13.1[89] has been linked with type II diabetes[9,90]. However other studies do not show any association with a diabetic phenotype[91,92]. Development of the cardiovascular system and TGFb signalling are linked to GLUT-10 function[93] and mutations are associated with altered angiogenesis and arterial tortuosity syndrome[93,94] as a consequence of a loss of regulation of smooth muscle mitochondrial antioxidants production in the absence of functional GLUT-10[89].
GLUT-12 (SLC2A12) was originally identified in the MCF-7 breast cancer epithelial cell line[95]. It is expressed in insulin sensitive tissues in humans and rodents including adipose tissue, skeletal muscle (major expression in type 1 oxidative fibres) and heart[72,88,96-98] as well as human chondrocytes[99]. GLUT-12 is also found in placenta, small intestine, heart and tumours with a high metabolic and capacity glucose utilisation[100-103]. In normal human muscle GLUT-12 undergoes PI3 kinase dependent translocation from an intracellular region to the plasma membrane[104]. Its expression in insulin sensitive tissues, and evidence that overexpression of GLUT-12 in mice improves glucose clearance rate and whole body insulin sensitivity[105] confirm that this transporter is insulin-sensitive. The GLUT-13 (SLC2A13) gene encodes a 629 amino acid protein, located on chromosome 12q12. It is a H+/myo-inositol co-transporter[11,106,107] also known as HMIT[108] in neuronal cells[106,108]. Although there are no known reports of glucose activity for GLUT-13[11], the rat gene contains motifs which are important for glucose transport activity (http://omim.org/entry/611036).
The data reviewed above reveal the widespread distribution and diverse function of extrahepatic transporter proteins but much less is known about their expression and function it the liver. Surprisingly, there are few studies documenting changes in expression and function in disease. Defining local expression of GLUTs in tissue will shed light on disease pathogenesis. For example, diabetes is associated with altered expression of GLUT-1, GLUT-2, GLUT-3 and GLUT-8 and GLUT-9 (reviewed in[8]) and abnormal GLUT-1 expression on tumour endothelium in HCC has prognostic and diagnostic significance[109-111]. A good example is the finding that diabetes in HCV is a consequence of virally induced downregulation of GLUT-1 and GLUT-2 on hepatocytes[30]. Similarly, transport of key substrates such as fructose has been linked to NAFLD[54]. Dysregulated glucose homeostasis and insulin resistance in NAFLD is associated with chronic organ damage affecting multiple hepatic cell types. The expression levels of transporters is not only regulated by insulin and glucose levels but also by cytokines including interleukin-6, which is increased in obesity and diabetes and can amplify insulin resistance via effects on GLUT-4[112]. The hexose transporters also play important roles in the function of cholangiocytes[113], endothelial cells and stellate cells[114]. Thus we summarise the current state of knowledge regarding hepatocellular expression of the GLUT family of proteins, in order to highlight their potential role in tissue homeostasis and disease (Table 1).
| Class | GLUT isoform | Hepatic expression | Subcellular expression/localisation | Protein/mRNA | Ref. |
| Class I | GLUT1 | Yes | Sinusoidal membrane of hepatocytes, protein restricted to hepatocytes proximal to the hepatic venule, also expressed on endothelial cells, kupffer cells and cholangiocytes; hepatocyte expression in HCC | Both | [110,113,115,116,120] |
| GLUT2 | Yes | Hepatocytes | Protein | [124-126] | |
| GLUT3 | Yes | Hepatocytes, bile canalicular membrane more enriched than sinusoidal membrane | Protein | [115,116,132] | |
| GLUT4 | Yes | Stellate cells | mRNA | [115,116,134] | |
| GLUT14 | No | ||||
| Class II | GLUT5 | Yes | Normal liver tissue hepatocytes (cytoplasmic) | Both | [51,115] |
| GLUT7 | No | [140] | |||
| GLUT9 | Yes | Majority of expression in hepatocytes of normal liver and HCC with cytoplasmic expression in pericentral areas | Protein | [51] | |
| GLUT11 | Yes | mRNA | [115] | ||
| Class III | GLUT6 | Yes | mRNA | [76] | |
| GLUT8 | Yes | Perivenous hepatocytes | Both | [115,143] | |
| GLUT10 | Yes | mRNA | [86,115] | ||
| GLUT12 | Yes | mRNA | [98] | ||
| GLUT13 | No |
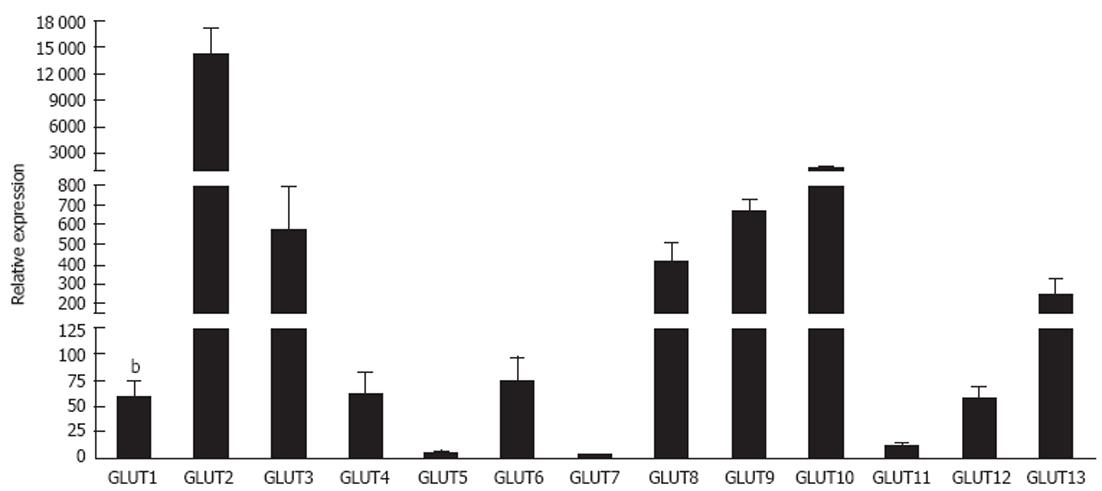
The widespread expression of GLUT-1 includes the liver although the precise cellular distribution remains controversial. Because hepatocytes are capable of gluconeogenesis their need for glucose uptake is modest. GLUT-1 is expressed on the sinusoidal membrane of rat and porcine[115,116] hepatocytes, and may be expressed to a greater extent than GLUT-2 during early post-natal development[117]. Expression of both GLUT-1 and GLUT-2 by foetal hepatocytes allows for efficient glycogenesis at low plasma glucose concentrations[118]. In adult animals, expression is strongest in the central acinar zones[119]. Transcription and microsomal expression of GLUT-1 is detected in periportal and perivenular hepatocytes but membrane localisation is restricted to hepatocytes proximal to the hepatic venule[120] under basal conditions. Our own microarray analysis of human normal livers confirms expression of GLUT-1 and GLUT-2 in total liver mRNA (Figure 1). In hepatocellular carcinoma, variable cytoplasmic GLUT-1 is detected and is has been used to distinguish between cholangiocarcinomas and hepatocellular carcinomas (HCC)[109] and has even been proposed as a therapeutic target for HCC[110]. Exposure of rodents to alcohol and high fat feeding results in increased GLUT-1 and decreased GLUT-2 expression in hepatocytes which presumably reflects changes in energy metabolism in response to the dietary changes[121].
Non-parenchymal cells, which cannot carry out gluconeogenesis, rely on glucose uptake rather than endogenous generation. GLUT-1 is the dominant receptor on both endothelial cells and Kupffer cells and levels increase in response to even brief exposure to LPS[122]. Interestingly, acute liver failure has been associated with increased GLUT-1 expression on cerebral vasculature in response to elevated circulating ammonia levels[123]. Cholangiocytes demonstrate basolateral expression of GLUT-1[113] which facilitates absorption of glucose from bile.
GLUT-2 fulfils the major glucose transport role in hepatocytes[36,124] (Figure 1). The protein localises to the sinusoidal plasma membrane of normal[23,125] and malignant hepatocytes. Historical reports suggest a Km for glucose transport of up to 66 mmol/L in intact rat hepatocytes[126] although contribution from other transporters likely contributes in this study since others report lower values between 10 mmol/L and 20 mmol/L[23]. GLUT-2 promotes rapid glucose efflux following gluconeogenesis. In the fasting state the liver produces glucose via glycogenesis or glycogenolysis with the conversion of glucose 6 phosphate into glucose preceding release via GLUT-2[23]. However in the fed state glucose and insulin levels rise and inhibit endogenous glucose production through effects on enzymes involved in gluconeogenesis. This is associated with removal of membrane GLUT-2 and a subsequent fall in GLUT-2 mediated release[23]. Excess glucose is stored as glycogen or converted to lipids and hepatocyte GLUT-2 and the insulin receptor are internalised together into endosomes in response to insulin[23,127,128]. In mice lacking GLUT-2 the rate of hepatic glucose production is not impaired indicating the presence of a facilitated diffusion-independent mechanism for glucose release[129]. Thus the major role of hepatocyte GLUT-2 is to regulate efflux rather than uptake of glucose. However, in obesity insulin resistance drives an increase in GLUT-2 levels that may further exacerbate metabolic dysfunction in NAFLD[130].
Much less is known about the hepatic expression and function of GLUT-3. GLUT-3 is expressed in porcine livers[115] and localised to the plasma membrane of rat hepatocytes. Expression is focussed on the bile canalicular membrane rather than the sinusoidal membrane[116]. Mice with GLUT-3 haploinsufficiency develop obesity and insulin resistance associated with hepatic steatosis, possibly as a consequence of foetal glucose insufficiency[131]. GLUT-3 expression is increased on both primary and metastatic hepatic tumours, which might reflect an increased need for glucose uptake in cancer[132]. Low levels of GLUT-3 have been reported in the human liver[36] and are supported by our microarray analysis but detailed human studies are lacking and little is known about changes in disease.
Whilst the liver is generally considered to lack significant expression of GLUT-4[133], a recent study reports expression of GLUT-4 mRNA in porcine liver[115]. Although there is little evidence for expression in hepatocytes, GLUT-4 has been detected in sinusoidal endothelial cells and stellate cells where it can mediate glucose uptake by semicarbazide sensitive amine oxidase mediated effects on insulin receptor signalling[134] which explains our findings of expression at mRNA level in humans (Figure 1). Expression on stellate cells is enhanced by leptin signalling[114] leading to HSC activation that may contribute to fibrogenesis in NAFLD. In contrast, in murine models of diet-induced obesity GLUT-4 mRNA is decreased in the liver[135] and cirrhosis is associated with decreased extrahepatic GLUT-4 mRNA[42]. Deletion of skeletal muscle GLUT-4 results in redirection of excess circulating glucose to the liver where is becomes fuel for conversion to lipid storage[136-138]. Thus glucose homeostasis is maintained by a complex relationship between intra- and extra hepatic levels of GLUT-4 regulated by metabolic activity and dietary intake. To date there are no published reports concerning expression of GLUT-14 in the liver.
GLUT-5 protein has been detected in human hepatocytes[51] although low to undetectable RNA levels in pigs[115] imply species-specific differences in GLUT-5 expression. Hepatic metastases from lung and breast cancer are GLUT-5 positive[132] but under normal conditions liver expression is minimal (Figure 1). A mechanistic link between elevations in GLUT-5 expression in small intestine and alterations in hepatic metabolism[139] has been suggested.
GLUT-7 was initially reported as a hepatic microsomal GLUT found in the endoplasmic reticulum, which facilitated the release of glucose formed in the process of gluconeogenesis and glycogenolysis for export into the blood[36,140]. However this has recently been challenged by studies showing that neither human nor rat livers contain GLUT-7 mRNA[141] and our data in Figure 1, suggesting that the previous findings were due to a cloning artefact. Definitive studies need to be performed to clarify the situation.
GLUT-9 has been detected in the cytoplasm of pericentral hepatocytes in normal human liver and in HCC[51]. The receptor appears to be functional for glucose transport because plasma membrane expression of GLUT-9 correlates with glucose influx in HepG2 cells[142] and GLUT-9 inactivation in mouse hepatocytes leads to hyperuricosuria[143]. Although GLUT-11 mRNA has been detected in porcine liver[115], there are no studies documenting expression of GLUT-11 in the human liver.
Little is known about the hepatic expression of the recently identified Class III transporters although our microarray data (Figure 1) is indicative of some degree of expression. Presence of mRNA for GLUT-6 has been described in hepatoma cell lines but has not been detected in normal human liver[76]. GLUT-8 mRNA has been detected in perivenous hepatocytes in pig[115] and mouse[144] livers where it may regulate glycolytic flux. Mice with type I diabetes show decreased expression whereas expression increases in insulin resistance and type II diabetes suggesting that expression is regulated by insulin[144]. Hepatic expression of GLUT-10 has been reported in pigs[115] and zebrafish[86] but we are unaware of any data in humans. GLUT-12 mRNA has been documented in all bovine tissues including the liver where levels are low compared to spleen and skeletal muscle[98], but again detailed cellular expression data is currently lacking. There are no known reports of GLUT-13 expression in the liver.
Systemic carbohydrate homeostasis is maintained by a complex relationship between organs such as the pancreas, intestine, muscle and liver. Intra- and extra hepatic levels of GLUT molecules are regulated in part by metabolic activity, dietary intake, and disease state. For example, diabetes is associated with altered expression of GLUT-1, GLUT-2, GLUT-3 and GLUT-8 and GLUT-9[8] and abnormal GLUT-1 expression on tumour endothelium in HCC[109-111] permits efficient glucose uptake by tumour cells even at low blood glucose concentrations. Chronic fructose intake drives glucose and glycogen storage, lipogenesis and production of lipogenic intermediates as well as promoting production of very-low-density lipoproteins[145]. This suggests that the reported hyperlipidaemic and hyperuricaemic effects of fructose, coupled with macrovesicular steatosis and lobular inflammation patterns[146] characteristic of human NAFLD seen in rodents fed high fructose diets, may be enhanced in the context of altered expression of fructose transporters within the hepatic parenchyma and especially so for individuals with high fructose intake or pre-existing hyperlipidaemia or metabolic syndrome. New data is increasingly suggesting the merits of targeting members of the GLUT family therapeutically. Thus overexpression of GLUT-1 in tumours, particularly those with poor prognosis has been suggested as a possible means to selectively inhibit tumour cell metabolism[147], although expression of GLUT-1 red blood cells will likely preclude use therapeutically. Similarly targeting of GLUT-3, which is involved in neovascularisation in glioblastoma has been suggested to prevent resistance to conventional therapy[148], and GLUT-4 is of particular interest in the context of diabetes and insulin resistance, with efforts underway to design therapeutics to enable appropriate glucose uptake independently of insulin stimulation[149]. Alterations in hepatic expression of GLUT transporters have been described in response to insulin resistance and hyperlipidaemia, alcohol consumption, viral infection and carcinogenesis, with diverse functions including biliary transport, fibrogenesis, urate transport and angiogenesis executed by family members in extraparenchymal cells. Combined with the central role of the liver in regulation of circulating carbohydrate therefore, future definition of the spatial, temporal and disease-specific expression of GLUTs within the liver microenvironment is key to understanding disease pathogenesis and potential hepatic complications of systemic inhibition.
Peer reviewer: Ming Li, Associate Professor, Health Sciences Center, Tulane University, 1430 Tulane Ave Sl-83, New Orleans, LA 70112, United States
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Augustin R. The protein family of glucose transport facilitators: It's not only about glucose after all. IUBMB Life. 2010;62:315-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 218] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Bell GI, Kayano T, Buse JB, Burant CF, Takeda J, Lin D, Fukumoto H, Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990;13:198-208. [PubMed] |
| 3. | Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr. 1996;16:235-256. [PubMed] |
| 4. | Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol. 2001;18:247-256. [PubMed] |
| 5. | Tirone TA, Brunicardi FC. Overview of glucose regulation. World J Surg. 2001;25:461-467. [PubMed] |
| 6. | Wu T, Pan T, Zheng Z, Chen T, Pan Y. [Relationship between Glut-1, Glut-3 expression and fluorodeoxyglucose uptake in NSCLC and benign pulmonary lesion.]. Zhongguo Fei Ai Za Zhi. 2008;11:555-558. [PubMed] |
| 7. | Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr. 1999;19:379-406. [PubMed] |
| 8. | Keembiyehetty C, Augustin R, Carayannopoulos MO, Steer S, Manolescu A, Cheeseman CI, Moley KH. Mouse glucose transporter 9 splice variants are expressed in adult liver and kidney and are up-regulated in diabetes. Mol Endocrinol. 2006;20:686-697. [PubMed] |
| 9. | McVie-Wylie AJ, Lamson DR, Chen YT. Molecular cloning of a novel member of the GLUT family of transporters, SLC2a10 (GLUT10), localized on chromosome 20q13.1: a candidate gene for NIDDM susceptibility. Genomics. 2001;72:113-117. [PubMed] |
| 10. | Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989;84:1620-1628. [PubMed] |
| 11. | Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113-128. [PubMed] |
| 12. | Gorovits N, Charron MJ. What we know about facilitative glucose transporters - Lessons from cultured cells, animal models, and human studies. Biochem Mol Biol Educ. 2003;31:163-172. [DOI] [Full Text] |
| 13. | Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence and structure of a human glucose transporter. Science. 1985;229:941-945. [PubMed] |
| 14. | Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Erythrocyte/HepG2-type glucose transporter is concentrated in cells of blood-tissue barriers. Biochem Biophys Res Commun. 1990;173:67-73. [PubMed] |
| 15. | Zhao FQ, Glimm DR, Kennelly JJ. Distribution of mammalian facilitative glucose transporter messenger RNA in bovine tissues. Int J Biochem. 1993;25:1897-1903. [PubMed] |
| 16. | Mueckler M. Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes. 1990;39:6-11. [PubMed] |
| 17. | Aghayan M, Rao LV, Smith RM, Jarett L, Charron MJ, Thorens B, Heyner S. Developmental expression and cellular localization of glucose transporter molecules during mouse preimplantation development. Development. 1992;115:305-312. [PubMed] |
| 18. | Chi MM, Pingsterhaus J, Carayannopoulos M, Moley KH. Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. J Biol Chem. 2000;275:40252-40257. [PubMed] |
| 19. | Smith TA. Facilitative glucose transporter expression in human cancer tissue. Br J Biomed Sci. 1999;56:285-292. [PubMed] |
| 20. | Hernández F, Navarro M, Encinas JL, López Gutiérrez JC, López Santamaría M, Leal N, Martínez L, Patrón M, Tovar JA. The role of GLUT1 immunostaining in the diagnosis and classification of liver vascular tumors in children. J Pediatr Surg. 2005;40:801-804. [PubMed] |
| 21. | Carvalho KC, Cunha IW, Rocha RM, Ayala FR, Cajaíba MM, Begnami MD, Vilela RS, Paiva GR, Andrade RG, Soares FA. GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics (Sao Paulo). 2011;66:965-972. [PubMed] |
| 22. | Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, Honma K, Suzuki T. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634-641. [PubMed] |
| 23. | Leturque A, Brot-Laroche E, Le Gall M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am J Physiol Endocrinol Metab. 2009;296:E985-E992. [PubMed] |
| 24. | Uldry M, Ibberson M, Hosokawa M, Thorens B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002;524:199-203. [PubMed] |
| 25. | Colville CA, Seatter MJ, Jess TJ, Gould GW, Thomas HM. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J. 1993;290:701-706. [PubMed] |
| 26. | Thorens B, Cheng ZQ, Brown D, Lodish HF. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990;259:C279-C285. [PubMed] |
| 27. | Orci L, Unger RH, Ravazzola M, Ogawa A, Komiya I, Baetens D, Lodish HF, Thorens B. Reduced beta-cell glucose transporter in new onset diabetic BB rats. J Clin Invest. 1990;86:1615-1622. [PubMed] |
| 28. | Weinstein SP, O'Boyle E, Fisher M, Haber RS. Regulation of GLUT2 glucose transporter expression in liver by thyroid hormone: evidence for hormonal regulation of the hepatic glucose transport system. Endocrinology. 1994;135:649-654. [PubMed] |
| 29. | Tsuda M, Kitasawa E, Ida H, Eto Y, Owada M. A newly recognized missense mutation in the GLUT2 gene in a patient with Fanconi-Bickel syndrome. Eur J Pediatr. 2000;159:867. [PubMed] |
| 30. | Kasai D, Adachi T, Deng L, Nagano-Fujii M, Sada K, Ikeda M, Kato N, Ide YH, Shoji I, Hotta H. HCV replication suppresses cellular glucose uptake through down-regulation of cell surface expression of glucose transporters. J Hepatol. 2009;50:883-894. [PubMed] |
| 31. | Kayano T, Fukumoto H, Eddy RL, Fan YS, Byers MG, Shows TB, Bell GI. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988;263:15245-15248. [PubMed] |
| 32. | Stuart CA, Wen G, Peng BH, Popov VL, Hudnall SD, Campbell GA. GLUT-3 expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E855-E861. [PubMed] |
| 33. | Guillet-Deniau I, Leturque A, Girard J. Expression and cellular localization of glucose transporters (GLUT1, GLUT3, GLUT4) during differentiation of myogenic cells isolated from rat foetuses. J Cell Sci. 1994;107:487-496. [PubMed] |
| 34. | Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242-E253. [PubMed] |
| 35. | Nishioka T, Oda Y, Seino Y, Yamamoto T, Inagaki N, Yano H, Imura H, Shigemoto R, Kikuchi H. Distribution of the glucose transporters in human brain tumors. Cancer Res. 1992;52:3972-3979. [PubMed] |
| 36. | Gould GW, Holman GD. The glucose transporter family: structure, function and tissue-specific expression. Biochem J. 1993;295:329-341. [PubMed] |
| 37. | Schmidt S, Hommel A, Gawlik V, Augustin R, Junicke N, Florian S, Richter M, Walther DJ, Montag D, Joost HG. Essential role of glucose transporter GLUT3 for post-implantation embryonic development. J Endocrinol. 2009;200:23-33. [PubMed] |
| 38. | Kipmen-Korgun D, Bilmen-Sarikcioglu S, Altunbas H, Demir R, Korgun ET. Type-2 diabetes down-regulates glucose transporter proteins and genes of the human blood leukocytes. Scand J Clin Lab Invest. 2009;69:350-358. [PubMed] |
| 39. | Birnbaum MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305-315. [PubMed] |
| 40. | James DE, Strube M, Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989;338:83-87. [PubMed] |
| 41. | Bell GI, Murray JC, Nakamura Y, Kayano T, Eddy RL, Fan YS, Byers MG, Shows TB. Polymorphic human insulin-responsive glucose-transporter gene on chromosome 17p13. Diabetes. 1989;38:1072-1075. [PubMed] |
| 42. | Holland-Fischer P, Greisen J, Grøfte T, Jensen TS, Hansen PO, Vilstrup H. Increased energy expenditure and glucose oxidation during acute nontraumatic skin pain in humans. Eur J Anaesthesiol. 2009;26:311-317. [PubMed] |
| 43. | McCall AL, van Bueren AM, Huang L, Stenbit A, Celnik E, Charron MJ. Forebrain endothelium expresses GLUT4, the insulin-responsive glucose transporter. Brain Res. 1997;744:318-326. [PubMed] |
| 44. | Mavros Y, Simar D, Singh MA. Glucose Tranporter-4 expression in monocytes: a systematic review. Diabetes Res Clin Pract. 2009;84:123-131. [PubMed] |
| 45. | Wu X, Freeze HH. GLUT14, a duplicon of GLUT3, is specifically expressed in testis as alternative splice forms. Genomics. 2002;80:553-557. [PubMed] |
| 46. | Shulman JM, Chipendo P, Chibnik LB, Aubin C, Tran D, Keenan BT, Kramer PL, Schneider JA, Bennett DA, Feany MB. Functional screening of Alzheimer pathology genome-wide association signals in Drosophila. Am J Hum Genet. 2011;88:232-238. [PubMed] |
| 47. | Wang W, Yu JT, Zhang W, Cui WZ, Wu ZC, Zhang Q, Tan L. Genetic association of SLC2A14 polymorphism with Alzheimer's disease in a Han Chinese population. J Mol Neurosci. 2012;47:481-484. [PubMed] |
| 48. | Corpe CP, Bovelander FJ, Munoz CM, Hoekstra JH, Simpson IA, Kwon O, Levine M, Burant CF. Cloning and functional characterization of the mouse fructose transporter, GLUT5. Biochim Biophys Acta. 2002;1576:191-197. [PubMed] |
| 49. | Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141-E145. [PubMed] |
| 50. | Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83:183-252. [PubMed] |
| 51. | Godoy A, Ulloa V, Rodríguez F, Reinicke K, Yañez AJ, García Mde L, Medina RA, Carrasco M, Barberis S, Castro T. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J Cell Physiol. 2006;207:614-627. [PubMed] |
| 52. | Douard V, Ferraris RP. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab. 2008;295:E227-E237. [PubMed] |
| 53. | Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713-725. [PubMed] |
| 54. | Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295:G987-G995. |
| 55. | Stuart CA, Howell ME, Yin D. Overexpression of GLUT5 in diabetic muscle is reversed by pioglitazone. Diabetes Care. 2007;30:925-931. [PubMed] |
| 56. | Li Q, Manolescu A, Ritzel M, Yao S, Slugoski M, Young JD, Chen XZ, Cheeseman CI. Cloning and functional characterization of the human GLUT7 isoform SLC2A7 from the small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;287:G236-G242. [PubMed] |
| 57. | Cheeseman C. GLUT7: a new intestinal facilitated hexose transporter. Am J Physiol Endocrinol Metab. 2008;295:E238-E241. [PubMed] |
| 58. | Manolescu A, Salas-Burgos AM, Fischbarg J, Cheeseman CI. Identification of a hydrophobic residue as a key determinant of fructose transport by the facilitative hexose transporter SLC2A7 (GLUT7). J Biol Chem. 2005;280:42978-42983. [PubMed] |
| 59. | Bibee KP, Illsley NP, Moley KH. Asymmetric syncytial expression of GLUT9 splice variants in human term placenta and alterations in diabetic pregnancies. Reprod Sci. 2011;18:20-27. [PubMed] |
| 60. | Schürmann A. Insight into the "odd" hexose transporters GLUT3, GLUT5, and GLUT7. Am J Physiol Endocrinol Metab. 2008;295:E225-E226. [PubMed] |
| 61. | Evans SA, Doblado M, Chi MM, Corbett JA, Moley KH. Facilitative glucose transporter 9 expression affects glucose sensing in pancreatic beta-cells. Endocrinology. 2009;150:5302-5310. [PubMed] |
| 62. | Doege H, Bocianski A, Joost HG, Schürmann A. Activity and genomic organization of human glucose transporter 9 (GLUT9), a novel member of the family of sugar-transport facilitators predominantly expressed in brain and leucocytes. Biochem J. 2000;350 Pt 3:771-776. [PubMed] |
| 63. | Phay JE, Hussain HB, Moley JF. Strategy for identification of novel glucose transporter family members by using internet-based genomic databases. Surgery. 2000;128:946-951. [PubMed] |
| 64. | Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem. 2004;279:16229-16236. [PubMed] |
| 65. | Li S, Sanna S, Maschio A, Busonero F, Usala G, Mulas A, Lai S, Dei M, Orrù M, Albai G. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 2007;3:e194. [PubMed] |
| 66. | Bibert S, Hess SK, Firsov D, Thorens B, Geering K, Horisberger JD, Bonny O. Mouse GLUT9: evidences for a urate uniporter. Am J Physiol Renal Physiol. 2009;297:F612-F619. [PubMed] |
| 67. | Stark K, Reinhard W, Neureuther K, Wiedmann S, Sedlacek K, Baessler A, Fischer M, Weber S, Kaess B, Erdmann J. Association of common polymorphisms in GLUT9 gene with gout but not with coronary artery disease in a large case-control study. PLoS One. 2008;3:e1948. [PubMed] |
| 68. | Liu WC, Hung CC, Chen SC, Lin MY, Chen LI, Hwang DY, Chang JM, Tsai JC, Chen HC, Hwang SJ. The rs1014290 polymorphism of the SLC2A9 gene is associated with type 2 diabetes mellitus in Han Chinese. Exp Diabetes Res. 2011;2011:527520. [PubMed] |
| 69. | Wu X, Li W, Sharma V, Godzik A, Freeze HH. Cloning and characterization of glucose transporter 11, a novel sugar transporter that is alternatively spliced in various tissues. Mol Genet Metab. 2002;76:37-45. [PubMed] |
| 70. | Scheepers A, Schmidt S, Manolescu A, Cheeseman CI, Bell A, Zahn C, Joost HG, Schürmann A. Characterization of the human SLC2A11 (GLUT11) gene: alternative promoter usage, function, expression, and subcellular distribution of three isoforms, and lack of mouse orthologue. Mol Membr Biol. 2005;22:339-351. [PubMed] |
| 71. | Sasaki T, Minoshima S, Shiohama A, Shintani A, Shimizu A, Asakawa S, Kawasaki K, Shimizu N. Molecular cloning of a member of the facilitative glucose transporter gene family GLUT11 (SLC2A11) and identification of transcription variants. Biochem Biophys Res Commun. 2001;289:1218-1224. [PubMed] |
| 72. | Stuart CA, Yin D, Howell ME, Dykes RJ, Laffan JJ, Ferrando AA. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab. 2006;291:E1067-E1073. [PubMed] |
| 73. | Gaster M, Handberg A, Schürmann A, Joost HG, Beck-Nielsen H, Schrøder HD. GLUT11, but not GLUT8 or GLUT12, is expressed in human skeletal muscle in a fibre type-specific pattern. Pflugers Arch. 2004;448:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | McBrayer SK, Cheng JC, Singhal S, Krett NL, Rosen ST, Shanmugam M. Multiple myeloma exhibits novel dependence on GLUT4, GLUT8, and GLUT11: implications for glucose transporter-directed therapy. Blood. 2012;119:4686-4697. [PubMed] |
| 75. | Lisinski I, Schürmann A, Joost HG, Cushman SW, Al-Hasani H. Targeting of GLUT6 (formerly GLUT9) and GLUT8 in rat adipose cells. Biochem J. 2001;358:517-522. [PubMed] |
| 76. | Kayano T, Burant CF, Fukumoto H, Gould GW, Fan YS, Eddy RL, Byers MG, Shows TB, Seino S, Bell GI. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 1990;265:13276-13282. [PubMed] |
| 77. | Aerni-Flessner LB, Otu MC, Moley KH. The amino acids upstream of NH(2)-terminal dileucine motif play a role in regulating the intracellular sorting of the Class III transporters GLUT8 and GLUT12. Mol Membr Biol. 2011;28:30-41. [PubMed] |
| 78. | Widmer M, Uldry M, Thorens B. GLUT8 subcellular localization and absence of translocation to the plasma membrane in PC12 cells and hippocampal neurons. Endocrinology. 2005;146:4727-4736. [PubMed] |
| 79. | Schürmann A, Koling S, Jacobs S, Saftig P, Krauss S, Wennemuth G, Kluge R, Joost HG. Reduced sperm count and normal fertility in male mice with targeted disruption of the ADP-ribosylation factor-like 4 (Arl4) gene. Mol Cell Biol. 2002;22:2761-2768. [PubMed] |
| 80. | Burant CF, Davidson NO. GLUT3 glucose transporter isoform in rat testis: localization, effect of diabetes mellitus, and comparison to human testis. Am J Physiol. 1994;267:R1488-R1495. [PubMed] |
| 81. | Angulo C, Rauch MC, Droppelmann A, Reyes AM, Slebe JC, Delgado-López F, Guaiquil VH, Vera JC, Concha II. Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C. J Cell Biochem. 1998;71:189-203. [PubMed] |
| 82. | Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, Devaskar SU, Moley KH. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci U S A. 2000;97:7313-7318. [PubMed] |
| 83. | Ibberson M, Uldry M, Thorens B. GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. J Biol Chem. 2000;275:4607-4612. [PubMed] |
| 84. | Reagan LP, Gorovits N, Hoskin EK, Alves SE, Katz EB, Grillo CA, Piroli GG, McEwen BS, Charron MJ. Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proc Natl Acad Sci U S A. 2001;98:2820-2825. [PubMed] |
| 85. | Shin BC, McKnight RA, Devaskar SU. Glucose transporter GLUT8 translocation in neurons is not insulin responsive. J Neurosci Res. 2004;75:835-844. [PubMed] |
| 86. | Chiarelli N, Ritelli M, Zoppi N, Benini A, Borsani G, Barlati S, Colombi M. Characterization and expression pattern analysis of the facilitative glucose transporter 10 gene (slc2a10) in Danio rerio. Int J Dev Biol. 2011;55:229-236. [PubMed] |
| 87. | Dawson PA, Mychaleckyj JC, Fossey SC, Mihic SJ, Craddock AL, Bowden DW. Sequence and functional analysis of GLUT10: a glucose transporter in the Type 2 diabetes-linked region of chromosome 20q12-13.1. Mol Genet Metab. 2001;74:186-199. [PubMed] |
| 88. | Wood IS, Hunter L, Trayhurn P. Expression of Class III facilitative glucose transporter genes (GLUT-10 and GLUT-12) in mouse and human adipose tissues. Biochem Biophys Res Commun. 2003;308:43-49. [PubMed] |
| 89. | Lee YC, Huang HY, Chang CJ, Cheng CH, Chen YT. Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: mechanistic insight into arterial tortuosity syndrome. Hum Mol Genet. 2010;19:3721-3733. [PubMed] |
| 90. | Ghosh S, Watanabe RM, Valle TT, Hauser ER, Magnuson VL, Langefeld CD, Ally DS, Mohlke KL, Silander K, Kohtamäki K. The Finland-United States investigation of non-insulin-dependent diabetes mellitus genetics (FUSION) study. I. An autosomal genome scan for genes that predispose to type 2 diabetes. Am J Hum Genet. 2000;67:1174-1185. [PubMed] |
| 91. | Bento JL, Bowden DW, Mychaleckyj JC, Hirakawa S, Rich SS, Freedman BI, Segade F. Genetic analysis of the GLUT10 glucose transporter (SLC2A10) polymorphisms in Caucasian American type 2 diabetes. BMC Med Genet. 2005;6:42. [PubMed] |
| 92. | Rose CS, Andersen G, Hamid YH, Glümer C, Drivsholm T, Borch-Johnsen K, Jørgensen T, Pedersen O, Hansen T. Studies of relationships between the GLUT10 Ala206Thr polymorphism and impaired insulin secretion. Diabet Med. 2005;22:946-949. [PubMed] |
| 93. | Willaert A, Khatri S, Callewaert BL, Coucke PJ, Crosby SD, Lee JG, Davis EC, Shiva S, Tsang M, De Paepe A. GLUT10 is required for the development of the cardiovascular system and the notochord and connects mitochondrial function to TGFβ signaling. Hum Mol Genet. 2012;21:1248-1259. [PubMed] |
| 94. | Cheng CH, Kikuchi T, Chen YH, Sabbagha NG, Lee YC, Pan HJ, Chang C, Chen YT. Mutations in the SLC2A10 gene cause arterial abnormalities in mice. Cardiovasc Res. 2009;81:381-388. [PubMed] |
| 95. | Rogers S, Macheda ML, Docherty SE, Carty MD, Henderson MA, Soeller WC, Gibbs EM, James DE, Best JD. Identification of a novel glucose transporter-like protein-GLUT-12. Am J Physiol Endocrinol Metab. 2002;282:E733-E738. [PubMed] |
| 96. | Macheda ML, Williams ED, Best JD, Wlodek ME, Rogers S. Expression and localisation of GLUT1 and GLUT12 glucose transporters in the pregnant and lactating rat mammary gland. Cell Tissue Res. 2003;311:91-97. [PubMed] |
| 97. | Zhou Y, Kaye PL, Pantaleon M. Identification of the facilitative glucose transporter 12 gene Glut12 in mouse preimplantation embryos. Gene Expr Patterns. 2004;4:621-631. [PubMed] |
| 98. | Miller PJ, Finucane KA, Hughes M, Zhao FQ. Cloning and expression of bovine glucose transporter GLUT12. Mamm Genome. 2005;16:873-883. [PubMed] |
| 99. | Richardson S, Neama G, Phillips T, Bell S, Carter SD, Moley KH, Moley JF, Vannucci SJ, Mobasheri A. Molecular characterization and partial cDNA cloning of facilitative glucose transporters expressed in human articular chondrocytes; stimulation of 2-deoxyglucose uptake by IGF-I and elevated MMP-2 secretion by glucose deprivation. Osteoarthritis Cartilage. 2003;11:92-101. [PubMed] |
| 100. | Ware B, Bevier M, Nishijima Y, Rogers S, Carnes CA, Lacombe VA. Chronic heart failure selectively induces regional heterogeneity of insulin-responsive glucose transporters. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1300-R1306. [PubMed] |
| 101. | Chandler JD, Williams ED, Slavin JL, Best JD, Rogers S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer. 2003;97:2035-2042. [PubMed] |
| 102. | Rogers S, Docherty SE, Slavin JL, Henderson MA, Best JD. Differential expression of GLUT12 in breast cancer and normal breast tissue. Cancer Lett. 2003;193:225-233. [PubMed] |
| 103. | Gude NM, Stevenson JL, Murthi P, Rogers S, Best JD, Kalionis B, King RG. Expression of GLUT12 in the fetal membranes of the human placenta. Placenta. 2005;26:67-72. [PubMed] |
| 104. | Stuart CA, Howell ME, Zhang Y, Yin D. Insulin-stimulated translocation of glucose transporter (GLUT) 12 parallels that of GLUT4 in normal muscle. J Clin Endocrinol Metab. 2009;94:3535-3542. [PubMed] |
| 105. | Ralphe JC, Nau PN, Mascio CE, Segar JL, Scholz TD. Regulation of myocardial glucose transporters GLUT1 and GLUT4 in chronically anemic fetal lambs. Pediatr Res. 2005;58:713-718. [PubMed] |
| 106. | Uldry M, Ibberson M, Horisberger JD, Chatton JY, Riederer BM, Thorens B. Identification of a mammalian H(+)-myo-inositol symporter expressed predominantly in the brain. EMBO J. 2001;20:4467-4477. [PubMed] |
| 107. | Scheepers A, Joost HG, Schürmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr. 2004;28:364-371. [PubMed] |
| 108. | Uldry M, Steiner P, Zurich MG, Béguin P, Hirling H, Dolci W, Thorens B. Regulated exocytosis of an H+/myo-inositol symporter at synapses and growth cones. EMBO J. 2004;23:531-540. [PubMed] |
| 109. | Roh MS, Jeong JS, Kim YH, Kim MC, Hong SH. Diagnostic utility of GLUT1 in the differential diagnosis of liver carcinomas. Hepatogastroenterology. 2004;51:1315-1318. [PubMed] |
| 110. | Amann T, Maegdefrau U, Hartmann A, Agaimy A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Schölmerich J, Oefner PJ. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544-1552. [PubMed] |
| 111. | Daskalow K, Pfander D, Weichert W, Rohwer N, Thelen A, Neuhaus P, Jonas S, Wiedenmann B, Benckert C, Cramer T. Distinct temporospatial expression patterns of glycolysis-related proteins in human hepatocellular carcinoma. Histochem Cell Biol. 2009;132:21-31. [PubMed] |
| 112. | Franckhauser S, Elias I, Rotter Sopasakis V, Ferré T, Nagaev I, Andersson CX, Agudo J, Ruberte J, Bosch F, Smith U. Overexpression of Il6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia. 2008;51:1306-1316. [PubMed] |
| 113. | Lazaridis KN, Pham L, Vroman B, de Groen PC, LaRusso NF. Kinetic and molecular identification of sodium-dependent glucose transporter in normal rat cholangiocytes. Am J Physiol. 1997;272:G1168-G1174. [PubMed] |
| 114. | Tang Y, Chen A. Curcumin prevents leptin raising glucose levels in hepatic stellate cells by blocking translocation of glucose transporter-4 and increasing glucokinase. Br J Pharmacol. 2010;161:1137-1149. [PubMed] |
| 115. | Aschenbach JR, Steglich K, Gäbel G, Honscha KU. Expression of mRNA for glucose transport proteins in jejunum, liver, kidney and skeletal muscle of pigs. J Physiol Biochem. 2009;65:251-266. [PubMed] |
| 116. | Shinoda Y, Suzuki T, Sugawara-Yokoo M, Nagamatsu S, Kuwano H, Takata K. Expression of sugar transporters by in vivo electroporation and particle gun methods in the rat liver: Localization to specific membrane domains. Acta Histochemica Et Cytochemica. 2001;34:15-24. |
| 117. | Goto M, Yoshioka T, Battelino T, Ravindranath T, Zeller WP. TNFalpha decreases gluconeogenesis in hepatocytes isolated from 10-day-old rats. Pediatr Res. 2001;49:552-557. [PubMed] |
| 118. | Levitsky LL, Zheng Q, Mink K, Rhoads DB. GLUT-1 and GLUT-2 mRNA, protein, and glucose transporter activity in cultured fetal and adult hepatocytes. Am J Physiol. 1994;267:E88-E94. [PubMed] |
| 119. | Tal M, Kahn BB, Lodish HF. Expression of the low Km GLUT-1 glucose transporter is turned on in perivenous hepatocytes of insulin-deficient diabetic rats. Endocrinology. 1991;129:1933-1941. [PubMed] |
| 120. | Bilir BM, Gong TW, Kwasiborski V, Shen CS, Fillmore CS, Berkowitz CM, Gumucio JJ. Novel control of the position-dependent expression of genes in hepatocytes. The GLUT-1 transporter. J Biol Chem. 1993;268:19776-19784. [PubMed] |
| 121. | Nanji AA, Fogt F, Griniuviene B. Alterations in glucose transporter proteins in alcoholic liver disease in the rat. Am J Pathol. 1995;146:329-334. [PubMed] |
| 122. | Spolarics Z, Pekala PH, Bagby GJ, Spitzer JJ. Brief endotoxemia markedly increases expression of GLUT1 glucose transporter in Kupffer, hepatic endothelial and parenchymal cells. Biochem Biophys Res Commun. 1993;193:1211-1215. [PubMed] |
| 123. | Bélanger M, Desjardins P, Chatauret N, Butterworth RF. Selectively increased expression of the astrocytic/endothelial glucose transporter protein GLUT1 in acute liver failure. Glia. 2006;53:557-562. [PubMed] |
| 124. | Axelrod JD, Pilch PF. Unique cytochalasin B binding characteristics of the hepatic glucose carrier. Biochemistry. 1983;22:2222-2227. [PubMed] |
| 125. | Thorens B, Weir GC, Leahy JL, Lodish HF, Bonner-Weir S. Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc Natl Acad Sci U S A. 1990;87:6492-6496. [PubMed] |
| 126. | Elliott KR, Bate AJ, Craik JD. Specificity of the rat hepatocyte monosaccharide transporter. Int J Biochem. 1984;16:1251-1253. [PubMed] |
| 127. | Eisenberg ML, Maker AV, Slezak LA, Nathan JD, Sritharan KC, Jena BP, Geibel JP, Andersen DK. Insulin receptor (IR) and glucose transporter 2 (GLUT2) proteins form a complex on the rat hepatocyte membrane. Cell Physiol Biochem. 2005;15:51-58. [PubMed] |
| 128. | Escrivá F, González-Rodriguez A, Fernández-Millán E, Rondinone CM, Alvarez C, Valverde AM. PTP1B deficiency enhances liver growth during suckling by increasing the expression of insulin-like growth factor-I. J Cell Physiol. 2010;225:214-222. [PubMed] |
| 129. | Guillam MT, Burcelin R, Thorens B. Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes. Proc Natl Acad Sci U S A. 1998;95:12317-12321. [PubMed] |
| 130. | González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946-1957. [PubMed] |
| 131. | Ganguly A, Devaskar SU. Glucose transporter isoform-3-null heterozygous mutation causes sexually dimorphic adiposity with insulin resistance. Am J Physiol Endocrinol Metab. 2008;294:E1144-E1151. [PubMed] |
| 132. | Kurata T, Oguri T, Isobe T, Ishioka S, Yamakido M. Differential expression of facilitative glucose transporter (GLUT) genes in primary lung cancers and their liver metastases. Jpn J Cancer Res. 1999;90:1238-1243. [PubMed] |
| 133. | Nevado C, Valverde AM, Benito M. Role of insulin receptor in the regulation of glucose uptake in neonatal hepatocytes. Endocrinology. 2006;147:3709-3718. [PubMed] |
| 134. | Weston CJ, Adams DH. Hepatic consequences of vascular adhesion protein-1 expression. J Neural Transm. 2011;118:1055-1064. [PubMed] |
| 135. | Hoffler U, Hobbie K, Wilson R, Bai R, Rahman A, Malarkey D, Travlos G, Ghanayem BI. Diet-induced obesity is associated with hyperleptinemia, hyperinsulinemia, hepatic steatosis, and glomerulopathy in C57Bl/6J mice. Endocrine. 2009;36:311-325. [PubMed] |
| 136. | Ciaraldi TP, Abrams L, Nikoulina S, Mudaliar S, Henry RR. Glucose transport in cultured human skeletal muscle cells. Regulation by insulin and glucose in nondiabetic and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1995;96:2820-2827. [PubMed] |
| 137. | Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest. 1998;101:2377-2386. [PubMed] |
| 138. | Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587-12594. [PubMed] |
| 139. | Sakar Y, Nazaret C, Lettéron P, Ait Omar A, Avenati M, Viollet B, Ducroc R, Bado A. Positive regulatory control loop between gut leptin and intestinal GLUT2/GLUT5 transporters links to hepatic metabolic functions in rodents. PLoS One. 2009;4:e7935. [PubMed] |
| 140. | Waddell ID, Zomerschoe AG, Voice MW, Burchell A. Cloning and expression of a hepatic microsomal glucose transport protein. Comparison with liver plasma-membrane glucose-transport protein GLUT 2. Biochem J. 1992;286:173-177. [PubMed] |
| 142. | Takanaga H, Chaudhuri B, Frommer WB. GLUT1 and GLUT9 as major contributors to glucose influx in HepG2 cells identified by a high sensitivity intramolecular FRET glucose sensor. Biochim Biophys Acta. 2008;1778:1091-1099. [PubMed] |
| 143. | Preitner F, Bonny O, Laverrière A, Rotman S, Firsov D, Da Costa A, Metref S, Thorens B. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci U S A. 2009;106:15501-15506. [PubMed] |
| 144. | Gorovits N, Cui L, Busik JV, Ranalletta M, Hauguel de-Mouzon S, Charron MJ. Regulation of hepatic GLUT8 expression in normal and diabetic models. Endocrinology. 2003;144:1703-1711. [PubMed] |
| 145. | Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58:754S-765S. [PubMed] |
| 146. | Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K, Hayashi S, Yamaji R, Inui H, Fukusato T, Yamanouchi T. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr. 2009;139:2067-2071. [PubMed] |
| 147. | Evans A, Bates V, Troy H, Hewitt S, Holbeck S, Chung YL, Phillips R, Stubbs M, Griffiths J, Airley R. Glut-1 as a therapeutic target: increased chemoresistance and HIF-1-independent link with cell turnover is revealed through COMPARE analysis and metabolomic studies. Cancer Chemother Pharmacol. 2008;61:377-393. [PubMed] |
| 148. | Le Calvé B, Rynkowski M, Le Mercier M, Bruyère C, Lonez C, Gras T, Haibe-Kains B, Bontempi G, Decaestecker C, Ruysschaert JM. Long-term in vitro treatment of human glioblastoma cells with temozolomide increases resistance in vivo through up-regulation of GLUT transporter and aldo-keto reductase enzyme AKR1C expression. Neoplasia. 2010;12:727-739. [PubMed] |
| 149. | Morgan BJ, Chai SY, Albiston AL. GLUT4 associated proteins as therapeutic targets for diabetes. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:25-32. [PubMed] |